Back to Journals » Orthopedic Research and Reviews » Volume 14
Osteochondral Allografts in Knee Surgery: Narrative Review of Evidence to Date
Authors Lai WC, Bohlen HL, Fackler NP , Wang D
Received 17 April 2022
Accepted for publication 8 August 2022
Published 11 August 2022 Volume 2022:14 Pages 263—274
DOI https://doi.org/10.2147/ORR.S253761
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Wilson C Lai,1 Hunter L Bohlen,1 Nathan P Fackler,1,2 Dean Wang1,3
1Department of Orthopaedic Surgery, UCI Health, Orange, CA, USA; 2Georgetown University School of Medicine, Washington, DC, USA; 3Department of Biomedical Engineering, University of California Irvine, Irvine, CA, USA
Correspondence: Dean Wang, Department of Orthopaedic Surgery, UCI Health, 101 The City Drive S. Pavilion III, 2nd Floor, Orange, CA, 92868, USA, Tel +1 714-456-7012, Email [email protected]
Abstract: Knee articular cartilage defects can result in significant pain and loss of function in active patients. Osteochondral allograft (OCA) transplantation offers a single-stage solution to address large chondral and osteochondral defects by resurfacing focal cartilage defects with mature hyaline cartilage. To date, OCA transplantation of the knee has demonstrated excellent clinical outcomes and long-term survivorship. However, significant variability still exists among clinicians with regard to parameters for graft acceptance, surgical technique, and rehabilitation. Technologies to optimize graft viability during storage, improve osseous integration of the allograft, and shorten recovery timelines after surgery continue to evolve. The purpose of this review is to examine the latest evidence on treatment indications, graft storage and surgical technique, patient outcomes and survivorship, and rehabilitation after surgery.
Keywords: cartilage, osteochondral allograft transplantation, survivorship, outcomes, rehabilitation
Introduction
It is estimated that 200,000 to 300,000 related surgical procedures to address symptomatic knee cartilage and osteochondral defects are performed each year in the United States.1–3 Articular cartilage injuries have a limited potential to heal, and progression of articular cartilage defects often lead to early-onset osteoarthritis and debilitating pain, especially for young and active patients.4 Multiple cartilage repair procedures for treating high-grade defects have been described, including microfracture, autologous chondrocyte implantation (ACI), osteochondral autograft transfer (OAT), and osteochondral allograft (OCA) transplantation.5 To date, OCA transplantation has been performed for over 40 years and is currently the gold standard treatment for addressing large (>2–4 cm2), full-thickness chondral or osteochondral defects of the knee (Figure 1).2,6 OCA transplantation has demonstrated excellent patient-reported outcomes, survivorship of 75–80% at 10 years, and a high rate of return to sport.7–11
Despite the current evidence in support of OCA transplantation, its application for certain conditions, such as early degenerative arthritis, remains controversial. Failures are characterized by inadequate osseous integration or delamination of the chondral surface, for which the exact mechanisms remain unknown. Furthermore, OCA transplantation will always be constrained by a limited graft supply, and technologies to optimize graft viability during storage and facilitate graft-host matching to limit articular surface step-off continue to evolve. This review examines the evidence to date regarding patient selection and contraindications for OCA transplantation, nuances of surgical technique, graft survivorship and patient outcomes, risks for failure, and rehabilitation and return to sport.
Patient Selection and Contraindications
Indications for OCA transplantation include patients with large symptomatic cartilage defects secondary to trauma, intraarticular fractures, osteonecrosis, osteochondritis dissecans (OCD), and revision of previously failed cartilage restoration procedures.2,12,13 Traditionally, OCA transplantation was reserved for the younger patient since patients >40 years old at time of surgery were associated with higher rates of graft failure, presumably due to pre-existing joint degeneration.5,14 Recently, multiple studies have found that patients ≥40 years old undergoing OCA transplantation can still have excellent patient-reported outcomes and improved quality of life, thereby delaying the need for prosthetic joint replacement for several years.14–16 Thus, in select older patients with only focal articular cartilage wear and whose activity levels not yet suitable for arthroplasty, OCA transplantation may be a reasonable treatment option.
