Back to Journals » International Journal of Nanomedicine » Volume 18
Osteoimmune Interactions and Therapeutic Potential of Macrophage-Derived Small Extracellular Vesicles in Bone-Related Diseases
Authors Liu N, Dong J, Li L, Liu F
Received 30 December 2022
Accepted for publication 19 April 2023
Published 26 April 2023 Volume 2023:18 Pages 2163—2180
DOI https://doi.org/10.2147/IJN.S403192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Video abstract of "Macrophage-derived small extracellular vesicles" [ID 403192].
Views: 63
Nan Liu, Jinlei Dong, Lianxin Li, Fanxiao Liu
Department of Orthopedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Fanxiao Liu, Department of Orthopedics, Shandong Provincial Hospital affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China, Tel/Fax +86-0531-68773195, Email [email protected]
Abstract: Due to the aging of the global population, the burden of bone-related diseases has increased sharply. Macrophage, as indispensable components of both innate immune responses and adaptive immunity, plays a considerable role in maintaining bone homeostasis and promoting bone establishment. Small extracellular vesicles (sEVs) have attracted increasing attention because they participate in cell cross-talk in pathological environments and can serve as drug delivery systems. In recent years, an increasing number of studies have expanded our knowledge about the effects of macrophage-derived sEVs (M-sEVs) in bone diseases via different forms of polarization and their biological functions. In this review, we comprehensively describe on the application and mechanisms of M-sEVs in various bone diseases and drug delivery, which may provide new perspectives for treating and diagnosing human bone disorders, especially osteoporosis, arthritis, osteolysis, and bone defects.
Keywords: small extracellular vesicles, macrophage, crosstalk, drug delivery, bone homeostasis
Introduction
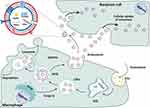
Extracellular vesicles (EVs) are nanovesicles with a lipid bilayer structure that are released by various cells.1 In recent years, EVs have attracted extensive attention as promising alternative mediators of cell communication, and can directly affect a number of physiological and pathological processes in target cells.2–4 As they have numerous advantages over conventional synthetic carriers, EVs have been considered as a next-generation drug delivery platform, opening new frontiers for modern drug delivery.5,6 EVs can be categorized into multiple subtypes based on their origins and physical characteristics.7 Among these different subtypes of EVs, small EVs (sEVs) have been one of the most widely studied subtypes in recent years.8
sEVs, with a diameter of 30–200 nm, are released by different cells, originate from the endocytic pathway7 and widely distributed in a variety of body fluids.9,10 All cells can release sEVs regardless of whether they are in a state of normal or pathological conditions. Due to their different sizes, levels, contents and origins, sEVs have great heterogeneity and target specific organs or cells, leading to different biological functions.11 As natural endogenous nanocarriers, intercellular communication through sEVs is a crucial process for tissue progression.12 sEVs, which are considered mediators of a novel mode of cell-to-cell communication, participate in intercellular signaling by transferring their encapsulated bioactive components such as RNAs, proteins, metabolites, nucleic acids, and lipids to regulate cellular behavior as a kind of signal, or by traveling to a distant body site to contribute to homeostasis and disease and are endocytosed by target cells, leading to changes in gene expression and cellular function in state of health and disease.1,13,14
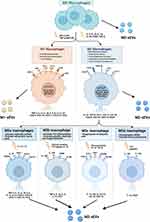
Macrophages are indispensable immune cells that contribute to both proinflammatory and anti-inflammatory processes and are involved in both tissue destruction and regeneration. Macrophages play an indispensable role in both innate immune responses and adaptive immunity and are distributed in all tissues of the body, with high plasticity and heterogeneity according to the tissue and organ in which they reside.15 In response to various stimuli, macrophages can switch phenotypes from unpolarized (M0) to polarized (M1 and M2) states and play unique roles in different stages of disease status and tissue healing.4,16–18 Moreover, the latest opinions tend to describe that M2 macrophages can be further divided into four subtypes (M2a, M2b, M2c and M2d) according to different stimulations.19,20 (Figure 1) Macrophages perform functions that are usually related to their secretion of sEVs and microRNAs (miRNAs) carried by sEVs (Figure 2). Moreover, recent studies have demonstrated that sEVs derived from macrophages play crucial roles in different biological processes, such as the immune response,21 signal transduction,22 cell proliferation,23 and angiogenesis,8 and promote or inhibit a range of diseases, such as orthopedic disorders,24 chronic inflammation,25 cardiovascular disease,26 metabolic disorders and tumors.1,11,27 Based on the current knowledge, this article reviews the status of investigations of sEVs secreted from macrophages and their potential application to bone diseases and aims to describe novel approaches for bone disease treatment in clinical practice.
