Back to Journals » Cancer Management and Research » Volume 12
Outcomes and Experiences of Child-Bearing Women with Nasopharyngeal Carcinoma
Authors Ma L, Chen F , Kong X, Xu T, Fei Z, Fang W, Wang B, Wu H
Received 31 May 2020
Accepted for publication 14 August 2020
Published 4 September 2020 Volume 2020:12 Pages 8047—8054
DOI https://doi.org/10.2147/CMAR.S265371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Liqin Ma,1,* Fei Chen,2,* Xiangquan Kong,3,* Ting Xu,2 Zhaodong Fei,1 Weining Fang,1 Binyi Wang,1 Haixia Wu2
1Department of Radiation Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, Fuzhou, People’s Republic of China; 2Fujian Medical University, Fuzhou, People’s Republic of China; 3Department of Radiation Oncology, Fujian Medical University Affiliated Xiamen Humanity Hospital, Fuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liqin Ma
Department of Radiation Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, No. 420, Fuma Road, Fuzhou, Fujian 350014, People’s Republic of China
Tel +8613609537603
Fax +86 591-83660063
Email [email protected]
Purpose: Nasopharyngeal carcinoma (NPC) is more common among women in Southeast Asia. An important issue is whether it is safe for them to bear children after treatment and when it is safe to do so. We conducted this study to explore the relation between fertility and prognosis in child-bearing women with NPC.
Patients and Methods: Child-bearing women were defined as young women between the ages of 18 and 30. A total of 127 eligible child-bearing NPC patients were identified from December 2003 to December 2014. The patients were divided into two groups, depending on whether or not they had post-therapeutic births. The Kaplan–Meier method was used for survival analyses. The Log rank test was used to compare two survival curves and the independent significances of different prognostic factors were assessed by Cox proportional hazards regression analysis.
Results: The 5-year overall survival (OS) and disease-free survival (DFS) in the Childbirth group were significantly higher than those in the Non-Childbirth group (100% vs 88.8%, P = 0.026 and 100% vs 77.5%, P = 0.007, respectively). In the Childbirth group, no difference was found in the 5-year DFS between different birth interval times, from 1 to 5 years after treatment. The clinical stage was identified as the risk factor of OS (HR = 101.725, 95% CI: 2.160– 4790.910, P = 0.019), and consequent childbirth after treatment was associated with favorable DFS (HR = 0.148, 95% CI: 0.034– 0.643, P = 0.011).
Conclusion: Post-therapeutic birth did not increase the mortality risk of child-bearing women with NPC. There was no significant correlation between the subsequent birth time window after treatment and the prognosis.
Keywords: nasopharyngeal carcinoma, fertility, child-bearing mortality risk, post-treatment
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common cancers and it occurs with much greater frequency in Southern China.1 The risk for males is two to three times higher than that for females.2,3 Radiotherapy (RT) is the main treatment for NPC,1–3 and intensity-modulated radiation therapy (IMRT) can achieve excellent 5-year local control rates, ≥90% for T3 disease and 74%–80% for T4 disease.4 Chemotherapy has made a small, but significant, contribution to improve overall survival (OS) and event-free survival.5 Concurrent chemoradiotherapy has been the standard treatment for advanced NPC.5–7 It was reported that women showed better survival outcomes than men and the survival of patients younger than 40 years old was better than older patients.8 As the probability of survival improves, female NPC patients of child-bearing age may consequently consider conception after recovery.
However, cancer treatments may cause gonadal toxicities,9,10 which is especially emphasized in female patients of child-bearing age.11 In previous studies, among 24 females who treated for Hodgkin’s disease in childhood, 17patients (70.8%) had normal gonadal function with regular menstrual periods.12 Besides, chemotherapy has a negative influence on pregnancy outcomes13 and increases the incidence of neonatal neutropenia.14 It was unclear whether subsequent childbearing would increase the mortality risk of women with carcinoma. Related studies are rare and no investigator has yet answered the question. This retrospective analysis is aimed at exploring the relation between fertility and prognosis in child-bearing patients with NPC. It aims to provide further reference for this subgroup of patients who plan to have children after treatment.