Contraindications for OCA transplantation include uncorrected limb malignment and/or ligamentous instability, body mass index (BMI) >35 kg/m2, smoking, alcohol abuse, chronic steroid use, advanced osteoarthritis within any compartment (Kellgren-Lawrence grade >2), and systematic autoimmune or inflammatory joint disease (Table 1).2,17 Bakay et al found in their study that all grafts with unaddressed 10°–15° varus malalignment after OCA transplantation failed in the short-term at a mean follow-up of 19 months.18 Other groups have similarly reported that uncorrected coronal malalignment is a risk factor for failure that can result in an almost seven times higher failure rate.19 The benefits of corrective osteotomies in isolation have been well documented and shown to slow the degenerative process by redistributing abnormal loads across the joint, promoting cartilage-like repair, and providing long-term relief in patients with cartilage defects and unicompartmental osteoarthritis.20 Thus, corrective osteotomy reduces excessive joint reactive forces on the transplanted osteochondral allograft, which may facilitate graft incorporation. Advanced osteoarthritis and inflammatory arthropathy are absolute contraindications to OCA transplantation due to the prominent inflammatory and catabolic environment of the joint, resulting in biologic destruction of any transplanted allografts. Giannini et al also reported that 86% of patients who underwent OCA transplantation for end-stage osteoarthritis were eventually revised to a total knee arthroplasty within 2 years postoperatively.21
 |
Table 1 Indications and Contraindications for Osteochondral Allograft Transplantation |
Graft Storage, Matching, and Surgical Technique
To promote the long-term repair efficacy of OCAs, storage conditions that preserve cellular viability are essential. The fundamental paradigm of fresh OCA transplantation is that viable allograft chondrocytes survive storage and subsequent transplantation while maintaining their metabolic activity and sustaining their surrounding extracellular matrix (ECM), thereby providing the patient with a biologically viable and mechanically functional hyaline articular cartilage surface. Chondrocyte viability of OCAs at the time of implantation is therefore considered to be critical to graft survivorship and successful outcomes.22 Gross et al found in 35 fresh OCA specimens that early graft failures were associated with lack of chondrocyte viability.23 The importance of chondrocyte viability is further underscored by the high short-term failure rates observed with decellularized osteochondral allografts.24,25 Currently, after the mandatory serologic and microbiologic testing periods, allografts are stored at 4°C for durations between 15 and 43 days prior to expiration, although some authors have found that 4°C storage >28 days may be detrimental to repair outcomes.26–28 Pallante et al suggested that higher storage temperatures at 37°C may result in more viable cells and better biological performance than 4°C-stored grafts.29 Alternative storage conditions, such as the Missouri Osteochondral Allograft Preservation System (MOPS) which stores grafts at 25°C in a low-oxygen, dexamethasone-based environment, are being investigated to allow for prolonged graft storage times while maintaining viability.30
Graft-to-recipient size matching has traditionally thought to be an important consideration for OCA transplantation. Condyle-specific matching was performed to ensure optimal surface congruity to match size, curvature, and shape to minimize articular step-off, edge loading, and risk of graft failure.31,32 However, the majority of cartilage lesions being treated are located on the medial femoral condyle, while only 25% of the graft supply is available in the form of medial femoral hemicondyles.33 Due to this dilemma in graft supply, cadaveric studies have explored the use of donor plugs from lateral femoral hemicondyles to fill medial condylar defects and found no significant differences between donor plugs from medial and lateral femoral hemicondyles, with both having excellent surface matches.33,34 This was verified clinically, as Wang et al discovered in 27 patients treated with non-orthotopic grafts that reoperation rate, failure rate, and outcome scores did not differ with 50 patients treated with orthotopic grafts.31 Therefore, condyle-specific matching, particularly for a single, smaller plug, may not be necessary. Lateral femoral hemicondyle grafts are generally wider and can be used to treat larger defects, are more readily available, and can lead to decreased storage time with fresher grafts and preserved cellular viability.28,31 Additionally, surgeons have traditionally tried to match grafts to the recipient in the anterior-posterior (AP) and medial-lateral (ML) dimensions to optimize articular congruity, but practically this has proven to be difficult. Typically, an AP graft-recipient mismatch correlates with a mismatch in radius of curvature between graft and recipient, which can lead to articular incongruity and increased susceptibility of graft failure.35 However, a clinical study in 69 patients treated with OCA transplantation showed that the magnitude of graft-recipient AP mismatch was not associated with graft failure or patient-reported outcomes.35 For smaller defects and plugs, it appears that condyle-specific or AP-dimension matching may not be needed in order to adequately resurface the articular cartilage surface with minimal step-off. Some authors have purported other parameters that may be better for graft-recipient size matching, such as the condylar or tibia plateau width measured on radiographs or MRI/computed tomography.12,36 In the upper extremity, preoperative quantitative MRI to optimize congruency between donor osteochondral graft and recipient has also been described for treating Kienböck’s disease.37
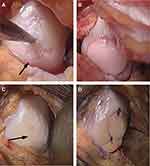
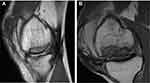
The success of OCA transplantation heavily relies on restoration of articular cartilage congruity and osseous integration of the allograft through a lengthy process of creeping substitution.38–40 It has been well established that high impaction forces during insertion and proud grafts lead to chondrocyte death and higher contact forces, respectively, and therefore both should be avoided. Regarding the optimal thickness of the OCA, studies have demonstrated that there is a balance between having a sufficient depth of bone to achieve proper press-fit fixation while limiting the bone volume to better facilitate creeping substitution and osseous integration. In a cadaveric study, Babu et al demonstrated that 15 mm diameter OCAs that were 7 mm in thickness were superior to those 4 mm in thickness in mean tensile force to failure and resistance to pull-out.41 Furthermore, they showed 7 mm thick donor plugs had a mean resistance to pull-out and subsidence comparable to 10 mm thick donor plugs.41 Ackermann et al reported that OCAs <5 mm in depth were 4.9 times more likely to have subchondral cystic changes at the host-graft junction compared to thicker grafts.38 It is theorized that thinner grafts have increased early mechanical instability, micromotion, and excessive loads leading to cyst formation, abnormal graft integration, and failure.38,42 Conversely, the authors also found that 40% of grafts >9 mm in thickness presented with a residual osseous cleft which may negatively affect osseous integration.38 Given these findings, the ideal OCA harvest thickness may be between 6 and 10 mm (2–3 mm of cartilage and 3–6 mm of bone) as reported in a consensus among an expert group.12 Surgical harvesting of elliptical OCAs has recently been developed to resurface entire condylar surfaces using a single graft (Figure 2). However, early failures have been observed, presumably due to the higher volume and thickness of allograft bone compared to stacked or snowman grafts, leading to poor osseous incorporation (Figure 3).
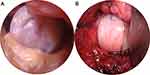 |
Figure 2 (A) Gross photo of an osteochondral lesion on the femoral condyle. (B) Treatment of the lesion with an elliptical osteochondral allograft (OCA) transplantation. |
Given the fresh nature of OCA, intraoperative preparation of the donor OCAs with pulsatile saline lavage is thought to remove antigenic marrow elements and decrease immunogenicity, thereby improving graft incorporation.43 Several studies have found that washing of specimens reduces rate of bacterial infection and transmission of blood borne viruses such as HIV and Hepatitis C, and prion disease.44–48 Ibrahim et al found that simple intraoperative washing of morcellised bone allograft with pulse lavage on average removed 70.5% protein, 95.2% fat, and 68.4% DNA compared to controls leading to reduced risks of blood borne disease transmission.48 Greater lavage duration was also found to be correlated with increased marrow removal in one study.49 Furthermore, Dunlop et al reported that washing of allografts to remove fat and marrow fluid produced stronger compact grafts that were more resistant to shear forces than controls.44 In addition, in a goat model, rinsing of allografts with saline resulted in higher bone and total tissue ingrowth compared with non-rinsed grafts.50 In contrast, Ambra et al reported that pulsatile lavage of OCAs did not significantly reduce DNA and bone marrow content, particularly for thicker 10-mm plugs.51 Other techniques, such as the use of CO2, to remove antigenic elements from donor grafts are currently being investigated.
