Back to Journals » Clinical Ophthalmology » Volume 13
Outcome of Descemet membrane endothelial keratoplasty for graft failure after Descemet stripping automated endothelial keratoplasty
Authors Agha B, Shajari M, Slavik-Lencova A, Kohnen T , Schmack I
Received 11 November 2018
Accepted for publication 18 January 2019
Published 25 March 2019 Volume 2019:13 Pages 553—559
DOI https://doi.org/10.2147/OPTH.S194185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bishr Agha, Mehdi Shajari, Anna Slavik-Lencova, Thomas Kohnen, Ingo Schmack
Department of Ophthalmology, Goethe-University, Frankfurt am Main, Germany
Purpose: To investigate the efficacy and safety of Descemet membrane endothelial keratoplasty (DMEK) for corneal decompensation following primary Descemet stripping automated endothelial keratoplasty (DSAEK).
Methods: This was a retrospective case series of 15 patients that underwent DMEK surgery for corneal decompensation after failed DSAEK. Main outcome parameter was corrected distance visual acuity (CDVA) after DMEK and DSAEK. Secondary outcome measures included central corneal thickness (CCT), endothelial cell density (ECD), rebubbling rate, and primary graft failure after DMEK. Explanted DSAEK grafts were evaluated by light microscopy.
Results: The mean (±SD) time period between DSAEK and DMEK surgery was 15±8 months (range, 6–31 months). Preoperative CDVA was 1.72±0.62 (logMAR). After DMEK, CDVA improved significantly to 0.78±0.48 at 1 month and to 0.23±0.24 after 12 months (P=0.022). Visual acuity data after DMEK were significantly better compared to preoperative values. The average CCT after DMEK decreased significantly from 869±210 µm (preoperative) to 505±45 µm (1 month postoperative) (P<0.001) and remained stable over 12 months. The ECD decreased from 2,589±209/mm2 (preoperative) to 1,691±589/mm2 (12 months postoperative). Rebubbling DMEK was required in three patients (=20%).
Conclusion: DMEK represents a feasible and safe procedure in achieving better functional results compared to DSAEK. Visual acuity and optical quality can be effectively reestablished after unsuccessful primary DSAEK surgery even in patients with long-standing corneal decompensation. Further investigations are required to validate the preliminary clinical findings.
Keywords: DMEK, DSAEK, corneal edema, corneal transplantation
Introduction
Nowadays, endothelial keratoplasty (EK) is considered the gold standard for treatment of patients with diseased corneal endothelium as observed in Fuchs’ endothelial corneal dystrophy and bullous keratopathy.1,2 The two most commonly applied surgical techniques, Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK), are characterized by fast visual recovery, high postoperative optical quality, and a low rate of primary or secondary graft failure and corneal rejection.2–4 The main differences between both methods in regard to intraoperative manipulation and functional outcome are related to the different composition and thickness of the lamellar grafts.5–10 In contrast to DSAEK, DMEK grafts are only composed of the corneal endothelium and Descemet membrane.5–7 They principally lack any stromal layers. As a result, DMEK surgery usually provides a better final visual quality related in parts to lower higher-order aberrations and improved contrast sensitivity, faster visual rehabilitation, and a higher level of patient satisfaction1.1–14 Newer techniques such as Ultrathin-DSAEK (UT-DSAEK) try to overcome these limitations by further reduction of the stromal thickness.15–17 In addition, histopathologic studies demonstrated that DMEK is feasible for reestablishing optical quality even in eyes with unsuccessful previous DSAEK or Descemet stripping endothelial keratoplasty.8,18–20 Nevertheless, the visual outcome of secondary DMEK for corneal decompensation following failed DSEK/DSAEK surgery is limited partially due to structural alterations of host stroma, donor-to-host interface, and the lamellar graft itself.8,19–21 Although intergroup comparisons between DSAEK and DMEK patients have substantially been performed,10–13 there are currently no data available specifically comparing efficacy parameters in DMEK patients with previous DSAEK surgery. Therefore, we analyzed the functional outcome of patients with DMEK for corneal decompensation after previous successful DSAEK procedure.
Materials and methods
All study data were collected and analyzed in accordance with the policies and procedures of the institutional review board of the Goethe-University Frankfurt, Germany, and the tenets of the declaration of Helsinki. The institutional review board waived the need for obtaining patient consent to review their medical records because patient data confidentiality was guaranteed throughout the study.