Patients and Methods
Patient Characteristics
The characteristics of the patients are presented in Table 1. From December 2003 to December 2014, a total of 127 female NPC patients of child-bearing age, diagnosed in Fujian Cancer Hospital, were enrolled. The main inclusion criteria consisted of the following: (i) histopathology confirmed primary NPC patients; (ii) complete clinical information and medical history, adequate clinical examination, and laboratory data; (iii) received entire treatment in our center (radical IMRT or two-dimensional conventional radiotherapy, with or without chemotherapy); (iv) absence of distant metastasis before, or during, treatment; (v) no evidence of carcinoma from another source or other severe disease; (vi) complete follow-up data; and (vii) patients no more than 30 years old. Patients were reclassified according to the eighth edition of the American Joint Committee on Cancer staging system. For every eligible female NPC patient, the year of completion of the treatment and subsequent years were screened for live births. Patients were divided into the Childbirth group and Non-Childbirth group, dependent on whether they had post-therapeutic births. The data included age; tumor, node, metastasis (TNM) stages; details of the chemotherapy and radiotherapy; details of the childbirth; and date of death. Data of this study were collected in a manner that the subjects could not be identified, so that no informed consent was required. The retrospective analysis received approval from the ethics committee of the Fujian Cancer Hospital (YKT2019-027-01) and was conducted in accordance with the Declaration of Helsinki.
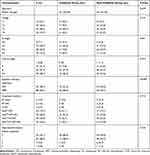 |
Table 1 Patients and Tumor Characteristics |
Treatment
All the therapeutic schemes regarding chemotherapy and radiotherapy were based on the clinical TNM stages, physicians’ absolute discretion, and patients’ choices.
Radiotherapy
Thirty-nine patients were treated with two-dimensional conventional radiotherapy, with a 1.8 or 2 Gy daily fraction and 5 fractions per week. Phase 1 of the conventional radiotherapy consisted of two fields of 36–40 Gy and the fields were then reduced off-cord to 50 Gy. Phase 2 brought a total dose of 68–72 Gy to the nasopharynx. Node negative patients received 50–54 Gy to the neck and node-positive patients were boosted to 66–70 Gy at the positive lymph node region. The other 88 patients received intensity-modulated radiotherapy, with a prescribed dose of 70 Gy in 31–35 fractions, at 2.0–2.25 Gy/fraction, to the planning target volume of the gross primary tumor volume and the nodal gross tumor volume. The target volume and radiotherapy dose were implemented using institutional treatment protocol as previously reported.15 Gross tumor volume (GTV) was outlined according to the information from computed tomography (CT) and magnetic resonance (MR) images and visible tumor. Clinical target volume 1 (CTV-1) was defined as the high-risk region including the GTV and 6 mm margin for potential microscopic disease. Clinical target volume 2 (CTV-2) was designed for regions potentially involved including nasopharyngeal cavity, maxillary sinus, pterygopalatine fossa, posterior ethmoid sinus, parapharyngeal space, skull base, anterior third of clivus and cervical, vertebra, inferior sphenoid sinus and cavernous sinus. Organs at risk (OARs) include brain stem, spinal cord, optic nerve, parotid and so on.
Chemotherapy
For patients at stage II–IV B, the concurrent chemotherapy regimen comprised two cycles of platinum-based chemotherapy (80mg/m2, on day 1, every 3 weeks). Patients at stages III–IV underwent two cycles of neoadjuvant chemotherapy which consisted of gemcitabine (1000 mg/m2, on days 1 and 8) plus cisplatin (80 mg/m2, on day 2); or paclitaxel (135 mg/m2, day 1) plus cisplatin (80 mg/m2, day 2). Concurrent platinum-based chemotherapy was administered to every patient as a standard treatment. Neoadjuvant and/or adjuvant chemotherapy were applied, according to the physician’s discretion. Neoadjuvant or adjuvant chemotherapy consisted of gemcitabine with cisplatin or paclitaxel with cisplatin. Chemotherapy was repeated every 3 weeks.
Follow-Up and Statistical Analysis
The data were collected every 3 months within the first 2 years and every 6 months thereafter. The follow-up period was from the diagnosis to the end of the follow-up or the date of death. The final date of the follow-up was January 2020. The pretreatment and treatment data were calculated and compared using Chi-square or Fisher’s texts and then processed using GraphPad Prism 8 software and R tool (Version 3.6.1). The Kaplan–Meier method was used for the survival analyses. The Log rank test was used to calculate the significance of the differences between the two survival curves. Cox proportional hazards regression analysis was used to assess the independent significance of different prognostic factors. Any difference was considered to be statistically significant if the p value was <0.05, based on two-sided tests. The primary clinical endpoint was OS and progression-free survival, defined as the date from diagnosis to death from any cause, or to disease progression or death from any cause, whichever occurred first.
