Back to Journals » Research and Reports in Urology » Volume 14
Outcomes and Prognostic Factors of Patients with Urothelial Carcinoma Undergoing Radical Cystectomy and pT0 in the Final Histology Without Neoadjuvant Chemotherapy
Authors Rodler S, Buchner A , Eismann L, Schulz GB, Marcon J, Ledderose S, Schlenker B , Stief CG, Karl A, Jokisch JF
Received 10 May 2022
Accepted for publication 26 July 2022
Published 1 August 2022 Volume 2022:14 Pages 281—290
DOI https://doi.org/10.2147/RRU.S374068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Severin Rodler,1 Alexander Buchner,1 Lennert Eismann,1 Gerald Bastian Schulz,1 Julian Marcon,1 Stephan Ledderose,2 Boris Schlenker,1 Christian G Stief,1 Alexander Karl,3 Jan-Friedrich Jokisch1
1Department of Urology, Klinikum der Universität München, Munich, Germany; 2Department of Pathology, Klinikum der Universität München, Munich, Germany; 3Department of Urology, Barmherzige Brüder Krankenhaus München, Munich, Germany
Correspondence: Severin Rodler, Department of Urology, Klinikum der Universität München, Marchioninistr. 15, München, 81377, Germany, Tel +089 4400 73530, Fax +089 4400 76532, Email [email protected]
Purpose: Absence of tumor in the final histopathology after radical cystectomy (RC) is a rare but potentially favorable outcome. Therefore, we aimed to analyze outcomes and prognostic factors of patients with urothelial carcinoma (UC) undergoing RC and T0 in the final histology without neoadjuvant chemotherapy at a high-volume academic center.
Patients and Methods: We retrospectively analyzed patients undergoing RC for pure UC between 2004 and 2020. Cancer-specific survival (CSS) and overall survival (OS) were calculated using Kaplan–Meier analysis and group comparison by Log rank test. Potential prognostic factors were analyzed using univariate Cox regression models.
Results: A total of 1051 patients with UC underwent RC. 72 patients (6.7%) showed pT0 in the final histology. Across all T-stages, 5-year CSS was significantly different with 88% for pT0, 80% for pTa/pTis, 78% for pT1, 76% for pT2, 51% for pT3 and 27% for pT4 in our cohort (p=0.001). Neither instillation therapy (HR 0.31, 95% CI 0.07– 1.43), number of TURB prior RC (HR 1.47, 95% CI 0.25– 6.18), use of photodynamic diagnostics (PDD) (HR 0.64, 95% CI 0.14– 3.02), performing a second resection (HR 0.87, 95% CI 0.27– 2.86), muscle-invasive disease prior RC at any TURB (HR 0.7, 95% CI 0.2– 2.39) or muscle-invasive disease in the TURB prior RC (HR 1.0, 0.31– 3.29) were associated with CSS in univariate analysis.
Conclusion: pT0 reveals a survival benefit in patients undergoing RC for UC and therefore presents a distinctive tumor entity. As clinical and cystoscopic characteristics do not improve patient stratification, further research is warranted to define risk groups in this specific tumor entity.
Keywords: bladder cancer, radical cystectomy, pT0 status after cystectomy
Plain Language Summary
We performed a study with patients undergoing radical cystectomy for bladder cancer. The absence of tumor in the final histology report is a favorable condition after radical cystectomy and is associated with improved outcomes.