Promotion of Osteogenesis
sEVs are lipid bilayer nanovesicles that are secreted by most cell types and control intercellular communication. Increasing data suggest that macrophages can establish an optimal microenvironment to reduce inflammation and promote osteogenesis through a paracrine mechanism.28 sEVs derived from macrophages have been investigated as potential immunomodulatory agents in clinical therapy.29,30 A large number of studies have demonstrated that sEVs can be used in cell-free therapy to effectively improve bone disease and solve the problems (immune rejection, insufficient bone mass, and poor stability) caused by traditional bone implants and stem cell therapy.31,32
In preclinical investigations, sEVs from macrophages with different polarization phenotypes were found to have different effects on bone metabolism.4 Based on the observations that macrophages contribute to bone regeneration, macrophage polarization to the M1 phenotype may be essential during the early phases of osteoinduction and bone regeneration, and the M2 phenotype may foster continued bone regeneration.30 Huang et al demonstrated that sEVs secreted by M1 macrophages promoted the proliferation and osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells (BMSCs). However, the sEVs secreted by M2 macrophages impaired the proliferation of BMSCs. M0-sEVs exhibit no considerable influence on the proliferation of BMSCs.33 Another study indicated that sEVs from M1 macrophages attenuated cementoblast mineralization, while sEVs from M2 macrophages facilitated cementoblast mineralization.4 During tissue healing and regeneration, biomimetic intrafibrillarly mineralized collagen (IMC) was found to regulate M2 macrophage polarization and secrete sEVs. Human BMSCs were cultured in osteogenic medium supplemented with IMC-sEVs. IMC-sEVs promoted BMSC osteogenic differentiation by increasing the expression levels of BMP2 and Smad5 (one of the classic proteins of the osteogenic pathway). Blockade of sEV secretion by GW4869 significantly impaired BMSC osteogenesis.26
In recent years, the function and potential application of miRNAs in osteogenic differentiation have attracted increasing attention. MiR-690 enriched in M2-sEVs could facilitate osteogenesis and reduce adipogenesis by upregulating the levels of IRS-1 and TAZ, suggesting a potential role of M2-sEVs as a therapeutic tool for bone loss.34 Macrophage-derived sEVs containing miR-381 suppress the osteogenic differentiation of BMSCs by reducing the levels of ALP and Runx2, and the effect could be reversed by a miR-381 inhibitor. Mg2+ is essential for the maintenance of physiological homeostasis in tissues and organs and has been verified to significantly decrease miR-381 expression and further facilitate the osteogenic differentiation of BMSCs.35 IL-10 is considered to be a crucial regulator of bone homeostasis and inflammatory conditions, playing a prominent role in regulating osteoblast/osteoclast differentiation and function. M2-sEVs could upregulate IL-10 cytokine expression in BMSCs and bone marrow-derived macrophages (BMDMs) by delivering IL-10 mRNA to cells directly, leading to the activation of the cellular IL-10/IL-10R pathway to regulate cell differentiation and bone metabolism and preventing bone loss in murine periodontitis models.36
Promotion of Osseointegration
Angiogenesis and osteogenesis are essential preconditions for achieving favorable osseointegration of the bone implants. Increasing evidence has demonstrated that osteoimmunomodulation mediated by macrophage-derived sEVs plays a pivotal role in angiogenesis and osteogenesis.37,38 sEVs can be internalized by various cells participating in bone formation, such as endothelial cells and osteoblasts, to intervene in the osseointegration.
In one study, sEVs derived from bone morphogenetic protein 2 (BMP2)- stimulated macrophages were used to modify titanium nanotube implants. The incorporation dramatically increased the expression of early osteoblastic differentiation markers, ALP, Runx2 and BMP2; promoted the osteogenic differentiation of BMSCs; activated autophagy of hBMSCs; and altered the secretion of cytokines associated with bone remodeling, indicating the important effects of macrophage-sEVs in osseointegration.39 Titanium and titanium alloys are the most widely used implant materials in clinics due to their good biocompatibility and mechanical properties and have an important influence on osseointegration. Mouse RAW264.7 cells stimulated with titanium disks (Ti6Al4V) were found to polarize into the anti-inflammatory M2 phenotype. sEVs derived from M2 macrophages induced upregulation of osteoblast differentiation marker genes Runx2 and CoL-1 in MC3T3-E1 cells. Moverover, several miRNAs involved in the progress of osseointegration were upregulated or downregulated, indicating that M2-sEVs played an important role in the process of osteoimmunity-promoting osseointegration.40 Another study confirmed that sEVs extracted from macrophages cultured with titania nanotube arrays anodized with a voltage of 40 V (TNA-40) can significantly upregulate the ALP activity and osteogenic-related gene expression of BMSCs, promote endothelial cell (EC) migration, and enhance angiogenic capacity through the interaction between miR-3473e and the target gene Akt1.41 The immunosuppressive and anti-inflammatory molecule ADA2AR has been proven to enhance the proliferation and migration of vascular endothelial cells (VECs) and promote angiogenesis and the selection of M-sEVs during bone healing. However, the underlying mechanism warrants further research.16 sEVs derived from alternatively activated M2 macrophages can induce in situ direct conversion of classic activated M1 macrophages into reprogrammed M2-like phenotype to promote cutaneous wound healing and repair by enhancing angiogenesis and re-epithelialization.42
Promotion of Tissue Repair and Regeneration
Macrophage-derived sEVs may serve as an emerging functional tool in biomaterial-mediated endogenous bone regeneration. Macrophages with different polarization phenotypes influence cementoblast mineralization through sEVs, M1 macrophages attenuate mineralization, and M2 macrophages enhance mineralization.43 In a diabetic wound rat model, macrophage-derived sEVs exerted anti-inflammatory effects by inhibiting the secretion of proinflammatory enzymes and cytokines, weakening the infiltration of inflammatory cells, and inducing endothelial cell proliferation and migration to improve angiogenesis and re-epithelialization to further promote diabetic wound repair.44
M-sEVs and Bone Diseases
Macrophages have been recognized as essential regulators of bone homeostasis.45,46 M-sEVs play a crucial role in bone diseases by interfering with osteoblast differentiation, osteogenesis, angiogenesis, cell proliferation, apoptosis, the immune response, and inflammatory factors. sEVs derived from different macrophage subtypes perform diverse functions (M0-sEVs in Figure 3 and Table 1, M1-sEVs in Figure 4 and Table 2, M2-sEVs in Figure 5 and Table 3).