To further improve the bony incorporation of OCAs, biologic augmentation through bone marrow aspirate concentrate (BMAC) has been explored, but the evidence overall is still lacking. BMAC contains osteoprogenitor cells and osteoinductive factors that promote an anabolic, anti-inflammatory environment thought to enhance bony healing.52,53 These growth factors and osteoprogenitor cells have also been found to accelerate the time-to-union of fractures.54 Other animal studies and clinical trials have shown that BMAC may be a viable option to augment cartilage maturation and improve osseous union.55–58 Oladeji et al first reported in 15 patients that the addition of BMAC to large femoral condylar OCAs prior to implantation led to superior radiographic integration to bone and less sclerosis than controls during the first 6 months postoperatively.52 In contrast, Wang et al found that the soaking of OCAs in BMAC prior to transplantation did not improve osseous integration or decrease cystic changes at mean 6 months and 12 months follow-up on magnetic resonance imaging (MRI).59 Other studies have since corroborated the lack of an augmentative effect with BMAC treatment of OCAs.60 Ultimately, soaking of OCAs in BMAC before implantation may be too simplistic, and further research is needed to target the growth factors within BMAC that can augment osseous integration of OCAs.59
Graft Survivorship and Patient Outcomes
The use of OCA transplantation has increased within recent years as the results of medium- and long-term studies have demonstrated good outcomes and graft survivorship.61 These studies report an average rate of survival of 86.7% in 582 patients, 78.7% in 686 patients, 72.8% in 343 patients, and 67.5% in 180 patients at 5, 10, 15, and 20 years, respectively.61 Graft failure is typically defined as the requirement of revision surgery or progression to either unicompartmental or total knee arthroplasty.61,62 Rates of failure are influenced by a multitude of factors including gender, age, BMI, defect size and location, and revision cases.17,61,63,64 Patient-reported outcomes are impacted by a variety of characteristics, including history of prior knee surgeries, baseline physical activity, and preoperative pain levels.65,66 Interestingly, patient-reported outcomes have not been shown to be influenced by factors such as age, gender, or size of the defect.62,66–68 The most commonly reported outcomes for OCA are the postoperative modified d’Aubign-Postel, Knee Society function, International Knee Documentation Committee (IKDC), and the Lysholm score, all of which typically show significant improvements postoperatively after OCA transplantation.61
When considering outcomes based on etiology, OCD in particular poses unique challenges to surgical management due to its large irregular shape, location in the periphery of the femoral condyle, and increased depth of bony involvement.69 OCD is largely a pediatric disease, and nonoperative management has proven to be effective for most patients prior to the closure of the epiphyseal plates.69,70 However, once skeletal maturity is reached, outcomes of nonoperative management worsen and therefore surgical management of OCD is increasing in popularity.65 OCA transplantation has demonstrated promising results for this group, with studies showing 95–97% survival at 5 years and 90% survival at 10 years.70,71 Patient-reported outcomes in this cohort are similar to the general OCA population in that knee outcome scores, such as Knee Injury and Osteoarthritis Outcome Score and IKDC, all improve postoperatively.72 Importantly, satisfaction in this treatment group is generally high, with recent studies reporting average satisfaction rates ranging from 81% to 95%.70,71 While the early data supporting OCA for the treatment of OCD are promising, studies examining longer term survival in this population are needed since the average age of patients in some studies undergoing OCA transplantations for OCD is just 23.9 years.72
Current methods for cartilage repair other than OCA include subchondral marrow stimulation, ACI, and OAT. When these procedures fail, OCA has been shown to be an acceptable salvage procedure, with 91% graft survival at 3.5 years, 82% at 10 years, and 74.9% at 15 years for salvage cases.64,73 Functional outcomes in this cohort have been shown to improve considerably and patient satisfaction is rated as good to excellent for a majority of patients who choose to undergo OCA transplantation for salvage of cartilage repair.64 Not surprisingly, the degree of osseous graft integration on MRI has been correlated with better outcomes in patients undergoing the salvage OCA transplantation procedure.73
While OCA has demonstrated its utility as a salvage procedure when other modes of cartilage repair fall short, once OCA fails, the treating surgeon is left with limited options, including revision OCA transplantation. Currently, two studies have reported on the outcomes in this scenario. Horton et al examined a series of 33 patients undergoing revision for OCA transplantation with a mean 10 years follow-up.74 This group defined failure as progression to partial or total knee arthroplasty and reported a failure rate of 39% at 5.5 years, with a reoperation rate of 67%. A more recent study by Davey et al examined a series of 9 patients undergoing revision OCA surgery and found a failure rate of 11% at 3.8 years and a reoperation rate of 50%.75 However, larger, prospective cohort studies will ultimately help with determining the best practices for revision cases of OCA transplantation.