Introduction
Bladder cancer (BC) presents the 10th most common tumor entity worldwide.1 According to European guidelines, radical cystectomy (RC) with regional pelvic lymph node dissection presents the established surgical standard therapy for patients with muscle invasive bladder cancer (MIBC) or high-risk non-muscle invasive bladder cancer (NMIBC).2 Several clinical parameters, such as preoperative thrombocytosis, platelet-to-leukocyte ratio, CRP-levels or ASA-Score were found to be prognostically relevant. Nevertheless, cancer stage and lymph node status still remain the strongest prognostic indicator in BC patients´ oncological outcome.3,4 Thereby, the absence of tumor in the final histology (pT0) has gained interest in recent years. Although previous series describe a 5– 25% rate of RC patients showing no evidence of malignancy at the postoperative histopathological examination, uncertainty remains concerning the clinical outcome of pT0 stage. Several groups indicate that pT0 in RC specimens are associated with improved oncological outcome compared with those with residual disease in final histology.5–10 However, Amling et al described an inferior prognosis for pT0 patients compared with patients presenting pTIS or pTa in RC specimen in a small sample cohort.11 Mainly retrospective studies described the oncological outcome of pT0 disease to be associated with varying risk factors including lymph node metastases or gender.8,12 Interestingly, some patients progress even though there is no evidence of residual tumor at the time of RC. Hence pT0 presents an unique and contentious pathological stage invariably challenging treating physicians.7
So far, most studies have failed to elucidate clinical and pathological prognostic factors that lead to pT0. Therefore, the aim of this study is to provide a conclusive analysis of the clinical and prognostic impact of pT0 on patients after RC for UC. Besides, this study focuses on the potential impact of preoperative clinical findings on pT0 disease and therefore tries to provide additional insights on the natural history of pT0 in RC specimens without prior neoadjuvant chemotherapy.
Materials and Methods
Patients Selection and Collection of Data
Inclusion criteria encompassed patients who underwent RC in a curative intent for urothelial cancer of the bladder only and without neoadjuvant treatment as previous chemotherapy or radiotherapy between April 2004 and December 2020. Further, variant histologies have been excluded as they might harbor a different biological behavior than pure UC. All patients underwent RC with a standardized open surgical approach as described before.13 Preoperative staging included either computed tomography (CT) or magnetic resonance imaging (MRI) of abdomen and chest. Only patients with absence of metastatic disease were included.
Preoperative Intravesical Findings and Treatment
A comprehensive revision of patients’ medical history concerning prior transurethral procedures and adjuvant intravesical therapy was performed. Beside the number of transurethral resections (TURB) and the performance of a second resection, the utilization of photodynamic diagnostic (PDD) was documented. Cystoscopic findings were estimated tumor size, associated Cis, highest and last t-stage respectively. Furthermore, adjuvant intravesical therapies with mitomycin c (MMC) and Bacillus Calmette–Guérin (BCG) were documented.
Pathological Evaluation
RC specimens and separately collected pelvic lymph nodes were processed in histopathological standard procedures at our Institute of Pathology and classified by pathologists according to the respective WHO classifications and Union for International Cancer Control (UICC) TNM staging systems (6th–8th edition).14 Pathological characteristics (T stage, N stage) were determined based on the archived histopathological reports. To check and ensure the accuracy and validity of the histopathological findings, accessible archival material from patients who had a tumor classified as pT0 was microscopically re-examined.
Follow-up
The follow-up regimes were adapted to the recommendations of EAU guidelines and included CT or MRI scans of thorax, abdomen and small pelvis, as well as analyses of blood samples.2 The clinical follow-up was performed at our outpatient clinic as well as using regular postal and personal validation questionnaires that were sent to the patient twice during the first postoperative year and annually afterwards. All patients were followed up until death or lost to follow-up. Cause of death was defined by the treating physicians or death certificate.
Statistical Analysis
Kaplan–Meier method was used to calculate cancer-specific survival. Log rank test was performed to analyze survival differences between groups. Potential prognostic factors were analyzed using univariate Cox regression models. A p-value of less than 0.05 was considered statistically significant. Calculations were performed using MedCalc 20 statistical software (MedCalc, Ostend, Belgium).