Study design and data collection
This was a retrospective comparative case series of patients undergoing DMEK for graft failure after previous successful DSAEK surgery. Medical files of all eligible patients treated with secondary DMEK between November 2014 and March 2016 were reviewed. Data collected included age, sex, corrected distance visual acuity (CDVA) after successful DSAEK as well as before and 1 month, 3 months, 6 months, and 12 months after DMEK surgery. CDVA was assessed using decimal charts and finally converted to logMAR (logarithmic minimal angle of resolution) values. Secondary outcome parameters were central corneal thickness (CCT) and endothelial cell density (ECD) measurements which were obtained by Pentacam AXL (Oculus GmbH, Wetzlar, Germany) and endothelial microscopic evaluation (Oculus/Nidek CEM-530, Oculus GmbH, Wetzlar, Germany). Patients with insufficient postoperative data collection and those with primary graft failure after DSAEK were excluded from further evaluation.
Corneal donor tissue
Donor corneas were provided by various German eye banks and organ-cultured for up to 27 days prior to transplantation. All corneal donors were older than 58 years of age. The minimum ECD prior to transplantation was 2,200 cell/mm2 or higher. The characteristics of the donor corneas are summarized in Table 1.
Surgical procedures
All surgeries were carried out by an experienced surgeon (TK).
Standard DSAEK
DSAEK grafts were prepared using sequential microkeratome cuts (400 and 100 μm) according to the corneal thickness of the corneal donor (Schwind eye-tech-solutions GmbH, Kleinostheim, Germany). The resulting median thickness of the DSAEK grafts was 90 μm (range 50–150 μm). After removal of the recipients Descemet membrane within a diameter of 8 mm, DSAEK graft (diameter 7.5 mm) were injected and centered. The anterior chamber was filled with air to achieve graft attachment.
Standard DMEK
DMEK graft preparation was performed by a single surgeon (IS) using a standardized technique as previously described,6 stained with trypan blue (VisionBlue staining solution, Dutch Ophthalmic, Zuidland, the Netherlands), and stored in a conic glass implantation cartridge (Geuder AG, Heidelberg, Germany). Diameters of the DMEK grafts were 8.0 mm and 7.75 mm depending on the white-to-white distance of the individual eyes.
Prior to DMEK implantation, DSAEK grafts were carefully mobilized with a blunt spatula and subsequently explanted via a 2.2 mm corneal incision. Adjacent remnants of recipient Descemet membrane were stained with trypan blue (VisionBlue staining solution) and removed within an overall diameter of 9.0 mm. After injection of the DMEK lamellae, grafts were carefully unfolded and centered over the pupil. Afterward, 20% sulfur hexafluoride gas was installed between the iris surface and endothelial cell layer of the DMEK lamellae until the grafts were completely unfolded and firmly attached to the posterior corneal stroma of the recipient. The amount of the anterior chamber gas fill ranged between 80% and 90%. All DMEK surgeries were uneventful. No intraoperative complications occurred. Intraocular pressure control was routinely performed within one to two hours after surgery. Patients were asked to stay predominantly supine during the following two to three days.
Clinical evaluation
CDVA was assessed using decimal charts and converted to logMAR (logarithmic minimal angle of resolution) values. Data of CDVA after DSAEK were compared with CDVA measurements obtained before DMEK as well as 1 month, 3 months, 6 months, and 12 months after DMEK surgery. CCT and ECD measurements were performed by Pentacam AXL (Oculus GmbH, Wetzlar, Germany) and endothelial microscopic evaluation (Oculus/Nidek CEM-530, Oculus GmbH, Wetzlar, Germany). All patients were examined by slit-lamp biomicroscopy before and after DMEK surgery. Indications for additional gas injections (rebubbling) were based on slit-lamp examination and anterior segment optical coherence tomography (Visante OCT, Carl Zeiss, Meditec, Jena, Germany). Patients with partial lamellar detachment of more than one-third of the total graft diameter or more than 3 clock hours were scheduled for rebubbling.
Histopathologic analysis
Explanted DSAEK grafts were submitted for histopathologic evaluation. Specimens were fixed in buffered 10% formaldehyde solution (pH 7.2), dehydrated, embedded in paraffin, cut in serial sections (6 μm), and stained with H&E and periodic acid-Schiff (PAS) for further evaluation. Main parameters examined were ECD, lamellar thickness, and secondary alterations such as retrocorneal membranes and cellular infiltrates.
Statistical analysis
Statistical analysis was performed using Excel for Mac (version 15.37, Microsoft, Inc., Redmond, WA, USA) and IBM SPSS software version 24 (IBM Corporation, Armonk, NY, USA). Differences of values were assessed by Wilcoxon test. A P-value <0.05 was considered statistically significant.