Lesions of the patellofemoral joint pose unique difficulties for OCA transplantation due to high joint reaction forces, presence of concomitant patellar instability or overload, and the undulating topography of the patellofemoral joint.76 As such, graft survival on the patella is typically lower than that on the condyles, with reports of survival being 87.9% at 5 years and 78.1% at 10 years.76,77 Mean rate of reoperation is 51.6%, with hardware removal being the most common reason for reoperation.77 However, patients undergoing OCA transplantation for lesions of the patellofemoral joint have reported a 89% satisfaction rate.78 Although outcomes for OCA in the patellofemoral joint are generally reported to be lower than OCA in other locations, patients still report improvement in functional outcome scores compared to their preoperative state.77 These results may be confounded as concomitant procedures, such as soft-tissue patellar stabilization or tibial tubercle osteotomy, may be concomitantly performed with OCA transplantation.77 Therefore, assessing the individual effect of OCA transplantation remains difficult in this cohort.
Historically, the use of OCA was reserved for the younger population due to worse outcomes reported in the >40 years age population. However, recent analyses have identified confounders that could have influenced these outcomes including history of prior surgeries, higher BMI, and long-standing defects.65,79 As the aging population seeks to remain active, it becomes increasingly important to understand outcomes of cartilage preservation in this cohort. Multiple studies have assessed outcomes of OCA in the >40 years old cohort and have found mixed results. Wang et al found graft survival to be lower in their older cohort, with 88% survival at 2 years and 73% at 4 years.14 However, Markus et al found a survival rate of 100% at 3.1 years and credits the difference in graft survival to their exclusion of patients with a history of knee osteoarthritis or inflammatory joint disease.79 With respect to outcomes, all four of the studies published on the >40 years old population found significant postoperative improvements in pain and functional scores as well as high satisfaction rates following the procedure.14,16,65,79
The trend in outcomes research explores patient-reported outcomes that are clinically meaningful to the patient, including specific factors associated with achieving minimal clinically important difference (MCID) and substantial clinical benefit (SCB).66 MCID is the smallest change in score from the outcome measurement that patients perceive as being beneficial, while SCB is the clinical value that the patient considers to be an improvement in their health status.66,80,81 Wang et al found that lower preoperative IKDC and Knee Outcome Survey–Activities of Daily Living (KOS-ADL) scores, lower preoperative 36-Item Short Form Health Survey pain scores, higher preoperative Marx Activity Rating Scale scores, and a history of ≤1 prior ipsilateral knee surgical procedure were predictive of achieving the MCID and/or SCB.66 The authors concluded that patients with high baseline physical activity levels and less overall preoperative pain or higher pain tolerances were likely do better postoperatively after OCA transplantation.66
Risk Factors for Failure
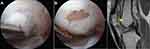
Understanding the risk factors associated with failure after OCA transplantation is helpful for surgeons during preoperative counseling and clinical surveillance.17 Failure of OCA transplantation can have many definitions, including subsequent revision cartilage surgery or arthroplasty, failure to achieve SCB or improvement to the level of MCID on patient-reported outcomes, or evidence of graft failure on postoperative imaging or second-look arthroscopy. In vivo mechanical failure of the transplanted graft typically occurs by osseous collapse or delamination of the articular cartilage surface (Figure 4).82 In a systematic review of 1036 patients, Familiari et al reported that bipolar defects, revision OCA, and patellar defects resulted in higher failure rates after OCA transplantation.61 A more recent systematic review and meta-analysis of 16 studies totaling 1401 patients by Kunze et al found that bipolar defects of the patellofemoral or tibiofemoral articulating surfaces, male sex, older age, and greater BMI were significantly associated with an increased failure rate after OCA transplantation.17 Chondral size defect or location, patellar defects, and concomitant procedures were not associated with increased risks of failure, which were in contrast to other studies.17,24,61,63 In a retrospective study, sex mismatch between donor and recipient was associated with a 3-times greater likelihood to fail at 5 years compared to same-sex transplantation.83
Regarding patient-specific factors, male sex has been identified as a significant risk factor for failure, with one study reporting that males were 4.18 times more likely to fail than females at a mean 3.6-year follow-up.14 BMI is also an important factor to consider during the preoperative counseling period. Traditionally, BMI ≥30 is a reported contraindication to cartilage repair procedures due to higher joint reactive forces and cartilage delamination leading to worse outcomes and failure.84,85 However, one study reported 2- and 5-year graft survivorship rates of 87% and 83%, respectively, in 31 young and athletic patients without significant comorbidities with a BMI ≥30 after OCA transplantation.86 Additionally, they reported high patient satisfaction rates and significant improvements in pain and physical functioning at 4.1 years follow-up and emphasized the importance of patient selection.86 Thus, BMI may not be a good parameter for obesity and the degree of joint reactive forces imparted to the transplanted graft.