Results
Patient Characteristics
A total of 1068 patients with urothelial carcinoma of the bladder and RC met the inclusion criteria and were included in the study. From this cohort, 72 patients (6.7%) presented with pT0 in the final histopathological report. The median age was 70 years (IQR 64–75) and the median follow-up time was 80 months (IQR 37–116). Thirty-one patients (43.1%) had muscle-invasive bladder cancer (≥T2) in the TURB prior RC. pT1 was observed in 25 patients (35%), pTis in 11 (15%) and pTa in 5 patients (7%). None of the patients revealed radiological signs of metastases prior RC. The median time interval between the first TURB and RC was 2.3 months (IQR 1.0–6 months). In 58 patients (81%) lymph nodes were found in the final histological specimen after RC. All patient characteristics are listed in Table 1.
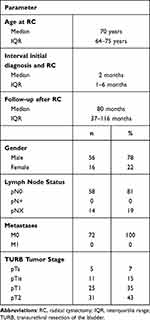 |
Table 1 Patient Characteristics of pT0 Cohort |
Survival Analysis
Five-year CSS was significantly different across all T-stages with 88% for pT0, 80% for pTa/pTis, 78% for pT1, 76% for pT2, 51% for pT3 and 27% for pT4 in our overall cohort of patients with urothelial carcinoma of the bladder (p=0.001). CSS compared only between pT0 and pTa/Tis (p=0.073) and between pT0 and pT1 (p=0.184) revealed no significant differences. However, CSS between pT0 and pT2 was significantly different (p=0.003) (Figure 1).
Anaylsis of Prognostic Factors in pT0 Disease
We performed univariate analysis for intravesical instillation therapy, surgical and technical aspects of the transurethral tumor resection prior RC, and pathological features of the TURB and RC. Regarding intravesical instillation therapy neither instillation therapy itself (HR 0.31, 95% CI 0.07–1.43), Bacillus Calmette–Guérin (BCG) (HR 0.38, 95% CI 0.08–1.78) or mitomycin administration (HR 0.43, 95% CI 0.05–3.39) was correlated significantly with CSS. Surgical and technical aspects of tumor resection as the number of TURB prior RC (HR 1.47, 95% CI 0.25–6.18), use of photodynamic diagnostics (PDD) (HR 0.64, HR 0.14–3.02), median time from first TURB (HR 1.02, 95% CI 0.31–3.34) and performing a second resection (HR 0.87, 95% CI 0.27–2.86) were not associated with CSS as well. Finally, pathological features such as simultaneous carcinoma in situ (CIS) (HR 0.21, 95% CI 0.03–1.61), muscle-invasive disease prior RC at any TURB (HR 0.7, 95% CI 0.2–2.39), muscle-invasive disease in the TURB prior RC (HR 1.0, 95% CI 0.31–3.29) and tumor size larger than 3 cm (HR 0.98, 95% CI 0.21–4.67) were not associated with CSS in the pT0 cohort (Table 2). Gender was analyzed after pT0 and was not associated with improved outcomes (Figure 2).
 |
Table 2 Potentially Prognostic Factors of pT0 |
Discussion
Only limited data exist on the outcome of patients with an absence of residual tumor in RC specimen. Whether pT0 confers a better survival rate compared with previous pathological stages, and how clinical characteristics determine pT0 stage, remains unclear. Postoperative pT0 status may be due to a complete TURB, tumor eradication by neoadjuvant therapy or pathological under-sampling in final specimen.5 Preexisting data on the oncological outcome of these patients remains non-homogeneous and not timely and therefore may not represent current practice patterns regarding quality of surgical resection.9
The primary objective of this series was therefore to evaluate a contemporary and homogeneous predefined cohort concerning oncological outcome of pT0 following RC. We therefore present the largest analysis of preoperative patient characteristics and prior intravesical treatments of patients presenting pT0 in final specimen. In order to consolidate the interpretation of the natural cause of pT0, patients with neoadjuvant treatment were excluded. A certain proportion of patients undergoing RC is not receiving neoadjuvant chemotherapy due to cisplatin ineligibility or patient preference. More recently, due to the COVID-19 pandemic, it has been recommended not to provide neoadjuvant chemotherapy in certain patients.15
Our cohort consisted of 72 patients with pT0 (6.7%), which is consistent with pT0 rates in the literature, where ranges from 5–25% are stated.7,9 In our series patients with pT0 after RC presented oncological outcomes identical to those showing non-muscle-invasive BC, such as pTa, pTiS and pT1. Besides that, survival of patients with pT0 in final specimens were significantly better than in patients remaining pT2 (88% vs 76%). Interestingly, pT0 down-staging showed this tendency towards better outcomes regardless of the prior tumor stage. Comparable findings were described by Volkmer et al who also did not observe survival differences among pT0 patients and those presenting pTa, pTis and pT1, respectively.8 Nevertheless, pT2 patients, who