Results
Patient Characteristics
A total of 127 consecutive patients at child-bearing age, with stage I–IVa NPC, were enrolled in the study. From the entire cohort, 38 patients had post-therapeutic births and were assigned to the Childbirth group, and 89 patients without post-therapeutic births were assigned to the Non-Childbirth group. All patients in the Childbirth group gave birth at least 1 year after treatment. Basic characteristics of all patients are presented in Table 1. For the entire cohort, the median age was 25 (range 18–30) years old. The median age was 23 (range 19–30) years old for the Childbirth group and 26 (range 18–30) years old for the Non-Childbirth group, respectively. There were no significant differences between the two groups in the age distribution (P = 0.107), T stage (P = 0.155), N stage (P = 0.461), clinical stage (P = 0.352), radiotherapy technique (P>0.999), treatment method (P = 0.715), and reproductive history before treatment (P = 0.232). The majority of the patients in both groups were at stages III–IV (84.2% vs 75.3%).
Influence of Post-Therapeutic Birth on Survival Outcomes
The median survival period of the Childbirth group was 11.0 years and only one patient died, while the median survival period in the Non-Childbirth group was 8.3 years, from which 15 patients (16.9%) died during follow-up. During the follow-up, relapse and metastasis occurred to one of the patients in the Childbirth group. Additionally, 2 patients (2.2%) experience a relapse, 11 patients (12.4%) had metastasis, and 3 patients (3.4%) had both a relapse and metastasis in the Non-Childbirth group. Between the Childbirth and Non-Childbirth groups, statistically significant differences were found in the 5-year OS rate and 5-year disease-free survival (DFS) (100% vs 88.8%, P = 0.026 and 100% vs 77.5%, P = 0.007, respectively) (Figure 1).
 |
Figure 1 Kaplan–Meier survival curves for the female patients with NPC in the Childbirth group and Non-Childbirth group. (A) overall survival, (B) disease-free survival. |
No difference was found in the 5-year DFS between different intervals, from the end of the treatment to the subsequent birth in the Childbirth group (Figure 2). To explore the impact of childbirth history before treatment, patients in the Non-Childbirth group were further divided into two subgroups. However, no significant differences were observed in the 5-year OS rate (88.9% vs 88.6%, P = 0.420) and 5-year DFS (82.2% vs 70.5%, P = 0.911) between the subgroups with and without a history of childbirth. Among the 45 patients with a history of childbirth in the Non-Childbirth group, 38 (84.4%) had one birth, 6 (13.3%%) had two births, and 1 (2.2%) had three births. No statistical difference was observed in the 5-year OS rate and 5-year DFS (P = 0.151 and P = 0.113, respectively).
 |
Figure 2 Kaplan–Meier survival curves for the female patients with NPC in different intervals between the accomplishment of treatment and subsequent birth. |
Multivariate Analyses of the Survival
Variables, including age, T stage, N stage, clinical stage, reproductive history, chemotherapy, and radiotherapy, were analyzed using multivariable analysis (Table 2). The clinical stage was found to be a risk factor of the OS (HR = 101.725, 95% CI: 2.160–4790.910, P = 0.019), and consequent childbirth after treatment was associated with favorable DFS (HR = 0.148, 95% CI: 0.034–0.643, P = 0.011).
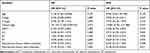 |
Table 2 Cox Regression Model of Multivariable Analysis for OS and DFS |
Discussion
The overall prognosis of NPC has been dramatically improved, owing to the advancements in patient management, including the improvement of RT technology, the broader application of chemotherapy, and staging systems with improved accuracy.4 Besides, Epstein-Barr virus (EBV) DNA has been used as tumor marker for NPC. The previous study has revealed that survival outcomes were significantly better in patients with undetectable EBV DNA level between pre-IMRT and mid/post IMRT.16 A previous randomized Phase 3 trial of gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma suggested that induction chemotherapy, added to chemoradiotherapy, had a significantly positive impact on recurrence-free survival and OS among patients with locoregionally advanced NPC.17 Similarly, Yang’s study came to the same conclusion—induction chemotherapy, followed by concurrent chemoradiotherapy, provided better DFS, distant metastasis-free survival and OS, compared with concurrent chemoradiotherapy alone in locoregionally advanced NPC.18 Moreover, the widespread application of IMRT has contributed to improved survival.19 With the combined use of IMRT and the addition of induction therapy and concurrent chemoradiotherapy, survival outcomes have been substantially improved in patients with NPC.