Results
The eyes of fifteen patients (female, n=8; male, n=7) treated with DMEK for insufficient optical quality secondary to corneal decompensation after previous DSAEK surgery were included in the study. Clinical indications for initial DSAEK surgery were Fuchs endothelial corneal dystrophy (n=12 eyes, 80%), pseudophakic bullous keratopathy (n=1 eye, 6.7%), and corneal decompensation of unknown reason (n=2 eyes, 13.3%). Single DSAEK was performed in most of the patients (n=10 eyes). Additional DSAEK surgery prior to DMEK was carried out in 5 of the 15 patients (2-times, n=4 patients; 3-times, n=1 patient). Reasons for repeat DSAEK included slow endothelial cell loss (ECL) and insufficient optical quality. The average time (±SD) interval between the most recent DSAEK procedure and DMEK surgery was 15±8 months (range, 6–31 months). The age of the patients at the time of DMEK surgery was 62–89 years (mean ± SD, 73.6±7.6 years).
CDVA
The best CDVA (mean ± SD) documented after DSAEK was 0.83±0.48. Over time, the average CDVA dropped significantly to 1.72±0.62 prior to DMEK surgery (P=0.004). One month after DMEK, CDVA (0.78±0.48) already improved significantly compared to the preoperative values (P=0.002). In addition, CDVA proved to be superior at all follow-up visits after DMEK in comparison to the best CDVA after DSAEK. Twelve months after DMEK the mean CDVA (±SD) was 0.23±0.24. The results are highlighted in Table 2 and Figure 1.
CCT
One month after DMEK, mean CCT (±SD) had significantly decreased from 869.0±209.9 μm (preoperative) to 505.3±44.8 μm (P<0.001). Minimum corneal thickness measurements were reached at three months after DMEK (458±23 μm). However, during the following nine months eyes showed a slight increase in mean CCT (511.7±67.7 μm), which was comparable to the average CCT data obtained at 1 month after DMEK (P=0.75). Table 3 and Figure 2 are providing a detailed overview of the results.
  | Table 3 Changes in CCT after DMEK |
ECD
Over the one year follow up period, mean (±SD) ECD of the donor corneas in DMEK eyes decreased significantly from 2,589.4±209.0 cells/mm2 (preoperative) to 1,691.4±589.3 cells/mm2 at twelve months after DMEK surgery (P=0.012). Average endothelial cell loss was 34.7%. The results are summarized in Table 4.
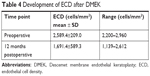  | Table 4 Development of ECD after DMEK |
Rebubbling rate
Lamellar graft detachment requiring intracameral gas injection (rebubbling) was present in three patients (20%). Indications for additional gas injections (rebubbling) were based on slit-lamp examination and anterior segment optical coherence tomography (Visante™ OCT, Carl Zeiss, Meditec, Jena, Germany). Patients with partial lamellar detachment of more than one-third of the total graft diameter or more than three clock hours were scheduled for rebubbling. Rebubbling was performed at one week (n=1), two weeks (n=1), and four weeks (n=1) after surgery. However, one patient eventually required regrafting for primary graft failure at six months after DMEK surgery. All remaining patients showed complete graft attachment within four to five days after surgery.
Histopathologic findings
The overall thickness of the explanted DSAEK grafts ranged between 110 and 230 μm. All explanted DSAEK lamellae demonstrated thickening of the corneal stroma due to stromal edema (Figure 3). In addition, stromal fibrosis was present in the majority of the specimens. Complete to subtotal loss of endothelial cells (endothelial cell count was between 0 and 7 per high-power field) was seen in all grafts (Figure 4). No cellular infiltrates suspicious of infectious or immunologic reaction were noted along the stromal interface or at the corneal endothelium.
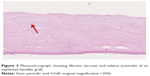  | Figure 3 Photomicrograph showing fibrosis (arrow) and edema (asterisk) of an explanted lamellar graft. |
Discussion
The findings of our study investigating the functional outcome of DMEK for corneal decompensation following DSAEK in the same cohort show that results after secondary DMEK are superior to those achieved in the course after DSAEK.