were down-staged to pT0 stage, also revealed significant improved CSS compared with stage-equivalent tumors (pT0 92.0% vs pT2 70.9%). Cho et al found similar survival benefits for pT0 following cT2 and in comparison to patients with residual MIBC.16 May et al also support our findings, by suggesting that only pT0 after cT2 BC shows significantly better CSS. There were no benefits in CSS for pT0 cases post NMIBC in previous TURB.17 Finally, Tilki et al presented a series of 228 pT0 patients after RC without prior neoadjuvant therapy.9 In their study, pT0 patients had outcomes similar to those with pTa and pTiS and therefore were significantly better than invasive tumor stages (≥pT1). Therefore, consolidated findings indicate that at any rate muscle-invasive tumor down-staged to pT0 may show significant improved oncological outcome, compared with patients with residual disease final specimen.
Controversially other authors implied that oncological outcomes of pT0 may only reflect the prior tumor stage.7,11 However, these series consisted of non-homogeneous cohorts, including at least one third of patients with NAC.7 This may have aggravated the data since NAC has been demonstrated to be associated with improved survival in univariate analysis, but fails to be prognostic in multivariate analysis compensating for confounders.18 Amling et al stated even a worsening of pT0 patients compared with pTa and pTIS patients, although no conclusive explanation for an impaired prognosis of these patients could be given.11
The impact of gender on survival after pT0 has been found to be independently prognostic by Tilki et al.9 Their 5-year recurrence-free survival was 92.2% for male patients and 77.7% for female patients. Moreover, a large body of evidence supports inferior survival rates by tumor stage and also a higher incidence and severity of disease for female patients.19 This might be due to delay of diagnosis, inequalities in healthcare and distinctions in treatment. Nevertheless, in our study, we do not observe a gender-associated difference in survival rates for pT0 stage. In line with this, results from a SEER-Medicare database showed no significant gender differences in survival rates for female patients presenting pT1–pT3 UC.20 This is consistent with our findings and therefore indicates that differences in sociodemographic characteristics might be responsible for a possible gender gap in tumor survival rates. Furthermore, the potential role of hormonally mediated differences in UC for women or an otherwise likelihood for female patients to harbor biologically more aggressive BC, remains uncertain.19
Tilki et al also revealed lymphatic spread as a prognostic feature.9 In our cohort, no patient with pT0 revealed lymph node metastases at the time of RC and therefore an analysis of this factor is not possible. Also 19% of the pT0 cohort had not received lymphadenectomy due to feasibility as prior pelvic or abdominal surgery and thereby limits further analysis of this potential factor in our cohort. The precise detection of lymphovascular invasion at the time of diagnosis is currently still challenging, as preoperative imaging modalities have a low staging accuracy.21,22 The rate of occult lymph node metastases is high (4%) and important to consider when counselling patients, because it directly impacts the survival of patients.23
Apart from initial tumor stage other local individual patient findings may offer insights on potential outcomes of pT0. We present a comprehensive and contemporary analysis of those findings. Interestingly, May et al found that a more recently performed TURB was associated with pT0 compared with older patient cohorts potentially treated with different surgical techniques. This emphasizes the need to investigate predictors in contemporary cohorts.17
The time from first diagnosis to RC or the number of performed TURB were not prognostic in our cohort. Moreover, it has been observed that the time from TURB to RC does not impact pT0 rates.24 Achieving pT0 might depend on prior radical transurethral resection of the tumor, rather than prior intravesical treatment or time between TURB and RC. Further, the use of advanced imaging technologies during cystoscopy such as PDD did not affect outcomes of patients. Prior use of instillation therapies has no prognostic impact on pT0 patients. This fact further highlights the unique surgical impact and potential need for radical approaches. Surgeon´s experience, and conducting standardized TURB procedures and completeness of TURB have been demonstrated to contribute to pT0 rates in RC.24 However, absence of tumor in the last TURB prior RC does not necessarily predict pT0 in the final histology after RC25 and therefore impacts bladder-preserving strategies in this setting.