In Eastern and Southeastern Asia, delivering a baby is of great traditional importance. The continuity of ethnicity is one of the key points in the traditional concepts of these regions. As traditional societies modernize, many women are choosing to have children later in life. Therefore, some female patients with NPC were nulliparous before diagnosis. Thus, whether and when to bear children after treatment are important issues for NPC patients, as female NPC patients have satisfying recoveries.
In our cohort, patients from the Childbirth group experienced significantly better outcomes, regarding the 5-year OS rate and 5-year DFS, than those of the Non-Childbirth group. Multivariate analysis shows that subsequent birth after treatment was indicative of decreased risk in terms of DFS. The data suggest that having a post-therapeutic birth is a factor for a better prognosis. A previous study on the effect of post-therapeutic birth shows that the actual survival of women who give birth after breast cancer seemed unexpectedly high, which suggests that successful pregnancy may have in some way been protective for breast carcinoma patients.20 Similarly, our study indicates that a successful pregnancy may have the same positive influence on NPC patients. Moreover, the result of the study is generally similar to previous studies, which indicated that childbirth itself may not negatively influence the survival outcomes in patients with pregnancy-associated NPC.21,22 One woman, who wished to become pregnant after her NPC treatment, generally had a satisfying body recovery. The previous study about childbearing and survival after breast carcinoma in young women also has revealed that relatively healthier women are more likely to become pregnant than women with adverse disease sequelae or poor prognosis,23 which is called “healthy mother effect” and it may account for the result that patients who had delivered a child had a better prognosis.
An earlier study showed that reproductive history was a factor that contributed to a worse prognosis in patients with breast cancer.24 To further investigate the influence of childbirth, we divided the Non-Childbirth group into two subgroups, based on their reproductive history before treatment. The survival outcomes of the 5-year OS rate (88.9% vs 88.6%, P = 0.420) and 5-year DFS (82.2% vs 70.5%, P = 0.911) between the two subgroups had no significant difference. For female NPC patients of child-bearing age who did not deliver a child after treatment, reproductive history was not a prognostic factor. The influence of reproductive history on female NPC patients seems to be less significant than for other types of cancer, such as breast cancer. Contrary to an earlier study, which indicated that women who had three pregnancies had a significantly increased risk for NPC, compared with those who had two pregnancies,25 no statistical difference was observed in the current study. Owing to the lack of data on abortions that may happen to women who were parous, or had an unsatisfying recovery after the treatment, patients without completed pregnancies were not identified in our cohort, which led to this difference.
Women delivering infants with different interval times, from 1 to 5 years after the treatment, showed no difference in the 5-year DFS (100%). This suggests that delivering from 1 to 5 years after treatment had no influence on prognoses. NPC patients who gave birth more than 6 years after treatment had the result of a worse DFS. However, this result is open to discussion because the sample size of patients in this subgroup is too small to draw a convincing conclusion. Furthermore, most of the patients were beyond the optimal age for childbearing and were not willing to be pregnant. Unfortunately, our cohort lacks the data on women who delivered within 1 year after treatment. A previous study showed that women fertilized during initial treatment had worse prognoses than those fertilized at least 2 years after radiation therapy,26 indicating that childbearing within 1 year of the treatment is not recommended. In the earlier finished study,
Xu B‘s research has confirmed that NPC is a high expression tumor of estrogen receptors (ER) and progestogen receptors (PR); the positive intensity of ER and PR was associated with the negative influence of NPC.27 This suggests that the level of these hormones may influence the prognoses of patients who had post-therapeutic births in different interval times.