 |
Table 1 Published Studies on Biogenesis of M0-Macrophage (M0-Mφ) Derived sEVs in Bone-Related Diseases |
 |
Table 2 Published Studies on Biogenesis of sEVs Derived from M1-Macrophage (M1-Mφ) in Bone-Related Diseases |
 |
Table 3 Published Studies on Biogenesis of sEVs Derived from M2-Macrophage (M2-Mφ) in Bone-Related Diseases |
 |
Figure 3 Published data on the biogenesis of sEVs derived from M0 macrophages in bone-related diseases. |
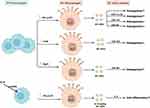 |
Figure 4 Published data on the biogenesis of sEVs derived from M1 macrophages in bone-related diseases. |
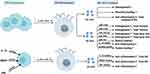 |
Figure 5 Published data on the biogenesis of sEVs derived from M2 macrophages in bone-related diseases. |
Bone Fracture or Defect
Bone fracture is a common clinical challenge and easily causes compromised physical activity, loss of productivity, and decreased quality of life. Osteoblast differentiation of MSCs is an important process during fracture healing. Studies have shown that during the early stage of the proinflammatory response, miRNA-21a-5p, which is overexpressed by M1-sEVs can promote the osteogenic differentiation of BMSCs.47 During the anti-inflammatory response that occurs during the late stages of the disease, M2-sEVs overexpressing miRNA-5106 were found to inhibit the expression of salt-induced kinases 2 and 3 (SIK2 and SIK3) to promote osteoblast differentiation and bone mineral deposition, and accelerate fracture healing.48 MiR-26a-5p carried by sEVs from M2 macrophages actively promoted the expression of the bone differentiation-related proteins ALP, RUNX-2, OPN, and CoL-2 in BMSCs, and the effect could be attenuated by the inhibitor GW4689. MiR-26a-5p in M2-sEVs can promote osteogenic differentiation and inhibit adipogenic differentiation, indicating a positive role during bone fracture healing.49
Bone defects resulting from fractures and disease are a medical concern, and are often unable to heal spontaneously by the body’s repair mechanisms. Macrophage-derived sEVs may serve as an emerging functional tool in biomaterial-mediated endogenous bone regeneration. MiR-155-encriched M1-sEVs were found to impair osteoblastic differentiation by reducing the expression of BMP2, BMP9 and RUNX2. MiR-378a contained in M2-sEVs acts as a positive regulator of osteogenesis by specifically activating upon the BMPS signal transduction pathway. The expression of BMP2 and BMP9 in MSCs increased after miR-378a was transferred into calvaria defect rats.4
Osteoporosis
Osteoporosis is considered as the most prevalent bone disorder in elderly people worldwide and is characterized by low bone mass, deterioration of bone tissue and decreased bone strength. When people are experience estrogen deficiency, aging, high levels of glucocorticoids, inflammation and hyperparathyroidism, the balance between the activities of bone-forming osteoblasts and bone-resorbing osteoclasts is disrupted, causing the occurrence of osteoporosis.14,50–52 Osteoporosis is more common in women than in men, and is a strong risk factor for fragility fractures in untreated people. It is estimated that 40% of postmenopausal women and 30% of older men (over 70 years of age) are at risk of experiencing osteoporotic fractures and the number continues to increase, posing tremendous medical and economic challenges to patients and society.53 Type 2 diabetes mellitus (T2DM) is a common cause of secondary osteoporosis. Microvascular complications of T2DM lead to reduced blood flow to bone and may contribute to bone loss and its fragility, leading to an increased risk of bone fracture.54 MiR-144-5p, which was derived from diabetic bone marrow-derived macrophage (dBMDM)-sEVs, could be transferred into BMSCs to suppress bone regeneration by targeting Smad1 and impair bone repair in vitro and vivo, increasing the risk of delayed bone union and nonunion and doubling the time of fracture healing associated with diabetes.55 The osteogenic differentiation of BMSCs is impaired under high-glucose and high-insulin (HGI) conditions in vitro. The effect is partially relieved by the intervention of M2 macrophage-derived sEVs through activating the Hedgehog signaling pathway. The ALP activity of BMSCs in the HGI group was found to be lower than that in the control group. After intervention with M2-sEVs, ALP activity was significantly higher than that in the HGI group. In addition, the expression of the osteogenesis-related proteins COL1A1 and GLI1 is upregulated after M2-sEV treatment, suggesting that M2-sEVs have therapeutic potential for the treatment of diabetic bone disease.56 In a mouse model of postmenopausal osteoporosis, miR-98 enriched in M1-sEVs could exacerbate bone loss by downregulating the expression of dual specificity phosphatase 1 (DUSP1) activating the JNK signaling pathway.57
Muscle Injury and Tendinopathy
Muscle injuries such as tendon tears and tendinopathies are common and easily cause considerable disability, pain, health care costs, and losses productivity. Following tendon injury, the development of fibrotic healing response impairs tendon function and restricts tendon motion. Peritendinous tissue fibrosis poses a major clinical problem in hand surgery. sEVs from macrophages are crucial factors in tissue microenvironment regulation following tissue injury. Macrophage-derived miR-21-5p-enriched sEVs lead to increased proliferation, migration, and fibrotic activity of tendon cells by inhibiting the expression of Smad7, providing potential targets for the prevention and treatment of tendon adhesion.58 MiR-501 overexpressed in M2-sEVs can be transferred into myoblasts, downregulate the expression of the transcription factor YY1, affect myoblast differentiation and promote the formation of myotubes, thus leading to regeneration of injured pubococcygeal muscle.59 M2 macrophage-derived circRNA-Ep400-containing sEVs inhibited miR-15b-5p but promoted the expression of Col-I, Col-III, α-SMA and FGF1/7/9 in both fibroblasts and tenocytes, thus promoting peritendinous fibrosis and providing novel therapeutics for tendon injury treatment.60
Inflammation and Bone Osteolytic Diseases