Rehabilitation and Return to Sport
Overall, OCA transplantation for the treatment of cartilage injury has resulted in excellent clinical results. However, the optimal postoperative rehabilitation protocols are not well established. Significant variation among postoperative protocols following OCA transplantation exists. A recent systematic review by Stark et al analyzing the postoperative protocols for 3451 knees across 62 studies explored the important factors in the rehabilitation protocol, including range of motion (ROM) in 56 studies, bracing in 37 studies, weightbearing restrictions in 62 studies, and return to sport criteria after OCA transplantation in 41 studies.87 Of the studies that reported ROM in this review, 78.6% of authors initiate motion within the 1st week postoperatively, with the remaining authors beginning motion during the 2nd or 4th week postoperatively.87 The majority of these authors allowed for unrestricted ROM at their respective time points. For patellofemoral lesions, more restrictive protocols may be indicated as multiple authors have advocated limiting postoperative ROM to <30–45o of flexion to avoid overload of the patella and trochlea.63,70,76,88 A study by Cameron et al advocated restricting ROM to 0–45o in the initial postoperative period with unrestricted ROM at 4 weeks postoperatively after OCA transplantation for patellofemoral lesions.88 In addition, nearly 50% of authors in Stark et al’s review supplemented their postoperative regimen with continuous passive motion (CPM) ranging from 1 to 8 weeks postoperatively, with a mean of 7 hours of daily use.87 Early postoperative ROM is not only critical in preventing stiffness and arthrofibrosis but thought to promote cartilage repair with the articular cartilage receiving nutrition through movement of synovial fluid.89–91 In terms of rehabilitation exercises, most surgeons initiate closed-chain and open-chain exercises at 4 and 8 weeks postoperatively, respectively.87
Multiple studies have also described the use of postoperative bracing, with durations ranging from 1 week to over 6 months.87 Objective criteria should be used to assess readiness for brace removal, including the return of adequate quadriceps strength as measured by the ability to perform a straight leg raise without extension lag.87 Patel et al recommended keeping the knee brace locked in extension during weightbearing to minimize shear forces on the knee joint until 2 weeks postoperatively when quadriceps control has been established, at which time the brace can be discontinued at week 4 postoperatively.92
Weightbearing protocols for condylar OCA transplantation also vary significantly among surgeons, with a recent trend of more permissive postoperative weightbearing protocols.87 Initial partial weightbearing was reported by only about 30% of authors, with the most common protocol initiating weightbearing at 2 weeks, and others withholding weightbearing until 6 weeks postoperatively.87 Limiting weightbearing in the early postoperative period may help avoid excessive forces that may displace the graft or cause apoptosis and proteoglycan loss within the articular cartilage; however, this must be balanced with the benefits of healthy moderate mechanical loading across the articular cartilage that promotes an anabolic chondrocyte response and transfer of nutrients from the synovial fluid.92–96 From a high-volume center, 20% foot flat weightbearing for 1–2 weeks is permitted postoperatively, followed by progression to full weightbearing and functional activity.92 For patellofemoral OCA transplantation, the majority of studies allowed for immediate weightbearing as tolerated in a knee brace locked in extension while restricting knee flexion postoperatively.76,87,97 The most appropriate time for when full weightbearing is permitted should be patient-specific as the physical and psychological benefits of early weightbearing and individual demand of the patient must be weighed against the potential excess strain on the transplanted graft and risk of failure. Return to sport following OCA transplantation presents a further challenge to the treating physician, as the physical demands across sport and competition level vary widely, as does the recovery process for each individual patient. This decision requires a multidisciplinary approach between the physician, physical therapists, athletic training staff, and athlete.92 Across 37 studies, the expected timeline from surgery to return to play ranged from 4 months to 1 year, with most authors (62.2%) favoring return to sport at 6 months.87 Though many protocols report time after surgery as the critical measure, objective functional assessment measures should be utilized instead. Quantitative measurements such as jump testing and movement screens aid in return to sport evaluations to determine strength and movement patterns in the surgical extremity.98 Furthermore, evaluation tools analyzing restoration of quadriceps strength, conditioning, and ability to perform sport specific-skills are important metrics in an athlete’s assessment prior to return to sport.92 One limitation of the included studies is the lack of standardized sport-specific protocols for return to play since the demands of different sports result in different loads across the knee joint. In addition to functional assessment tools, some authors have advocated for advanced imaging with MRI and CT to assess graft incorporation, with full incorporation required prior to advancing an athlete back to full activity.99
Overall, a high rate of return to sport has been achieved following OCA transplantation. In a study of 43 athletes at mean 2.5 years follow-up, 88% achieved some level of return to sport, with 79% achieving full return to preinjury level.100 Time to return to sport averaged 9.6 months postoperatively with factors negatively affecting return to sport being ages ≥25 years old and preoperative symptoms ≥1 year. A larger study by Nielsen et al of 142 patients at mean 6 years follow-up of both highly competitive and recreational athletes demonstrated a 75.2% return to sport, with 91% of patients expressing satisfaction with results of surgery.101 These results have been demonstrated in elite athletes as well. A case series by Balazs et al of 11 professional or collegiate basketball players undergoing OCA transplantation were able to achieve a return to play at preinjury level of competition in 80% of cases, with median time to return to play of 14 months.102
Summary
Overall, OCA transplantation has demonstrated excellent survivorship and patient-reported outcomes for treatment of large osteochondral defects, OCD, and failed cartilage procedures. Furthermore, the procedure may be indicated in select older patients with focal articular cartilage defects and activity levels not yet suitable for arthroplasty. Technologies to improve graft viability during storage and biologic incorporation of the allograft continue to evolve, which will hopefully further optimize patient outcomes and survivorship. Return to sport after OCA transplantation is possible at 6 months postoperatively and should include objective functional assessment measures and a multidisciplinary approach.