Due to the COVID-19 pandemic clinical relevance of pT0 seems very relevant since NAC was often not feasible.26 Therefore, we focused on a cohort without prior treatment. In addition, pT0 after pure surgical treatment might present a superior biological behavior, as localization, multifocality, invasiveness and especially lymphovascular invasion might be different compared with patients with other T stages after surgery.
The study is limited by its retrospective design. However, it presents with 1068 patients with pure urothelial carcinoma and complete follow-up in one of the largest, contemporary, single-center studies on this topic. Still, only 72 patients revealed pT0 in the final histology. This fact might cause underpower of this study potentially leading to p-values not reaching significance. Further, patients with variant histologies of bladder cancer have been excluded from this study. Conclusions regarding pT0 in those subgroups can therefore not be drawn from the present study.
Conclusion
pT0 at the time of RC is observed in a minority of patients and is associated with improved survival in a cohort of patients without neoadjuvant chemotherapy. However, some patients progress despite no evidence of disease at the time of RC. Clinical characteristics reveal no specific subgroup within pT0 patients to identify high-risk patients. Further research is warranted to elucidate prognostic factors, improve quality of resection, and ultimately improve patient outcomes.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Statement of Ethics
The study was performed in accordance with the Declaration of Helsinki.
Prior to initiation of the study, the study design was approved by the local ethics committee of LMU Munich (Reference number 20-179). Written informed consent to participate in the study was obtained from all participants.
Acknowledgments
We thank all patients included in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No specific funding was obtained for this project.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Witjes JA, Bruins HM, Cathomas R, et al. European Association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. doi:10.1016/j.eururo.2020.03.055
3. Rodler S, Buchner A, Ledderose ST, et al. Prognostic value of pretreatment inflammatory markers in variant histologies of the bladder: is inflammation linked to survival after radical cystectomy? World J Urol. 2021;39(7):2537–2543. doi:10.1007/s00345-020-03482-8
4. Svatek RS, Shariat SF, Novara G, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107(6):898–904. doi:10.1111/j.1464-410X.2010.09628.x
5. Hotston M, Patel S, Sohail M, Persad RA. What is the significance of pT0 at cystectomy? Surg Oncol. 2006;15(2):65–69. doi:10.1016/j.suronc.2006.07.005
6. Kassouf W, Spiess PE, Brown GA, et al. P0 stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol. 2007;52(3):769–776. doi:10.1016/j.eururo.2007.03.086
7. Thrasher JB, Frazier HA, Robertson JE, Paulson DF. Does of stage pT0 cystectomy specimen confer a survival advantage in patients with minimally invasive bladder cancer? J Urol. 1994;152(2 Pt 1):393–396. doi:10.1016/S0022-5347(17)32746-5
8. Volkmer BG, Kuefer R, Bartsch G
9. Tilki D, Svatek RS, Novara G, et al. Stage pT0 at radical cystectomy confers improved survival: an international study of 4430 patients. J Urol. 2010;184(3):888–894. doi:10.1016/j.juro.2010.04.081
10. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the national cancer data base. Int J Radiat Oncol Biol Phys. 2014;88(5):1048–1056. doi:10.1016/j.ijrobp.2014.01.001
11. Amling CL, Thrasher JB, Frazier HA, Dodge RK, Robertson JE, Paulson DF. Radical cystectomy for stages Ta, Tis and T1 transitional cell carcinoma of the bladder. J Urol. 1994;151(1):31–35. doi:10.1016/s0022-5347(17)34865-6
12. Palapattu GS, Shariat SF, Karakiewicz PI, et al. Cancer specific outcomes in patients with pT0 disease following radical cystectomy. J Urol. 2006;175(5):1645–1649. doi:10.1016/s0022-5347(05)00995-x
13. Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176(2):486–492. doi:10.1016/j.juro.2006.03.038