Inflammatory-mediated pathological bone osteolytic diseases including osteoarthritis (OA), rheumatoid arthritis (RA), immunoinflammatory osteolysis, aseptic prosthesis loosening, and periodontitis are major orthopedic diseases that cause disability and impose a substantial economic burden worldwide.61,62 Bone remodeling is a process involving osteoblast formation and osteoclast resorption to maintain bone architecture balance and systemic mineral homeostasis.63 Chronic inflammation caused by various factors can disrupt this delicate balance between osteoblasts and osteoclasts and disturb bone metabolism, causing osteolytic disorders.64 Macrophages play a substantial role in bone diseases caused by chronic inflammation.64 Proteomic studies have shown that macrophage-derived sEVs contain a large number of proteins, such as alarmins, chemokines, and inflammatory cytokines and play a critical role in bone remodeling as vehicles.65 Alarmins are endogenous molecules often detected in macrophage-sEVs that include annexins, galectins, heat-shock proteins and S100-alarmins, which are released upon cellular stress and activate the immune system.66–68 Alarmins play wide roles in osteocyte, osteoblast and osteoclast differentiation and function. Annexin2 in macrophage sEVs can stimulate bone resorption by promoting the overexpression of the pro-osteoclastogenic factors GM-CSF and RANKL, and facilitating osteoclast formation.69 Galectin-3 as a regulator of the osteoblast-osteoclast interaction inhibits osteoclastogenesis and regulates bone homeostasis.70 Increased HSP60 levels stimulated by IL-1β and TNF-α can promote osteoclast formation and activity by activating RANK-RANKL signaling by binding to TLR2.71 IL-1β, a critical inflammatory cytokine in the inflammatory cascade response, is also contained in macrophage-sEVs. It stimulates the production of various proinflammatory mediators, such as cytokines, chemokines, and matrix metalloproteinases (MMPs), leading to the continued progression of chronic inflammation.72,73 In inflammatory conditions, LPS/IFN-γ can stimulate macrophages to secrete endoplasmic reticulum aminopeptidase 1 (ERAP1) via sEVs to enhance the phagocytic and nitric oxide (NO) synthetic activities of macrophages and modify inflammatory reactions.74 Similarly, macrophages secrete pro- and anti-inflammatory mediators into the micro milieu to regulate bone homeostasis in inflammatory conditions. During the inflammatory phase, the production and release of sEVs are abundant and easily cause tissue cell hypoxia due to the increase in cell metabolism and the reduction in the levels of substrates. Under hypoxic conditions, sEVs enriched with miR-222 derived from M1 macrophages can be released to target BMSCs, inducing cell apoptosis and thereby regulating bone formation.75 MiR-222 has also been reported to inhibit endothelial cell migration, proliferation, and angiogenesis by suppressing the production of nitric oxide synthase.76 Under inflammatory conditions, chemokines and proinflammatory cytokines (IFN-γ, LPS, and IL-1β) are produced and induce polarization of M1 macrophages. MiR-155 is derived from M1 macrophage sEVs and transferred into endothelial cells, controlling endothelial cell migration and cell-cell connections, thereby regulating vascular permeability.38,77
Osteoarthritis
Osteoarthritis is the most common form of joint disease and is characterized by pain, cartilage destruction, synovial fibrosis osteophyte formation, and sometimes the swelling of certain joints such as the hand, spine, hip and knee, and is frequently accompanied by synovitis.78 Multiple studies have confirmed that macrophages play an important role in the occurrence of OA through inflammatory factors, cytokines, miRNA and proteins.79 The long noncoding RNA (lncRNA) MM2P induces M2 macrophage polarization, and promotes the spread of SOX9 enriched in M2-sEVs into chondrocytes, significantly enhancing the differentiation and function of chondrocytes, which promotes cartilage repair and supports a novel therapeutic strategy for OA.80 Knee OA (KOA) is a degenerative disease with the main symptoms of cartilage destruction and knee joint pain, resulting in the activation of the PI3K/Akt/mTOR signaling pathway in the body. sEVs derived from M2 macrophage intervention can inhibit the protein and mRNA expression of PI3K, Akt, and mTOR in KOA model rats and reduce cartilage injury, indicating that M2-sEVs may exert a therapeutic effect on KOA, but the mechanism is still unclear.81
Rheumatoid Arthritis
RA is one of the most widespread chronic inflammatory joint diseases with strong genetic predisposition factors and is characterized by synovitis, cartilage erosion and bone loss accompanied by pain, swelling, stiffness, and joint deformity, and can also damage extra-articular organs, such as the heart, kidney, lung and nervous system.82,83 MiR-223 is enriched in macrophage-sEVs under inflammatory conditions.84 As an innate immune regulatory factor, the expression of miR-223 is highest in the bone marrow cavity, playing an important role in the differentiation of granulocytes and macrophages during the differentiation of bone marrow. MiR-223 activates the immune system, promotes macrophage polarization to the anti-inflammatory M2 type, and induces the production of more sEVs.38,85 Another study revealed the multiple roles that miR-223 plays in bone metabolism by regulating osteoclast differentiation, representing a promising diagnostic tool for the treatment of bone diseases related to aberrant bone metabolism.86 Another study also showed that miR-223 derived from macrophages can induce the differentiation of monocytes, activate hematopoietic cell production in the marrow, and promote the release of more sEVs. Thus, this feedback mechanism may be an innate response element that regulates host defense and inflammation.87
Immunoinflammatory Osteolysis and Aseptic Loosening
Aseptic loosening is a common complication following total joint arthroplasty due to periprosthetic inflammatory osteolysis and is the leading cause of revision procedures.88,89 Wear particles released from the sliding surfaces of prosthetic materials induce a persistent local inflammatory response, thereby stimulating bone resorbing-osteoclast activities and bone loss around the implant.90,91 Macrophages activated by wear particulate debris release an array of cytokines and proinflammatory mediators in the joint fluid and facilitate the recruitment of inflammatory cells.92,93 MiR-155 can be stimulated by activators of proinflammatory M1 phenotype macrophages (LPS, IFN-α) and released to promote the secretion of inflammatory cytokines such as TNF-α and IL-12, thereby aggravating the inflammatory response.94 The production of miR-23a-5p-containing sEVs induced by RANKL effectively inhibits the expression of Runx2 and suppresses osteoblast differentiation.95
Periodontitis