Disclosure
Dr Dean Wang reports grants, personal fees from Vericel, personal fees from Newclip, personal fees from Mitek, grants from NIH, grants from NSF, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation a functional evaluation with long-term follow-up. Am J Sports Med. 2012. doi:10.1177/0363546511428778
2. Cavendish PA, Everhart JS, Peters NJ, Sommerfeldt MF, Flanigan DC. Osteochondral allograft transplantation for knee cartilage and osteochondral defects: a review of indications, technique, rehabilitation, and outcomes. JBJS Rev. 2019;7(6):e7–e7. doi:10.2106/JBJS.RVW.18.00123
3. Everhart JS, Campbell AB, Abouljoud MM, Kirven JC, Flanigan DC. Cost-efficacy of knee cartilage defect treatments in the United States. Am J Sports Med. 2020;48(1):242–251. doi:10.1177/0363546519834557
4. Heir S, Nerhus TK, Røtterud JH, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231–237. doi:10.1177/0363546509352157
5. Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265–273. doi:10.1177/1941738113508917
6. Hevesi M, Denbeigh JM, Paggi CA, et al. Fresh osteochondral allograft transplantation in the knee: a viability and histologic analysis for optimizing graft viability and expanding existing standard processed graft resources using a living donor cartilage program. Cartilage. 2021;13(1 Suppl):948S. doi:10.1177/1947603519880330
7. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi:10.1002/14651858.ED000142
8. Gross AE, McKee NH, Pritzker KPH, Langer F. Reconstruction of skeletal deficits at the knee. A comprehensive osteochondral transplant program. Clin Orthop Relat Res. 1983;174(174):96–106. doi:10.1097/00003086-198304000-00013
9. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435(435):79–87. doi:10.1097/01.BLO.0000165845.21735.05
10. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6(4):203–207. doi:10.1177/1947603515595072
11. Crawford ZT, Schumaier AP, Glogovac G, Grawe BM. Return to sport and sports-specific outcomes after osteochondral allograft transplantation in the knee: a systematic review of studies with at least 2 years’ mean follow-up. Arthroscopy. 2019;35(6):1880–1889. doi:10.1016/J.ARTHRO.2018.11.064
12. Görtz S, Tabbaa SM, Jones DG, et al. Metrics of OsteoChondral Allografts (MOCA) group consensus statements on the use of viable osteochondral allograft. Orthop J Sport Med. 2021;9(3):232596712098360. doi:10.1177/2325967120983604
13. Godin JA, Sanchez G, Cinque ME, Chahla J, Kennedy NI, Provencher MT. Osteochondral allograft transplantation for treatment of medial femoral condyle defect. Arthrosc Tech. 2017;6(4):e1239–e1244. doi:10.1016/J.EATS.2017.04.010
14. Wang D, Kalia V, Eliasberg CD, et al. Osteochondral allograft transplantation of the knee in patients aged 40 years and older. Am J Sports Med. 2018;46(3):581–589. doi:10.1177/0363546517741465
15. Degen RM, Coleman NW, Chang B, Tetreault D, Mahony GT, Williams RJ. Outcomes following structural grafting of distal femoral osteochondral injuries in patients aged 40 years and older. J Knee Surg. 2017;30(3):244–251. doi:10.1055/S-0036-1584534
16. Anderson DE, Robinson KS, Wiedrick J, Crawford DC. Efficacy of fresh osteochondral allograft transplantation in the knee for adults 40 years and older. Orthop J Sport Med. 2018;6(11):232596711880544. doi:10.1177/2325967118805441
17. Kunze KN, Manzi JS, Wright-Chisem J, Ramkumar P, Nwachukwu BU, Williams RJ. Risk factors for failure after osteochondral allograft transplantation of the knee: a systematic review and exploratory meta-analysis. Am J Sports Med. 2022;036354652110639. doi:10.1177/03635465211063901
18. Bakay A, Csönge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22(5):277–281. doi:10.1007/S002640050260
19. León SA, Mei XY, Safir OA, Gross AE, Kuzyk PR. Long-term results of fresh osteochondral allografts and realignment osteotomy for cartilage repair in the knee. Bone Joint J. 2019;101-B(1_Supple_A):46–52. doi:10.1302/0301-620X.101B1.BJJ-2018-0407.R1
20. Wakabayashi S, Akizuki S, Takizawa T, Yasukawa Y. A comparison of the healing potential of fibrillated cartilage versus eburnated bone in osteoarthritic knees after high tibial osteotomy: an arthroscopic study with 1-year follow-up. Arthroscopy. 2002;18(3):272–278. doi:10.1053/JARS.2002.30488
21. Giannini S, Buda R, Ruffilli A, et al. Failures in bipolar fresh osteochondral allograft for the treatment of end-stage knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2081–2089. doi:10.1007/S00167-014-2961-1
22. Cook JL, Dvm YZ, Stannard JP, et al. Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med. 2016;44(5):1260–1268. doi:10.1177/0363546516629434
23. Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KPH. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466(8):1863–1870. doi:10.1007/S11999-008-0282-8
24. Johnson CC, Johnson DJ, Garcia GH, et al. High short-term failure rate associated with decellularized osteochondral allograft for treatment of knee cartilage lesions. Arthroscopy. 2017;33(12):2219–2227. doi:10.1016/J.ARTHRO.2017.07.018
25. Farr J, Gracitelli GC, Shah N, Chang EY, Gomoll AH. High failure rate of a decellularized osteochondral allograft for the treatment of cartilage lesions. Am J Sports Med. 2016;44(8):2015–2022. doi:10.1177/0363546516645086
26. Pallante AL, Chen AC, Ball ST, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40(8):1814–1823. doi:10.1177/0363546512449321
27. Davidson PA, Rivenburgh DW, Dawson PE, Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored osteoarticular allografts. Am J Sports Med. 2007;35(7):1082–1090. doi:10.1177/0363546507299529
28. Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85(11):2111–2120. doi:10.2106/00004623-200311000-00008
29. Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37 degrees C than at 4 degrees C for osteochondral grafts. Am J Sports Med. 2009;37:24S–32S. doi:10.1177/0363546509351496
