Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis
Authors Kate S , Chougule A, Joshi A, Noronha V, Patil V, Dusane R, Solanki L, Tiwrekar P, Trivedi V , Prabhash K
Received 25 July 2018
Accepted for publication 10 December 2018
Published 29 January 2019 Volume 2019:10 Pages 1—10
DOI https://doi.org/10.2147/LCTT.S181406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Shruti Kate,1 Anuradha Chougule,2 Amit Joshi,1 Vanita Noronha,1 Vijay Patil,1 Rohit Dusane,3 Leena Solanki,1 Priyanka Tiwrekar,2 Vaishakhi Trivedi,1 Kumar Prabhash1
1Department of Medical Oncology, Tata Memorial Hospital, Mumbai, Maharashtra, India; 2Department of Molecular Pathology, Tata Memorial Hospital, Mumbai, Maharashtra, India; 3Department of Biostatistics, Clinical Research Secretariat, Tata Memorial Hospital, Mumbai, Maharashtra, India
Background: The significance of uncommon EGFR mutations in newly diagnosed advanced non-small-cell lung cancer (NSCLC) patients is incompletely known. We aimed to analyze the demographic profile, outcome, and treatment attributes of these patients.
Patients and methods: We retrospectively surveyed 5,738 advanced NSCLC patients who underwent EGFR testing in our center from 2013 to 2017 by in-house primer probes on real time PCR platform. Descriptive data were accumulated from electronic medical records. Survival plot was calculated using Kaplan–Meier method and compared between groups using log-rank test.
Results: Out of 1,260 EGFR mutation-positive patients, 83 (6.58%) had uncommon mutations in isolation or in various combinations. Uncommon mutations were more frequent in men, never-smokers, and adenocarcinomas. Overall, exon 18 G719X, exon 20 insertion, exon 20 T790M, exon 20 S768I, and exon 21 (L858R/L861Q) were present in 9.6%, 19.3%, 12%, 3.6%, and 3.6% patients, respectively. Dual mutation positivity was found in 50.6% patients. On classifying patients as per tyrosine kinase inhibitor (TKI) sensitivity, it was found that majority of the patients had a combination TKI sensitive and insensitive mutations. The median duration of follow-up was 13 months. Five patients were lost to follow-up. Median progression-free survival on first line therapy was 6.7 months (95% CI: 4.8–8.5). Median overall survival (OS) of patients who received TKI during the course of their disease was 20.2 months (95% CI: 11.4–28.9). Median overall survival (mOS) of the entire cohort was 15.8 months (95% CI: 10.1–21.5). Among all uncommon mutations, patients with dual mutations did better, with an mOS time of 22.6 months (95% CI: 8.2–37.0, P=0.005). It was observed that TKI sensitive/TKI insensitive dual mutations had a superior OS of 28.2 months (95% CI: 15.2–41.2, P=0.039) as compared to TKI sensitive and TKI insensitive EGFR mutations.
Conclusion: Uncommon EGFR mutations constitute a heterogeneous group, hence, it is imperative to understand each subgroup more to define optimal treatment.
Keywords: uncommon EGFR mutations, advanced NSCLC, tyrosine kinase inhibitors, complex EGFR mutations, dual EGFR mutations
Introduction
The discovery of somatic mutations in EGFR and use of targeted therapy with oral tyrosine kinase inhibitors (TKIs) have changed the landscape of management of advanced non-small-cell lung cancer (NSCLC) patients. The incidence of EGFR mutations differs significantly across different ethnicities with incidence of 10%–15% in North American and European populations to up to 62% in Asian population.1 The largest cohort study from India showed an overall EGFR mutation rate of 23% with a frequency of 20.4% and 29.8% in males and females, respectively.2
Overall, in frame deletions in exon 19 at the LeuArgGluAla sequence (E746-A750), and the exon 21-point mutation Leu858Arg (L858R), represent 85%–90% of all EGFR mutations in NSCLC and are conventionally referred to as the common, TKI sensitive mutations based on various large trials.3,4 Many other “uncommon” mutations have been reported, including G719X in exon 18 (G719C, G719S, and G719A), L861Q in exon 21, S768I in exon 20, and exon 20 insertions, the predictive significance of which is still unclear. Though it is known that, the incidence of exon 20 T790M mutation can be as high as 50% in patients who develop resistance first generation TKIs – erlotinib or gefitinib, rarely de novo mutation can be found in newly diagnosed patients.5
On the basis of preclinical trials some of these uncommon mutations are considered to be partially sensitive to first generation TKI, while others are referred to as resistant to the first and second generation TKI. The frequency of these uncommon EGFR mutations (both TKI sensitive and resistant) has been reported around 1%–10%, although frequency of compound mutations could be as high as 30% of the total EGFR mutated patients.6 In Indian population, the incidence of exon 18 and 20 mutations has been reported as 7% and 3%, respectively, in a cohort of 210 EGFR mutated patients with only two patients harboring mutation in exon 20 along with exon 21.2
There is limited data available on the tumor biology, prognosis, and impact of various treatments on these patients. It is unlikely that we will have a randomized study due to limited number of patients. Information on these patients will help us understand these rare mutations and also help in treatment decision-making in the clinic. Hence, we planned to analyze the clinical profile, outcome, and treatment attributes of this unique group of patients.