Chronic periodontitis is one of the most common diseases of the oral and maxillofacial regions and is caused by bacterial infection and the host immune-inflammatory response, leading to alveolar bone absorption and tooth loosening and shedding.96 Porphyromonas gingivalis (P.g) is the major pathogenic bacterium involved in chronic periodontitis. A recent study revealed that P.g-LPS-activated macrophage-derived sEVs can inhibit BMSC osteogenic differentiation by mediating inflammatory stimulation. P.g LPS-stimulated macrophages produced an inflammatory response and released sEVs. After BMSCs had taken up inflammatory sEVs, the gene and protein expression levels of TNF-α and IL-6 were significantly unregulated, but the expression of ALP, RUNX2, and COL-1 decreased, indicating an inhibitory effect on BMSCs in inflammatory states.97
Osteosarcoma and Tumor Bone Metastasis
Osteosarcoma (OS) is one of the most common primary malignant bone tumors in adolescents, and in general, it occurs in males more frequently than in females.98 sEVs from macrophages have been reported to play critical roles in regulating the proliferation, metastasis, and angiogenesis of osteosarcoma by participating in intercellular contacts and controlling cellular signaling.99 LIFR-AS1 as a newly described tumor-related lncRNA, has been reported to be released from macrophages to osteosarcoma cells via sEVs and further promote tumor progression by acting as a sponge in the miR-29a/NFIA pathway.100 Bone is one of the most common metastatic sites of breast cancer. sEVs enriched in miR-223 secreted from IL-4-activated macrophages were found to promote breast cancer cell invasion through the miR-223/Mef2c/β-catenin pathway.101 sEVs containing miR‑660 derived from tumor-associated macrophages promoted the invasion and migration of breast cancer cells by inhibiting KLHL21 activity and then increased bone and lung lymph node metastasis.102
Drug Delivery
sEVs have been demonstrated to be endogenous delivery carriers with excellent biocompatibility, low cytotoxicity, and immunological inertness and are an ideal potential candidate for the treatment of many diseases. Numerous studies have shown that sEVs derived from macrophages can carry various drugs, such as protein, DNA and small molecular drugs, and play a role in inflammatory regulation. IL4-loaded sEVs exhibit high anti-inflammatory effects by modulating macrophage polarization. IL4-sEVs are absorbed by macrophages via endocytosis and regulate the M1/M2 status and suppress downstream fibroblast proliferation, pannus formation, and bone destruction in CIA mice. In the synovium of RA joints, IL4-sEV-infiltrated macrophages predominantly secrete proinflammatory mediators, which are expected to contribute to the in vivo anti-inflammatory effects and are considered for the treatment of chronic inflammatory rheumatoid arthritis.103,104 Another study showed M2 sEVs/pDNA/BSP nanoparticles using M2-sEVs as a platform for the codelivery of IL-10 pDNA and the chemotherapeutic drug betamethasone sodium phosphate (BSP), which can effectively increase IL-10 secretion, attenuate the production of proinflammatory cytokines (IL-1β, TNF-α), promote macrophage polarization from a proinflammatory to an anti-inflammatory state, and efficiently reduce joint damage for RA treatment.105 Engineered M2-sEVs loaded with FDA-approved hexyl 5-aminolevulinate hydrochloride (HAL) exhibited excellent inflammatory-tropism and anti-inflammatory effects via surface-bonded chemokine receptors and anti-inflammatory cytokines derived from M2 macrophages. The encapsulated HAL can facilitate the biosynthesis of anti-inflammatory carbon monoxide and bilirubin and further enhance anti-inflammatory effects.106 Curcumin is a natural polyphenol with anti-inflammatory, antineoplastic and antioxidant activities. Curcumins complexed with sEVs (Cur-sEVs) have been found to have increased stability and bioavailability. Macrophages treated with Cur-sEVs produced significantly lower levels of IL-6 and TNF-α than macrophages treated with curcumin or sEV treatment alone, indicating a higher anti-inflammatory activity.107 Dexamethasone sodium phosphate encapsulated in M-sEVs (Dex/sEVs) showed an anti-inflammatory effect on macrophages by suppressing the expression of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) and increasing the levels of anti-inflammatory cytokines (IL-10), promoting the bone and cartilage repair and significantly reducing the incidence of inflamed joints in CIA mice.108 For the application of sEVs to achieve good therapeutic effects, safe manufacturing, quality control, and scalable mass production solutions are needed. Thus, optimized technologies need to be developed in the future to facilitate the clinical use of sEV delivery.
Discussion and Future Perspectives
Orthopedic diseases constitute one of the major causes of disability and impose a considerable economic burden worldwide. Macrophages play an important role in maintaining bone homeostasis and promoting bone establishment. sEVs derived from macrophages, which act as mediators of intercellular communication are potential immunomodulatory agents investigated in the context of basic research and clinical therapy. This paper describes the contribution of sEVs derived from macrophages in bone diseases and provides a theoretical basis for further research and the clinical application of sEVs as ideal candidates for drug delivery systems in the treatment of orthopedic diseases in the future. sEVs, as an important medium of communication between cells, can mediate bone metabolism through the paracrine transmission of various bioactive substances.30,109 We focused on miRNAs and intracellular proteins as key mediators of sEV effects on bone homeostasis. In addition, due to the advantages of lower toxicity and immunogenicity, higher biocompatibility, natural targeting ability, and good biological barrier permeability, their carrier functions can be used for more complex scenarios than lipid nanoparticles. M-sEVs have been used as a vehicle for the delivery of drugs with remarkable inflammatory tropism and anti-inflammatory effects.110 However, it is difficult to determine the exact dose and timing for application, and basic research and preclinical exploration of the specific mechanism of sEVs for intercellular communication under physiological and pathological conditions are still an immature stage. The contents of sEVs are complex, which hinders further exploration of their mechanisms of action. The exact mechanisms of sEVs still need to be further investigated and the application of engineered sEVs for clinical regeneration requires continued study of safety, feasibility and mass productivity.