30. Stoker AM, Stannard JP, Kuroki K, et al. Validation of the Missouri osteochondral allograft preservation system for the maintenance of osteochondral allograft quality during prolonged storage. Am J Sports Med. 2018;46:58–65. doi:10.1177/0363546517727516
31. Wang D, Jones KJ, Eliasberg CD, Pais MD, Rodeo SA, Williams RJ. Condyle-specific matching does not improve midterm clinical outcomes of osteochondral allograft transplantation in the knee. J Bone Joint Surg Am. 2017;99(19):1614–1620. doi:10.2106/JBJS.16.01542
32. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32(2):317–320. doi:10.1177/0363546503261730
33. Mologne TS, Cory E, Hansen BC, et al. Osteochondral allograft transplant to the medial femoral condyle using a medial or lateral femoral condyle allograft: is there a difference in graft sources? Am J Sports Med. 2014;42(9):2205–2213. doi:10.1177/0363546514540446
34. Urita A, Cvetanovich GL, Madden BT, et al. Topographic matching of osteochondral allograft transplantation using lateral femoral condyle for the treatment of medial femoral condyle lesions: a computer-simulated model study. Arthroscopy. 2018;34(11):3033–3042. doi:10.1016/J.ARTHRO.2018.05.039
35. Wang D, Coxe FR, Balazs GC, et al. Graft-recipient anteroposterior mismatch does not affect the midterm clinical outcomes of osteochondral allograft transplantation of the femoral condyle. Am J Sports Med. 2018;46(10):2441–2448. doi:10.1177/0363546518782939
36. Bernstein DT, O’Neill CA, Kim RS, et al. Osteochondral allograft donor-host matching by the femoral condyle radius of curvature. Am J Sports Med. 2017;45(2):403–409. doi:10.1177/0363546516671519
37. Barber L, Koff MF, Virtue P, Lipman JP, Hotchkiss RJ, Potter HG. The use of MRI modeling to enhance osteochondral transfer in segmental Kienböck’s disease. Cartilage. 2012;3(2):188–193. doi:10.1177/1947603511415842
38. Ackermann J, Merkely G, Shah N, Gomoll AH. Decreased graft thickness is associated with subchondral cyst formation after osteochondral allograft transplantation in the knee. Am J Sports Med. 2019;47(9):2123–2129. doi:10.1177/0363546519851098
39. Pallante AL, Görtz S, Chen AC, et al. Treatment of articular cartilage defects in the goat with frozen versus fresh osteochondral allografts: effects on cartilage stiffness, zonal composition, and structure at six months. J Bone Joint Surg Am. 2012;94(21):1984–1995. doi:10.2106/JBJS.K.00439
40. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635–640. doi:10.1177/0363546513516747
41. Babu JM, Hodax JD, Fadale PD, Owens BD. Osteochondral allograft transplantation: identifying the biomechanical impact of using shorter grafts and pulsatile lavage on graft stability. J Knee Surg. 2020;33(1):29–33. doi:10.1055/S-0038-1676501
42. Chen Y, Wang T, Guan M, et al. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr Cartil. 2015;23(12):2174–2183. doi:10.1016/j.joca.2015.07.012
43. Ullmark G. Bigger size and defatting of bone chips will increase cup stability. Arch Orthop Trauma Surg. 2000;120(7–8):445–447. doi:10.1007/S004029900122
44. Dunlop DG, Brewster NT, Gopal Madabhushi SP, Usmani AS, Pankaj P, Howie CR. Techniques to improve the shear strength of impacted bone graft: the effect of particle size and washing of the graft. J Bone Joint Surg Am. 2003;85(4):639–646. doi:10.2106/00004623-200304000-00009
45. Kwong FNK, Ibrahim T, Power RA. Incidence of infection with the use of non-irradiated morcellised allograft bone washed at the time of revision arthroplasty of the Hip. J Bone Joint Surg Br. 2005;87(11):1524–1526. doi:10.1302/0301-620X.87B11.16354
46. Simonds RJ. HIV transmission by organ and tissue transplantation. AIDS. 1993;7:S35–38. doi:10.1097/00002030-199311002-00008
47. Conrad EU, Gretch DR, Obermeyer KR, et al. Transmission of the hepatitis-C virus by tissue transplantation. J Bone Joint Surg Am. 1995;77(2):214–224. doi:10.2106/00004623-199502000-00007
48. Ibrahim T, Qureshi A, McQuillan TA, Thomson J, Galea G, Power RA. Intra-operative washing of morcellised bone allograft with pulse lavage: how effective is it in reducing blood and marrow content? Cell Tissue Bank. 2012;13(1):157–165. doi:10.1007/S10561-011-9241-9
49. Sun Y, Jiang W, Cory E, et al. Pulsed lavage cleansing of osteochondral grafts depends on lavage duration, flow intensity, and graft storage condition. PLoS One. 2017;12(5). doi:10.1371/JOURNAL.PONE.0176934
50. Van der Donk S, Weernink T, Buma P, Aspenberg P, Slooff TJJH, Schreurs BW. Rinsing morselized allografts improves bone and tissue ingrowth. Clin Orthop Relat Res. 2003;408(408):302–310. doi:10.1097/00003086-200303000-00041
51. Felipe Ambra L, de Girolamo L, Gomoll AH. Pulse lavage fails to significantly reduce bone marrow content in osteochondral allografts a histological and DNA quantification study. Am J Sports Med. 2019;47(11):2723–2728. doi:10.1177/0363546519864716
52. Oladeji LO, Stannard JP, Cook CR, et al. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med. 2017;45(12):2797–2803. doi:10.1177/0363546517715725
53. Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res. 2015;2015:1–17. doi:10.1155/2015/752510
54. Gianakos A, Ni A, Zambrana L, Kennedy JG, Lane JM. Bone marrow aspirate concentrate in animal long bone healing: an analysis of basic science evidence. J Orthop Trauma. 2016;30(1):1–9. doi:10.1097/BOT.0000000000000453
55. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286–299. doi:10.1177/1947603510392023
56. Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: a comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91–98. doi:10.1177/0363546515609597
57. Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. doi:10.2106/JBJS.I.01284
58. Stoker AM, Baumann CA, Stannard JP, Cook JL. Bone marrow aspirate concentrate versus platelet rich plasma to enhance osseous integration potential for osteochondral allografts. J Knee Surg. 2018;31(4):314–320. doi:10.1055/S-0037-1603800