Conclusion
The results of this study demonstrate that post-therapeutic birth does not increase the mortality risk of child-bearing patients with NPC. Multivariate analysis showed that having a post-therapeutic birth was a significant prognostic factor in female NPC patients for a better DFS. Further analyses indicated that an applicable interval time between the completion of treatment and subsequent birth is 1–5 years. Based on this study, post-therapeutic birth is not associated with a worse prognosis. In contrary, successful pregnancy and post-therapeutic birth may have in some way been protective to patients. The final decision on whether to deliver a child or not entirely depends on the choice of individual patient and her family.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi:10.1016/S0140-6736(05)66698-6
2. Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–668. doi:10.1016/j.ijrobp.2010.03.024
3. Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi:10.1016/j.radonc.2012.08.013
4. Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi:10.1200/JCO.2015.60.9347
5. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase iii randomized intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. doi:10.1200/JCO.1998.16.4.1310
6. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21(4):631–637. doi:10.1200/JCO.2003.06.158
7. Sun XS, Liu SL, Luo MJ, et al. The association between the development of radiation therapy, image technology, and chemotherapy, and the survival of patients with nasopharyngeal carcinoma: a cohort study from 1990 to 2012. Int J Radiat Oncol Biol Phys. 2019;105(3):581–590. doi:10.1016/j.ijrobp.2019.06.2549
8. Sham JS, Choy D. Prognostic factors of nasopharyngeal carcinoma: a review of 759 patients. Br J Radiol. 1990;63:51–58. doi:10.1259/0007-1285-63-745-51
9. Garsi JP, Schlumberger M, Rubino C, et al. Therapeutic administration of 131I for differentiated thyroid cancer, radiation dose to ovaries and outcome of pregnancies. Radioprotection. 2008;43(5). doi:10.1051/radiopro:2008698
10. Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117(10 Suppl):2301–2310. doi:10.1002/cncr.26045
11. Lee BC, Yen RF, Lin CL, Liang JA, Lin MC, Kao CH. Pregnancy incidence in female nasopharyngeal carcinoma survivors of reproductive age: a population-based study. Medicine. 2016;95(20):e3729. doi:10.1097/MD.0000000000003729
12. Zaletel LZ, Bratanic N, Jereb B. Gonadal function in patients treated for Hodgkin’s disease in childhood. Radiol Oncol. 2010;44(3):187–193. doi:10.2478/v10019-010-0034-8
13. Danet C, Araujo M, Bos-Thompson MA, et al. Pregnancy outcomes in women exposed to cancer chemotherapy. Pharmacoepidemiol Drug Saf. 2018;27(12):1302–1308. doi:10.1002/pds.4689
14. La Nasa M, Gaughan J, Cardonick E. Incidence of neonatal neutropenia and leukopenia after in utero exposure to chemotherapy for maternal cancer. Am J Clin Oncol. 2019;42(4):351–354. doi:10.1097/COC.0000000000000527
15. Chen C, Fei Z, Pan J, Bai P, Chen L. Significance of primary tumor volume and T-stage on prognosis in nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Jpn J Clin Oncol. 2011;41(4):537–542. doi:10.1093/jjco/hyq242
16. Prayongrat A, Chakkabat C, Kannarunimit D, Hansasuta P, Lertbutsayanukul C. Prevalence and significance of plasma Epstein-Barr Virus DNA level in nasopharyngeal carcinoma. J Radiat Res. 2017;58(4):509–516. doi:10.1093/jrr/rrw128
17. Zhang Y, Chen L, Hu G-Q, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–1135. doi:10.1056/NEJMoa1905287
18. Yang Q, Cao SM, Guo L, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. doi:10.1016/j.ejca.2019.07.007
19. Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
20. Verkooijen HM, Lim GH, Czene K, et al. Effect of childbirth after treatment on long-term survival from breast cancer. Br J Surg. 2010;97(8):1253–1259. doi:10.1002/bjs.7131
21. Zhang L, Liu H, Tang LQ. Prognostic effect of pregnancy on young female patients with nasopharyngeal carcinoma: result from a match cohort analysis. Oncotarget. 2016;7(6):21913–21921. doi:10.18632/oncotarget.8008
22. Cheng YK, Zhang F, Tang LL, et al. Pregnancy associated nasopharyngeal carcinoma: a retrospective case-control analysis of maternal survival outcomes. Radiother Oncol. 2015;116(1):125–130. doi:10.1016/j.radonc.2015.06.008
23. Mueller BA, Simon MS, Deapen D, Kamineni A, Malone KE, Daling JR. Childbearing and survival after breast carcinoma in young women. Cancer. 2003;98(6):1131–1140. doi:10.1002/cncr.11634
24. Phillips KA, Milne RL, Friedlander ML, et al. Prognosis of premenopausal breast cancer and childbirth prior to diagnosis. J Clin Oncol. 2004;22(4):699–705. doi:10.1200/JCO.2004.07.062
25. Feng R-M, Chang ET, Liu Z, et al. Reproductive history and risk of nasopharyngeal carcinoma: a population-based case–control study in southern China. Oral Oncol. 2019;88:102–108. doi:10.1016/j.oraloncology.2018.11.025
26. Star J, Malee MP. Pregnancy complicated by nasopharyngeal carcinoma. Obstet Gynecol. 1999;94(5):845.
27. Xu B, Hu P, Wu Q, et al. [Relationship between expression of estrogen receptor progesterone receptor and the biological characteristics of nasopharyngeal carcinoma]. Lin Chuang Er Bi Yan Hou Ke Za Zhi = J Clin Otorhinolaryngol. 1999;13(8):347–349.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