Conclusion
M-sEVs play important roles in the mechanism of bone disease by interfering with osteogenesis, angiogenesis, epithelialization, cell proliferation and migration, differentiation, the immune response, inflammatory factors and others. They participate in a variety of pathways associated with bone homeostasis and can be used as drug carriers. It is believed that M-sEVs have great therapeutic potential in all aspects of medical treatment in the future development of biotechnology.
Funding
This work was partially supported by the Natural Science Foundation of Shandong Province (No. ZR2021QH307; No. ZR2021MH013), China Scholarship Council (CSC) (NO. 201808080126), the incubation fund of Shandong Provincial Hospital (grant number 2020FY019), Young Scholars Program of Shandong Provincial Hospital, the Shandong Province Major Scientific and Technical Innovation Project (No. 2021SFGC0502), and the Jinan Clinical Medical Science and Technology Innovation Plan (NO. 202019168). The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
2. Shahin HI, Radnaa E, Tantengco OAG, et al. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cells dagger. Biol Reprod. 2021;105(2):464–480. doi:10.1093/biolre/ioab088
3. Kim SD, Kang SA, Kim YW, et al. Screening and functional pathway analysis of pulmonary genes associated with suppression of allergic airway inflammation by adipose stem cell-derived extracellular vesicles. Stem Cells Int. 2020;2020:5684250. doi:10.1155/2020/5684250
4. Kang M, Huang CC, Lu Y, et al. Bone regeneration is mediated by macrophage extracellular vesicles. Bone. 2020;141:115627. doi:10.1016/j.bone.2020.115627
5. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi:10.1038/s41565-021-00931-2
6. Liu H, Zhang Q, Wang S, et al. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact Mater. 2022;14:169–181. doi:10.1016/j.bioactmat.2021.12.006
7. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
8. Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228(2):R57–71. doi:10.1530/JOE-15-0201
9. Ye J, Liu X. Macrophage-derived small extracellular vesicles in multiple diseases: biogenesis, function, and therapeutic applications. Front Cell Dev Biol. 2022;10:913110. doi:10.3389/fcell.2022.913110
10. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi:10.1038/nri855
11. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
12. Moller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20(12):697–709. doi:10.1038/s41568-020-00299-w
13. Gong L, Chen B, Zhang J, et al. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J Extracell Vesicles. 2020;9(1):1800971. doi:10.1080/20013078.2020.1800971
14. Liang G, Kow ASF, Tham CL, Ho YC, Lee MT. Ameliorative effect of tocotrienols on perimenopausal-associated osteoporosis-A review. Antioxidants. 2022;11:11. doi:10.3390/antiox11112179
15. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
16. Wang D, Wang J, Zhou J, Zheng X. The role of adenosine receptor A2A in the regulation of macrophage exosomes and vascular endothelial cells during bone healing. J Inflamm Res. 2021;14:4001–4017. doi:10.2147/JIR.S324232
17. Chen K, Jiao Y, Liu L, et al. Communications between bone marrow macrophages and bone cells in bone remodeling. Front Cell Dev Biol. 2020;8:598263. doi:10.3389/fcell.2020.598263
18. Pieters BCH, Cappariello A, van den Bosch MHJ, et al. Macrophage-derived extracellular vesicles as carriers of alarmins and their potential involvement in bone homeostasis. Front Immunol. 2019;10:1901. doi:10.3389/fimmu.2019.01901
19. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi:10.1038/ni.1937
20. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi:10.1155/2015/816460
21. Bai ZZ, Li HY, Li CH, Sheng CL, Zhao XN. M1 macrophage-derived exosomal MicroRNA-326 suppresses hepatocellular carcinoma cell progression via mediating NF-kappaB signaling pathway. Nanoscale Res Lett. 2020;15(1):221. doi:10.1186/s11671-020-03432-8
22. Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15(2–3):260–271. doi:10.1002/pmic.201400234
23. Zhu G, Xia Y, Zhao Z, et al. LncRNA XIST from the bone marrow mesenchymal stem cell derived exosome promotes osteosarcoma growth and metastasis through miR-655/ACLY signal. Cancer Cell Int. 2022;22(1):330. doi:10.1186/s12935-022-02746-0
24. Zhou QF, Cai YZ, Lin XJ. The dual character of exosomes in osteoarthritis: antagonists and therapeutic agents. Acta Biomater. 2020;105:15–25. doi:10.1016/j.actbio.2020.01.040
25. Yang R, Liao Y, Wang L, et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi:10.3389/fimmu.2019.02346
26. Liu S, Chen J, Shi J, et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol. 2020;115(2):22. doi:10.1007/s00395-020-0781-7
27. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi:10.1186/s13578-019-0282-2
28. Liu A, Jin S, Fu C, et al. Macrophage-derived small extracellular vesicles promote biomimetic mineralized collagen-mediated endogenous bone regeneration. Int J Oral Sci. 2020;12(1):33. doi:10.1038/s41368-020-00100-6
29. McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of Uptake. J Circ Biomark. 2015;4:7. doi:10.5772/61186
30. Meng F, Xue X, Yin Z, et al. Research progress of exosomes in bone diseases: mechanism, diagnosis and therapy. Front Bioeng Biotechnol. 2022;10:866627. doi:10.3389/fbioe.2022.866627
31. Bjorge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomater Sci. 2017;6(1):60–78. doi:10.1039/c7bm00479f
32. Huang J, Xiong J, Yang L, et al. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale. 2021;13(19):8740–8750. doi:10.1039/d1nr01314a
33. Huang X, Lan Y, Shen J, Chen Z, Xie Z. Extracellular vesicles in bone homeostasis: emerging mediators of osteoimmune interactions and promising therapeutic targets. Int J Biol Sci. 2022;18(10):4088–4100. doi:10.7150/ijbs.69816
34. Li Z, Wang Y, Li S, Li Y. Exosomes derived from M2 macrophages facilitate osteogenesis and reduce adipogenesis of BMSCs. Front Endocrinol. 2021;12:680328. doi:10.3389/fendo.2021.680328
35. Zhu Y, Zhao S, Cheng L, et al. Mg(2+) -mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J Orthop Res. 2022;40(7):1563–1576. doi:10.1002/jor.25189
36. Chen X, Wan Z, Yang L, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnology. 2022;20(1):110. doi:10.1186/s12951-022-01314-y
37. Bai L, Zhao Y, Chen P, et al. Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration. Small. 2021;17(4):e2006287. doi:10.1002/smll.202006287
38. Xiao Y, Ding Y, Zhuang J, et al. Osteoimmunomodulation role of exosomes derived from immune cells on osseointegration. Front Bioeng Biotechnol. 2022;10:989537. doi:10.3389/fbioe.2022.989537
39. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. doi:10.1016/j.actbio.2019.01.006
40. Zhang T, Jiang M, Yin X, Yao P, Sun H. Mechanism of exosomes involved in osteoimmunity promoting osseointegration around titanium implants with small-scale topography. Front Bioeng Biotechnol. 2021;9:682384. doi:10.3389/fbioe.2021.682384
41. Wang Z, Zhao F, Zhao Y, Bai L, Hang R. Simultaneously enhanced osteogenesis and angiogenesis via macrophage-derived exosomes upon stimulation with titania nanotubes. Biomater Adv. 2022;134:112708. doi:10.1016/j.msec.2022.112708
42. Kim H, Wang SY, Kwak G, et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6(20):1900513. doi:10.1002/advs.201900513
43. Zhao Y, Huang Y, Liu H, et al. Macrophages with different polarization phenotypes influence cementoblast mineralization through exosomes. Stem Cells Int. 2022;2022:4185972. doi:10.1155/2022/4185972
44. Li M, Wang T, Tian H, et al. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Bio. 2019;47(1):3793–3803. doi:10.1080/21691401.2019.1669617
45. Zhang Q, Sun W, Li T, Liu F. Polarization behavior of bone macrophage as well as associated osteoimmunity in glucocorticoid-induced osteonecrosis of the femoral head. J Inflamm Res. 2023;16:879–894. doi:10.2147/JIR.S401968
46. Zhang Q, Lu S, Zhou D, Dong J, Liu F. PTGS2 identified as a biomarker of glucocorticoid-induced osteonecrosis of the femoral head and an enhancer of osteogenesis. Genes & Diseases. 2023;10:14–17. doi:10.1016/j.gendis.2022.01.005
47. Liu K, Luo X, Lv ZY, et al. Macrophage-derived exosomes promote bone mesenchymal stem cells towards osteoblastic fate through microRNA-21a-5p. Front Bioeng Biotechnol. 2021;9:801432. doi:10.3389/fbioe.2021.801432
48. Xiong Y, Chen L, Yan C, et al. M2 Macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J Nanobiotechnology. 2020;18(1):66. doi:10.1186/s12951-020-00622-5
49. Bin-Bin Z, Da-Wa ZX, Chao L, et al. M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic differentiation of bone mesenchymal stem cells. J Orthop Surg Res. 2022;17(1):137. doi:10.1186/s13018-022-03029-0
50. Arceo-Mendoza RM, Camacho PM. Postmenopausal osteoporosis: latest guidelines. Endocrinol Metab Clin North Am. 2021;50(2):167–178. doi:10.1016/j.ecl.2021.03.009
51. Liu F, Dong J, Zhang P, Zhou D, Zhang Q. Transcriptome sequencing reveals key genes in three early phases of osteogenic, adipogenic, and chondrogenic differentiation of bone marrow mesenchymal stem cells in rats. Front Mol Biosci. 2021;8:782054. doi:10.3389/fmolb.2021.782054
52. Zhang Q, Dong J, Zhang P, Zhou D, Liu F. Dynamics of transcription factors in three early phases of osteogenic, adipogenic, and chondrogenic differentiation determining the fate of bone marrow mesenchymal stem cells in rats. Front Cell Dev Biol. 2021;9:768316. doi:10.3389/fcell.2021.768316
53. Mitchell PJ, Chan DD, Lee JK, Tabu I, Alpuerto BB. The global burden of fragility fractures - what are the differences, and where are the gaps. Best Pract Res Clin Rheumatol. 2022;36:101777. doi:10.1016/j.berh.2022.101777
54. Hygum K, Starup-Linde J, Langdahl BL. Diabetes and bone. Osteoporos Sarcopenia. 2019;5(2):29–37. doi:10.1016/j.afos.2019.05.001
55. Zhang D, Wu Y, Li Z, et al. MiR-144-5p, an exosomal miRNA from bone marrow-derived macrophage in type 2 diabetes, impairs bone fracture healing via targeting Smad1. J Nanobiotechnology. 2021;19(1):226. doi:10.1186/s12951-021-00964-8
56. Zhang C, Bao LR, Yang YT, Wang Z, Li Y. M2巨噬细胞外泌体对高糖高胰岛素条件下小鼠骨髓间充质干细胞成骨分化的影响 [Role of M2 macrophage exosomes in osteogenic differentiation of mouse bone marrow mesenchymal stem cells under high-glucose and high-insulin]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022;53(1):63–70. Chinese. doi:10.12182/20220160207
57. Yu L, Hu M, Cui X, et al. M1 macrophage-derived exosomes aggravate bone loss in postmenopausal osteoporosis via a microRNA-98/DUSP1/JNK axis. Cell Biol Int. 2021;45(12):2452–2463. doi:10.1002/cbin.11690
58. Cui H, He Y, Chen S, et al. Macrophage-Derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 Pathway. Mol Ther Nucleic Acids. 2019;14:114–130. doi:10.1016/j.omtn.2018.11.006
59. Zhou M, Li B, Liu C, et al. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(PtB):108223. doi:10.1016/j.intimp.2021.108223
60. Yu Y, Sun B, Wang Z, et al. Exosomes from M2 macrophage promote peritendinous fibrosis posterior tendon injury via the MiR-15b-5p/FGF-1/7/9 pathway by delivery of circRNA-Ep400. Front Cell Dev Biol. 2021;9:595911. doi:10.3389/fcell.2021.595911
61. Ponzetti M, Rucci N. Updates on osteoimmunology: what’s new on the cross-talk between bone and immune system. Front Endocrinol. 2019;10:236. doi:10.3389/fendo.2019.00236
62. Terkawi MA, Matsumae G, Shimizu T, et al. Interplay between inflammation and pathological bone resorption: insights into recent mechanisms and pathways in related diseases for future perspectives. Int J Mol Sci. 2022;23:3. doi:10.3390/ijms23031786
63. Uenaka M, Yamashita E, Kikuta J, et al. Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat Commun. 2022;13(1):1066. doi:10.1038/s41467-022-28673-2
64. Garcia-Martin R, Wang G, Brandao BB, et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601(7893):446–451. doi:10.1038/s41586-021-04234-3
65. Hu Y, Wang Y, Chen T, et al. Exosome: function and application in inflammatory bone diseases. Oxid Med Cell Longev. 2021;2021:6324912. doi:10.1155/2021/6324912
66. Hassani K, Olivier M, Milon G. Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl Trop Dis. 2013;7(5):e2185. doi:10.1371/journal.pntd.0002185
67. Cypryk W, Lorey M, Puustinen A, Nyman TA, Matikainen S. Proteomic and bioinformatic characterization of extracellular vesicles released from human macrophages upon influenza A virus infection. J Proteome Res. 2017;16(1):217–227. doi:10.1021/acs.jproteome.6b00596
68. Cypryk W, Ohman T, Eskelinen EL, Matikainen S, Nyman TA. Quantitative proteomics of extracellular vesicles released from human monocyte-derived macrophages upon beta-glucan stimulation. J Proteome Res. 2014;13(5):2468–2477. doi:10.1021/pr4012552
69. Li F, Chung H, Reddy SV, et al. Annexin II stimulates RANKL expression through MAPK. J Bone Miner Res. 2005;20(7):1161–1167. doi:10.1359/JBMR.050207
70. Li YJ, Kukita A, Teramachi J, et al. A possible suppressive role of galectin-3 in upregulated osteoclastogenesis accompanying adjuvant-induced arthritis in rats. Lab Invest. 2009;89(1):26–37. doi:10.1038/labinvest.2008.111
71. Koh JM, Lee YS, Kim YS, et al. Heat shock protein 60 causes osteoclastic bone resorption via toll-like receptor-2 in estrogen deficiency. Bone. 2009;45(4):650–660. doi:10.1016/j.bone.2009.06.007