Patients and methods
Ethics
This was a retrospective analysis of lung cancer patients who were treated at our center between January 2013 and December 2017. Patients with EGFR mutations were retrieved from the medical oncology molecular laboratory database. The study was conducted in accordance with the Declaration of Helsinki. The institutional review board (IRB) and the ethics committee (EC) of Tata Memorial Center (TMC) – Advanced Center for Treatment, Research and Education in Cancer (ACTREC; Mumbai, India) approved the project of lung cancer audit (No. 108) during the 21st TMC-ACTREC IRB meeting. Since this was a retrospective analysis, the IRB and the EC waived the need for an informed consent. Patient records/information were anonymized and de-identified prior to analysis. EGFR exon 19 and L858R point mutations in exon 21 were classified as common TKI sensitive EGFR mutations. Exon 18 G719X, exon 20 T790M mutation, exon 20 insertions, exon 20 S768I, and exon 21 L861R were classified as uncommon EGFR mutations and subclassified as single or dual, if they were present in isolation or in combination with other mutations. The uncommon EGFR mutations were further classified as per their known sensitivity to first and second generation oral TKI treatment. Exon 18 G719X, exon 20 768I, and exon 21 L861Q were referred to as predicted TKI sensitive uncommon mutations, while exon 20 insertions and exon 20 T790M mutations were referred as predicted TKI insensitive uncommon mutations. Patient’s clinical and demographic profile (age, sex, smoking history, performance status, and tumor histology) was noted from the lung cancer audit database. The sample used for EGFR analysis was classified as a tissue block (if biopsy specimen was used), fluid cell block (if patient had a positive pleural or pericardial fluid), or blood (if none were available). Treatment characteristics were obtained from the electronic medical records. Treatment responses were defined as partial response, complete response, stable disease, and progressive disease according to the response evaluation criteria in solid tumors version 1.1 criteria. Computed tomography scan of chest and abdomen was performed every 2–3 months for assessment of response to treatment. Response rates were calculated by combining the patients who had a complete response or partial response among patients who were evaluated clinically and radiologically. All patients who could be assessed radiologically at least at the first evaluation time point after starting a therapy, were considered as evaluable in the final analysis. Final date for data collection on follow-up was April 26, 2018. Overall survival (OS) was measured from the start of any treatment to the day of death or date of last follow-up. Progression-free survival (PFS) with first line therapy was calculated from the date of start of first line therapy to the date of progression (radiological or clinical) or death. PFS on oral TKI therapy was calculated from the date of start of oral TKI to the date of progression (radiological or clinical) or death.
EGFR mutation testing was done using a nested-PCR method with in-house primer (TaqMan) probes, the details of which have been published earlier by our group.2
Statistical analysis
SPSS version 24 was used for the analysis. Demography was analyzed by descriptive statistics. Percentages were calculated for specific mutations. Survival curve was plotted by Kaplan–Meier method and was compared between groups using log-rank test.
Results
Demographic and clinical profile
A total of 1,260 patients (21.9%) were found to have an EGFR activating mutation, out of the 5,738 advanced NSCLC patients who underwent testing at our center. Of these 1,260 patients, 83 (6.58%) patients had uncommon mutations in isolation or in various combinations. The demographic and clinical profile of the study cohort is depicted in Table 1. It was observed that uncommon mutations were more frequent in men, never-smokers, and adenocarcinomas.