14. Amin MB, Edge S, Greene F. AJCC Cancer Staging Manual. Springer; 2016.
15. Ribal MJ, Cornford P, Briganti A, et al. European Association of urology guidelines office rapid reaction group: an organisation-wide collaborative effort to adapt the European Association of urology guidelines recommendations to the coronavirus disease 2019 era. Eur Urol. 2020;78(1):21–28. doi:10.1016/j.eururo.2020.04.056
16. Cho KS, Seo JW, Park SY, et al. The prognostic significance of pathologic stage T0 on organ-confined bladder transitional cell carcinoma following radical cystectomy. Urol Int. 2008;81(4):394–398. doi:10.1159/000167835
17. May M, Bastian PJ, Burger M, et al. Multicenter evaluation of the prognostic value of pT0 stage after radical cystectomy due to urothelial carcinoma of the bladder. BJU Int. 2011;108(8 Pt 2):E278–83. doi:10.1111/j.1464-410X.2011.10189.x
18. Chromecki TF, Cha EK, Shariat SF. Stage pT0 after radical cystectomy: are all patients equal? Eur Urol. 2011;60(3):603–604. doi:10.1016/j.eururo.2011.05.050
19. Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105(3):300–308. doi:10.1111/j.1464-410X.2009.09076.x
20. Datta GD, Neville B, Datta NS, Earle C. Gender disparities in bladder cancer survival: an assessment of socio-demographic factors. Cancer Epidemiol Biomark Prev. 2006;15(12_Supplement):B38–B38.
21. Magers MJ, Kaimakliotis HZ, Barboza MP, et al. Clinicopathological characteristics of ypT0N0 urothelial carcinoma following neoadjuvant chemotherapy and cystectomy. J Clin Pathol. 2019;72(8):550–553. doi:10.1136/jclinpath-2019-205742
22. Rodler S, Solyanik O, Ingenerf M, et al. Accuracy and prognostic value of radiological lymph node features in variant histologies of bladder cancer. World J Urol. 2022;40(7):1707–1714. doi:10.1007/s00345-022-04010-6
23. van Hoogstraten LMC, van Gennep EJ, Kiemeney LALM, et al. Occult lymph node metastases in patients without residual muscle-invasive bladder cancer at radical cystectomy with or without neoadjuvant chemotherapy: a nationwide study of 5417 patients. World J Urol. 2022;40(1):111–118. doi:10.1007/s00345-021-03839-7
24. Mazaris E, Nafie S, Boustead G. Is TURBT able to cure high risk recurrent superficial or muscle invasive bladder cancer: factors resulting in pT0 radical cystectomy specimens. Int Braz J Urol. 2013;39(3):364–370. doi:10.1590/s1677-5538.Ibju.2013.03.09
25. Kukreja JB, Porten S, Golla V, et al. Absence of tumor on repeat transurethral resection of bladder tumor does not predict final pathologic t0 stage in bladder cancer treated with radical cystectomy. Eur Urol Focus. 2018;4(5):720–724. doi:10.1016/j.euf.2016.12.005
26. EAU MIBC guidelines panel: recommendations from the EAU MIBC guidelines panel applicable during the COVID-19 pandemic. Available from: https://uroweb.org/wp-content/uploads/Covid-19-EAU-MIBC-Recommendations.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