Previously, Weller et al20 focused on the outcome after DMEK for graft failure after DSAEK for corneal decompensation. In contrast to our study, the cohort undergoing secondary DMEK after failed DSAEK was compared to a control group with primary DMEK.20 Overall, preoperative visual acuity data (logMAR) were markedly better than in the present study (1.27±0.34 vs 1.72±0.62). Regardless of the poor preoperative visual acuity in our study, a CDVA of 0.23±0.24 was reached at twelve months after DMEK, which is comparable to the visual acuity data reported by Weller et al (0.19±0.08 at twelve months).20 The greater SD in our study might be explained due to a slightly older study population and a more advanced corneal decompensation although mean preoperative CCT was comparable. Another study, performed by Brockmann and associates, investigated the results of DMEK surgery for graft failure after Descemet stripping endothelial keratoplasty (DSEK).19 It was concluded that DMEK is a feasible choice in patients with prior DSEK and attributed visual limitations compared to primary DMEK among other factors to histopathologic changes like the deposition of matrix proteins within the corneal stroma and the stroma-to-stroma interface. Nevertheless, DSEK is a slightly different technique compared to DSAEK potentially resulting in different graft characteristics. In addition, preoperative visual acuity data were superior and mean corneal thickness measurements were thinner compared to our study. Another major difference between our study and both above mentioned studies is that we performed an intraindividual comparison of the best CDVA documented in the course after DSAEK to the results achieved after secondary DMEK in the same cohort. This is also a relevant finding because visual acuity could be improved by DMEK even though thin DSAEK grafts were used. The median thickness of the DSAEK grafts used in our study was 90 μm, which is generally considered as ultrathin DSAEK (UT-DSAEK).16 Hence our study does not support the conclusion from a previous study that UT-DSAEK can provide visual outcomes comparable with DMEK along with less frequent complications like graft detachments requiring rebubbling.15
It is already known that DMEK is technically feasible in eyes with prior DSAEK and potentially better functional results can be achieved.8,18,21 The major difference between the studies to our series, as well as the work by Weller and associates, is that DMEK was not performed when corneal decompensation occurred but rather to improve visual outcome by secondary DMEK. The different initial situation is especially relevant as surgical difficulty in DMEK is much more advanced when marked corneal edema is present, mainly because of poor visualization of the anterior chamber and graft orientation. It has also been shown by studies evaluating the outcome of repeat DMEK for corneal decompensation following graft detachment or failure after DMEK that it can be safely performed in the setting of marked corneal edema.22 In another report of three cases undergoing DMEK for the management of persistent corneal edema after Descemet stripping without EK in patients with Fuchs’ endothelial dystrophy DMEK also showed to be a reliable procedure.23
The rebubbling rate in our study was slightly higher than the rebubbling rate reported by Weller et al (20% vs 13%).20 We routinely used 20% sulfur hexafluoride (SF6) instead of air tamponade to improve the primary graft attachment rate. Sulfur hexafluoride is reported to reduce the number of rebubblings without negatively affecting clinical outcome after DMEK compared to the use of air.24 However, the difference concerning this aspect is likely owing to the small sample size in both studies. Of note, all rebubblings performed in this study were performed within one month after DMEK. This is in accordance to a recent published study investigating the clinical outcome after rebubbling for graft detachment after DMEK in a series of 760 DMEK surgeries with forty-one eyes requiring rebubbling.25 The authors concluded that visual outcomes may be similar to uncomplicated DMEK when rebubbling is performed within the first six to eight weeks after surgery.
Limitations of the present study are due to the small cohort size and the heterogeneous preoperative visual acuity data. Larger studies are mandatory to better evaluate if there are limitations concerning severity of preoperative corneal decompensation and visual acuity regarding the suitability of visual restoration by secondary DMEK after failed DSAEK.
Conclusion
This study demonstrates that DMEK is a feasible and successful procedure for improving visual acuity even in eyes with major visual limitations due to corneal decompensation after graft failure following previous DSAEK surgery.
Disclosure
BA, ASL, and IS have no financial interests. MS reports consultancy for Oculus, Oertli, Santen, and Zeiss. TK reports consultancy and research for Abbott/J&J, Alcon/Novartis, Oculentis, Oculus, Presbia, Schwind, and Zeiss; consultancy for Allergan, Bausch & Lomb, Dompé, Geuder, Med Update, Merck, Rayner, Santen, Staar, Tear Lab, Thea, Thieme, Uni-Med Verlag, and Ziemer; research for Avedro and Hoya; personal fees from Allergan, Bausch & Lomb, Dompé, Geuder, Med Update, Merck, Rayner, Santen, Staar, Tear Lab, Thea, Thieme, Uni-Med, and Ziemer; grants and other from Avedro and Hoya; and grants and personal fees from Carl Zeiss, Johnson&Johnson, Novartis/Alcon, Oculentis, Oculus, Schwind, Presbia, outside the submitted work. The authors report no other conflicts of interest in this work.