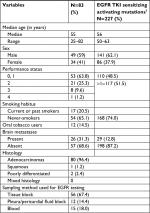  | Table 1 Demographic and clinical profile of the study cohort Note: Copyright ©2017. Dove Medical Press. Adapted from Noronha V, Choughule A, Patil VM, et al. Epidermal growth factor receptor exon 20 mutation in lung cancer: types, incidence, clinical features and impact on treatment. Onco Targets Ther. 2017;10:2903–2908.7 Abbreviation: TKI, tyrosine kinase inhibitor. |
It was observed that most patients in our study cohort had complex dual mutations (50.6%). Table 2 and Figure 1 describe the frequency of uncommon EGFR mutations and their distribution as per predicted sensitivity to first generation TKI.
  | Table 2 Uncommon EGFR mutation frequency and their distribution according to predicted sensitivity to oral TKI Abbreviation: TKI, tyrosine kinase inhibitor. |
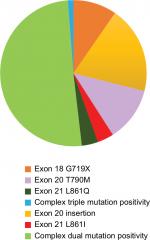  | Figure 1 Distribution of uncommon EGFR mutations. |
Treatment pattern and outcome
The median duration of follow-up was 13 months. The PFS of the entire cohort on first line therapy was 6.7 (95% CI: 4.7–8.6) months. OS of the entire cohort was 15.8 months (95% CI: 10.1–21.5). Figure 2A and B represents the survival curves of the study population. Table 3 depicts the median progression-free survival (mPFS) and mOS of the cohort of various uncommon mutations.
Response to first line therapy
First line therapy comprised of oral TKI in 50.6% (42/83) patients, while 34.9% (29/83) received chemotherapy as their first line therapy. Seven patients presented with very poor performance status (Eastern Cooperative Oncology Group Performance Status ≥3) and were offered palliative care alone. Five patients were lost to follow-up after initial work up.
Response to first line therapy on the first assessment time point could be evaluated in 54 patients, out of whom 28 patients had a partial response, 14 patients had a stable disease, and 12 patients had a progressive disease.
The mPFS of patients who received oral TKI as first line therapy was 9.1 (95% CI: 4.6–13.6) months, while it was 6.7 (95% CI: 5.8–7.5) and 2.3 (95% CI: 0–6.4) months for those patients who received chemotherapy and palliative care as first line therapy, respectively (P=0.003).
Effectiveness of oral TKI
Overall 73.0% patients received oral TKI during the course of their disease. Response to TKI could be assessed in 42 patients out of whom 26 had developed a partial response, 7 had developed a stable disease, and 9 had developed a progressive disease on clinical and/or radiological assessment. Response rates to oral TKI varied from 0% to 50% among various groups (Table 4). Figure 3A and B depicts the response to TKI therapy as observed in different types of mutations. mPFS on TKI therapy was 9.1 (6.2–12.0) months. OS of patients who received oral TKI anytime during the course of their disease was 20.2 (95% CI: 11.4–28.9) months vs 12.9 (11.8–14.0) months for those who did not receive TKI therapy. This difference was statistically significant with a P-value of 0.049. While the mPFS of patients who had TKI sensitive single or dual mutations, was 12.8 and 9.1 months, respectively, it was 3.7 months for patients with TKI insensitive mutations. Majority of our patients received first generation TKI due to financial constraints. Table 5 depicts the type of TKI received by the patients and their survival. Majority of patients received first generation TKI and there was no statistically significant difference in survival among the patients who received first, second, or third generation TKI. Table 6 gives a description of all patients who received TKI anytime during the course of their disease along with the type of TKI received and their survival.
  | Table 4 Responsiveness to oral TKI Abbreviations: mPFS, median progression-free survival; NE, not estimable; RR, response rates; TKI, tyrosine kinase inhibitor. |
  | Table 5 Type of TKI and survival Abbreviations: mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; RR, response rates; TKI, tyrosine kinase inhibitor. |
Discussion
In our study, we found that the uncommon mutations comprised of 6.5% of our population of EGFR mutated patients. The available literature suggests that incidence rates of uncommon mutations could vary between 5.9% and 20.4%.8–10 So far, this is the largest study from India and second largest single institution study in literature to report the outcome of this rare subset of patients with EGFR mutations. The most frequent uncommon mutations that we observed in our cohort were complex dual mutations (50.6%) followed by exon 20 insertions (19.3%), exon 20 T790M (12.0%), and exon 18 G719X (9.6%). Within the subgroup of dual mutations, the largest subset of patients was comprised of exon 19 deletion in combination with exon 20 T790M mutation (20.4%) followed by exon 21 L858R mutation (18.4 %) also in combination with exon 20 T790M mutation. The largest study in literature so far done by Tu et al reported exon 20 insertion as the most frequent mutation followed by exon 18 G719X, either alone or in conjunction with other mutations followed by compound exon 21 L858R occurring in 31%, 21%, and 17% of patients with uncommon EGFR mutations, respectively.11
The overall mPFS and mOS of our cohort of patients with uncommon EGFR mutations were 6.7 (95% CI: 4.7–8.6) and 15.8 (95% CI: 10.1–21.5) months, respectively. Oral TKI was used in first line setting in 50.6% patients, and subsequently in second or third line setting in 22.4% patients. The mPFS and response rate on TKI therapy were 9.1 months (95% CI: 6.2–12.0 months) and 54.7%, respectively.