72. Pizzirani C, Ferrari D, Chiozzi P, et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109(9):3856–3864. doi:10.1182/blood-2005-06-031377
73. Xin Y, Wang W, Mao E, Yang H, Targeting LS. NLRP3 Inflammasome alleviates synovitis by reducing pyroptosis in rats with experimental temporomandibular joint osteoarthritis. Mediators Inflamm. 2022;2022:2581151. doi:10.1155/2022/2581151
74. Goto Y, Ogawa Y, Tsumoto H, et al. Contribution of the exosome-associated form of secreted endoplasmic reticulum aminopeptidase 1 to exosome-mediated macrophage activation. Biochim Biophys Acta Mol Cell Res. 2018;1865(6):874–888. doi:10.1016/j.bbamcr.2018.03.009
75. Qi Y, Zhu T, Zhang T, et al. M1 macrophage-derived exosomes transfer miR-222 to induce bone marrow mesenchymal stem cell apoptosis. Lab Invest. 2021;101(10):1318–1326. doi:10.1038/s41374-021-00622-5
76. Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29(1):12–15. doi:10.1016/j.tips.2007.10.014
77. Ortega FJ, Moreno M, Mercader JM, et al. Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin Epigenetics. 2015;7:49. doi:10.1186/s13148-015-0083-3
78. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi:10.1016/j.immuni.2014.06.008
79. Bondeson J, Blom AB, Wainwright S, et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–657. doi:10.1002/art.27290
80. Bai J, Zhang Y, Zheng X, et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11(9):763. doi:10.1038/s41419-020-02945-5
81. Da-Wa ZX, Jun M, Chao-Zheng L, et al. Exosomes Derived from M2 macrophages exert a therapeutic effect via inhibition of the PI3K/AKT/mTOR pathway in rats with knee osteoarthritic. Biomed Res Int. 2021;2021:7218067. doi:10.1155/2021/7218067
82. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
83. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. 2021;10:11. doi:10.3390/cells10112857
84. Krzywinska E, Stockmann C. Hypoxia, metabolism and immune cell function. Biomedicines. 2018;6:2. doi:10.3390/biomedicines6020056
85. Zhu X, Shen H, Yin X, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81. doi:10.1186/s13046-019-1095-1
86. Xie Y, Zhang L, Gao Y, Ge W, Tang P. The multiple roles of microrna-223 in regulating bone metabolism. Molecules. 2015;20(10):19433–19448. doi:10.3390/molecules201019433
87. Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–995. doi:10.1182/blood-2011-08-374793
88. Wang B, Li Q, Dong J, Zhou D, Liu F. Comparisons of the surface micromotions of cementless femoral prosthesis in the horizontal and vertical levels: a network analysis of biomechanical studies. J Orthop Surg Res. 2020;15(1):293. doi:10.1186/s13018-020-01794-4
89. Yan SG, Chevalier Y, Liu F, et al. Metaphyseal anchoring short stem Hip arthroplasty provides a more physiological load transfer: a comparative finite element analysis study. J Orthop Surg Res. 2020;15(1):498. doi:10.1186/s13018-020-02027-4
90. Hodges NA, Sussman EM, Stegemann JP. Aseptic and septic prosthetic joint loosening: impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:121127. doi:10.1016/j.biomaterials.2021.121127
91. Baranowska A, Plusa T, Baranowski P, Szymczak Z, Dudek J. Czy aseptyczne obluzowanie protez stawów jest aseptyczne?[Is aseptic loosening of joint prostheses aseptic?]. Pol Merkur Lekarski. 2022;50(299):318–322. Polish.
92. Nich C, Takakubo Y, Pajarinen J, et al. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res A. 2013;101(10):3033–3045. doi:10.1002/jbm.a.34599
93. Liu F, Dong J, Zhou D, Zhang Q. Identification of key candidate genes related to inflammatory osteolysis associated with vitamin E-Blended UHMWPE debris of orthopedic implants by integrated bioinformatics analysis and experimental confirmation. J Inflamm Res. 2021;14:3537–3554. doi:10.2147/JIR.S320839
94. Stanczyk J, Pedrioli DM, Brentano F, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58(4):1001–1009. doi:10.1002/art.23386
95. Yang JX, Xie P, Li YS, Wen T, Yang XC. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020;70:109504. doi:10.1016/j.cellsig.2019.109504
96. Chen YW, Hsieh O, Chen YA, Chiou LL, Chang PC. Randomized controlled clinical effectiveness of adjunct 660-nm light-emitting diode irradiation during non-surgical periodontal therapy. J Formos Med Assoc. 2020;119(1 Pt 1):157–163. doi:10.1016/j.jfma.2019.01.010
97. Song X, Xue Y, Fan S, Hao J, Deng R. Lipopolysaccharide-activated macrophages regulate the osteogenic differentiation of bone marrow mesenchymal stem cells through exosomes. PeerJ. 2022;10:e13442. doi:10.7717/peerj.13442
98. Rickel K, Fang F, Tao J. Molecular genetics of osteosarcoma. Bone. 2017;102:69–79. doi:10.1016/j.bone.2016.10.017
99. Liang X, Guo W, Ren T, et al. Macrophages reduce the sensitivity of osteosarcoma to neoadjuvant chemotherapy drugs by secreting Interleukin-1 beta. Cancer Lett. 2020;480:4–14. doi:10.1016/j.canlet.2020.03.019
100. Zhang H, Yu Y, Wang J, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021;21(1):192. doi:10.1186/s12935-021-01893-0
101. Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi:10.1186/1476-4598-10-117
102. Li C, Li R, Hu X, Zhou G, Jiang G. Tumor-promoting mechanisms of macrophage-derived extracellular vesicles-enclosed microRNA-660 in breast cancer progression. Breast Cancer Res Treat. 2022;192(2):353–368. doi:10.1007/s10549-021-06433-y
103. Takenaka M, Yabuta A, Takahashi Y, Takakura Y. Interleukin-4-carrying small extracellular vesicles with a high potential as anti-inflammatory therapeutics based on modulation of macrophage function. Biomaterials. 2021;278:121160. doi:10.1016/j.biomaterials.2021.121160
104. Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–485. doi:10.1038/nrrheum.2016.91
105. Li H, Feng Y, Zheng X, et al. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J Control Release. 2022;341:16–30. doi:10.1016/j.jconrel.2021.11.019
106. Wu G, Zhang J, Zhao Q, et al. Molecularly engineered macrophage-derived exosomes with inflammation tropism and intrinsic heme biosynthesis for atherosclerosis treatment. Angew Chem Int Ed Engl. 2020;59(10):4068–4074. doi:10.1002/anie.201913700
107. Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi:10.1038/mt.2010.105
108. Yan F, Zhong Z, Wang Y, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnology. 2020;18(1):115. doi:10.1186/s12951-020-00675-6
109. Zeng ZL, Xie H. Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater Transl. 2022;3(3):175–187. doi:10.12336/biomatertransl.2022.03.002
110. Xue X, Hu Y, Deng Y, Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv Funct Mater. 2021;31:19. doi:10.1002/adfm.202009432
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.