References
Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115(9):1525–1533. | ||
Price FW, Feng MT, Price MO. Evolution of endothelial keratoplasty: where are we headed? Cornea. 2015;34(Supp 10):S41–S47. | ||
Marques RE, Guerra PS, Sousa DC, Gonçalves AI, Quintas AM, Rodrigues W. DMEK versus DSAEK for Fuchs’ endothelial dystrophy: a meta-analysis. Eur J Ophthalmol. 2018;29(1):15–22. | ||
Pavlovic I, Shajari M, Herrmann E, Schmack I, Lencova A, Kohnen T. Meta-analysis of postoperative outcome parameters comparing Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Cornea. 2017;36(12):1445–1451. | ||
Heinzelmann S, Böhringer D, Eberwein P, Reinhard T, Maier P. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):515–522. | ||
Melles GRJ, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25(8):987–990. | ||
Dapena I, Ham L, Melles GRJ. Endothelial keratoplasty: DSEK/DSAEK or DMEK – the thinner the better? Curr Opin Ophthalmol. 2009;20(4):299–307. | ||
Ham L, Dapena I, van der Wees J, Melles GRJ. Secondary DMEK for poor visual outcome after DSEK: donor posterior stroma may limit visual acuity in endothelial keratoplasty. Cornea. 2010;29(11):1278–1283. | ||
Neff KD, Biber JM, Holland EJ. Comparison of central corneal graft thickness to visual acuity outcomes in endothelial keratoplasty. Cornea. 2011;30(4):388–391. | ||
Droutsas K, Lazaridis A, Papaconstantinou D, et al. Visual outcomes after Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial Keratoplasty – Comparison of specific matched pairs. Cornea. 2016;35(6):765–771. | ||
Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30(12):1382–1386. | ||
Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Am J Ophthamol. 2012;153(6):1082–1090. | ||
Hamzaoglu EC, Straiko MD, Mayko ZM, Sáles CS, Terry MA. The first 100 eyes of standardized Descemet stripping automated endothelial keratoplasty versus standardized Descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122(11):2193–2199. | ||
Turnbull AMJ, Tsatsos M, Hossain PN, Anderson DF. Determinants of visual quality after endothelial keratoplasty. Surv Ophthalmol. 2016;61(3):257–271. | ||
Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin Descemet’s stripping automated endothelial keratoplasty with the Microkeratome double-pass technique. Ophthalmology. 2013;120(6):1186–1194. | ||
Busin M, Albe E. Does thickness matter: ultrathin Descemet stripping automated endothelial keratoplasty. Curr Opin Ophthalmol. 2014;25:312–318. | ||
Dickman MM, Kruit PJ, Remeijer L, et al. A randomized multicenter clinical trial of ultrathin Descemet stripping automated endothelial keratoplasty (DSAEK) versus DSAEK. Ophthalmology. 2016;123(11):2276–2284. | ||
Dirisamer M, Parker J, Naveiras M, et al. Identifying causes for poor visual outcome after DSEK/DSAEK following secondary DMEK in the same eye. Acta Ophthalmol. 2013;91(2):131–139. | ||
Brockmann T, Brockmann C, Maier A-K, et al. Descemet membrane endothelial keratoplasty for graft failure after Descemet stripping endothelial keratoplasty: clinical results and histopathologic findings. JAMA Ophthalmol. 2015;133(7):813–819. | ||
Weller JM, Tourtas T, Kruse FE, Schlötzer-Schrehardt U, Fuchsluger T, Bachmann BO. Descemet membrane endothelial keratoplasty as treatment for graft failure after Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2015;159(6):1050–1057. | ||
Sorkin N, Showail M, Einan-Lifshitz A, et al. Outcomes of Descemet membrane endothelial keratoplasty in eyes with a previous Descemet stripping automated endothelial keratoplasty graft. Cornea. 2018;37(6):678–681. | ||
Baydoun L, van Dijk K, Dapena I, et al. Repeat Descemet membrane endothelial keratoplasty after complicated primary Descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122(1):8–16. | ||
Rao R, Borkar DS, Colby KA, Veldman PB. Descemet membrane endothelial keratoplasty after failed Descemet stripping without endothelial keratoplasty. Cornea. 2017;36(7):763–766. | ||
Schaub F, Enders P, Snijders K, et al. One-year outcome after Descemet membrane endothelial keratoplasty (DMEK) comparing sulfur hexafluoride (SF6) 20% versus 100% air for anterior chamber tamponade. Br J Ophthalmol. 2017;101(7):902–908. | ||
Gerber-Hollbach N, Baydoun L, López EF, et al. Clinical outcome of Rebubbling for graft detachment after Descemet membrane endothelial keratoplasty. Cornea. 2017;36(7):771–776. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