Favorable efficacy with oral TKI was observed among patients with exon 18 G719X mutation and dual mutations. On classifying patients further on the basis of TKI sensitivity, we observed that the response rates and mPFS on TKI therapy were highest for TKI sensitive dual and complex TKI sensitive and insensitive mutations. The mPFS on TKI therapy for exon 18 G719X mutation and dual mutations were 9.0 and 9.4 months, respectively. These results are also comparable with the mPFS observed in patients with common EGFR mutations treated in our institution with TKI and in concordance with similar studies in Chinese population.12,13 On the contrary, exon 20 insertions, exon 20 S768I, and exon 21 L861Q were associated with an unfavorable response to oral TKI, with mPFS <6 months on TKI therapy. Our interpretation of the dismal response and survival of exon 20 768I and exon 21 patients is limited by very small number of these patients. We observed that compound or complex mutations with co-occurring classical mutations had the best survival outcomes. Wu et al reported the largest cohort of complex mutations from which 32 patients were evaluable for TKI response (first generation); the overall response rate was 56%.14 Of ten patients with a PFS >10 months, seven harbored one classical mutation.14 Complex mutations appear to be more responsive to therapy and likely the activating mutation is the driver mutation in such patients rather than the uncommon mutation. We have tried to compare the results of this study with few others (Table 7), but since the composition of uncommon mutations for the purpose of estimation of survival times was different in each study, it is not possible to directly compare the results. Also, the nature of TKI used and chemotherapy regimes are different in each trial. Majority of our patients received first generation TKI due to financial constraints.
The choice of therapy in the first line setting is often limited by the performance status of the patient. Overall, we found that, there was a statistically significant difference in survival between patients who received TKI anytime during the course of their disease vs those who never received TKIs (20.2 vs 12.9 months, P-value =0.049). Hence, we suggest the use of oral TKI in such patients.
Conclusion
To summarize, uncommon EGFR mutations do constitute a distinct heterogeneous group with differential sensitivity and varied responses to treatment. We observed that patients with exon 18 G719X mutation and dual or compound uncommon mutations have a favorable response to oral TKI. We thus suggest use of oral TKI in these subgroups of patients with uncommon EGFR mutations.
Disclosure
The authors report no conflicts of interest in this work.
References
Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–162. | ||
Chougule A, Prabhash K, Noronha V, et al. Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One. 2013;8(10):e76164. | ||
Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. | ||
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957 | ||
Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. | ||
Syahruddin E, Wulandari L, Sri Muktiati N, et al. Uncommon EGFR mutations in cytological specimens of 1,874 newly diagnosed Indonesian lung cancer patients. Lung Cancer. 2018;9:25–34. | ||
Noronha V, Choughule A, Patil VM, et al. Epidermal growth factor receptor exon 20 mutation in lung cancer: types, incidence, clinical features and impact on treatment. Onco Targets Ther. 2017;10:2903–2908. | ||
Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25(1):126–131. | ||
Arrieta O, Cardona AF, Corrales L, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87(2):169–175. | ||
De Pas T, Toffalorio F, Manzotti M, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6(11):1895–1901. | ||
Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102. | ||
Patil VM, Noronha V, Joshi A, et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open. 2017;2(1):e000168. | ||
Shi J, Yang H, Jiang T, et al. Uncommon EGFR mutations in a cohort of Chinese NSCLC patients and outcomes of first-line EGFR-TKIs and platinum-based chemotherapy. Chin J Cancer Res. 2017;29(6):543–552. | ||
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821. | ||
Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