59. Wang D, Lin KM, Burge AJ, Balazs GC, Williams RJ. Bone marrow aspirate concentrate does not improve osseous integration of osteochondral allografts for the treatment of chondral defects in the knee at 6 and 12 months: a comparative magnetic resonance imaging analysis. Am J Sports Med. 2019;47(2):339–346. doi:10.1177/0363546518813915
60. Ackermann J, Mestriner AB, Shah N, Gomoll AH. Effect of autogenous bone marrow aspirate treatment on magnetic resonance imaging integration of osteochondral allografts in the knee: a matched comparative imaging analysis. Arthrosc - J Arthrosc Relat Surg. 2019;35(8):2436–2444. doi:10.1016/J.ARTHRO.2019.03.033
61. Familiari F, Cinque ME, Chahla J, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2018;46(14):3541–3549. doi:10.1177/0363546517732531
62. Matthews JR, Brutico JM, Abraham DT, et al. Differences in clinical and functional outcomes between osteochondral allograft transplantation and autologous chondrocyte implantation for the treatment of focal articular cartilage defects. Orthop J Sport Med. 2022;10(2):232596712110584. doi:10.1177/23259671211058425
63. Meric G, Gracitelli GC, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709–714. doi:10.1177/0363546514562549
64. Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage. 2015;6(2):98–105. doi:10.1177/1947603514566298
65. Frank RM, Lee S, Levy D, et al. Osteochondral allograft transplantation of the knee: analysis of failures at 5 years. Am J Sports Med. 2017;45(4):864–874. doi:10.1177/0363546516676072
66. Wang D, Chang B, Coxe FR, et al. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med. 2019;47(1):71–81. doi:10.1177/0363546518808030
67. Frank RM, Cotter EJ, Lee S, Poland S, Cole BJ. Do outcomes of osteochondral allograft transplantation differ based on age and sex? A comparative matched group analysis. Am J Sports Med. 2018;46(1):181–191. doi:10.1177/0363546517739625
68. Tírico LEP, McCauley JC, Pulido PA, Bugbee WD. Lesion size does not predict outcomes in fresh osteochondral allograft transplantation. Am J Sports Med. 2018;46(4):900–907. doi:10.1177/0363546517746106
69. Jones KJ, Cash BM, Arshi A, Williams RJ. Fresh osteochondral allograft transplantation for uncontained, elongated osteochondritis dissecans lesions of the medial femoral condyle. Arthrosc Tech. 2019;8(3):e267–e273. doi:10.1016/J.EATS.2018.10.023
70. Cotter EJ, Frank RM, Wang KC, et al. Clinical outcomes of osteochondral allograft transplantation for secondary treatment of osteochondritis dissecans of the knee in skeletally mature patients. Arthroscopy. 2018;34(4):1105–1112. doi:10.1016/J.ARTHRO.2017.10.043
71. Sadr KN, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation in patients with osteochondritis dissecans of the knee. Am J Sports Med. 2016;44(11):2870–2875. doi:10.1177/0363546516657526
72. Roberti Di Sarsina T, Fiore M, Coco V, et al. Fresh osteochondral allograft transplantation in osteochondritis dissecans in the knee joint. Life. 2021;11(11):1205. doi:10.3390/LIFE11111205
73. Wang T, Wang D, Burge AJ, et al. Clinical and MRI outcomes of fresh osteochondral allograft transplantation after failed cartilage repair surgery in the knee. J Bone Joint Surg Am. 2018;100(22):1949–1959. doi:10.2106/JBJS.17.01418
74. Horton MT, Pulido PA, McCauley JC, Bugbee WD. Revision osteochondral allograft transplantations: do they work? Am J Sports Med. 2013;41(11):2507–2511. doi:10.1177/0363546513500628
75. Davey A, Frank RM, Wang KC, Southworth TM, Cole BJ. Clinical outcomes of revision osteochondral allograft transplantation. Arthroscopy. 2019;35(9):2636–2645. doi:10.1016/J.ARTHRO.2019.03.055
76. Gracitelli GC, Meric G, Pulido PA, Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879–884. doi:10.1177/0363546514564144
77. Chahla J, Sweet MC, Okoroha KR, et al. Osteochondral allograft transplantation in the patellofemoral joint: a systematic review. Am J Sports Med. 2019;47(12):3009–3018. doi:10.1177/0363546518814236
78. Lin KM, Wang D, Burge AJ, Warner T, Jones KJ, Williams RJ. Osteochondral allograft transplant of the patella using femoral condylar allografts: magnetic resonance imaging and clinical outcomes at minimum 2-year follow-up. Orthop J Sport Med. 2020;8(10):232596712096008. doi:10.1177/2325967120960088
79. Markus DH, Hurley ET, Haskel JD, et al. High return to sport in patients over 45 years of age undergoing osteochondral allograft transplantation for isolated chondral defects in the knee. Cartilage. 2021;13(1_suppl):915S–919S. doi:10.1177/19476035211046008
80. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10(1):24. doi:10.1186/S13018-014-0144-X
81. Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008;90(9):1839–1847. doi:10.2106/JBJS.G.01095
82. Rauck RC, Wang D, Tao M, Williams RJ. Chondral delamination of fresh osteochondral allografts after implantation in the knee: a matched cohort analysis. Cartilage. 2019;10(4):402–407. doi:10.1177/1947603518777576
83. Merkely G, Farina EM, Leite CBG, et al. Association of sex mismatch between donor and recipient with graft survivorship at 5 years after osteochondral allograft transplantation. Am J Sports Med. 2022;50(3):681–688. doi:10.1177/03635465211068872
84. Lacy KW, Cracchiolo A, Yu S, Goitz H. Medial femoral condyle cartilage defect biomechanics: effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44(2):409–416. doi:10.1177/0363546515613517
85. Keng A, Sayre EC, Guermazi A, et al. Association of body mass index with knee cartilage damage in an asymptomatic population-based study. BMC Musculoskelet Disord. 2017;18(1). doi:10.1186/S12891-017-1884-7
86. Wang D, Rebolledo BJ, Dare DM, et al. Osteochondral allograft transplantation of the knee in patients with an elevated body mass index. Cartilage. 2019;10(2):214–221. doi:10.1177/1947603518754630
87. Stark M, Rao S, Gleason B, et al. Rehabilitation and return-to-play criteria after fresh osteochondral allograft transplantation: a systematic review. Orthop J Sport Med. 2021;9(7):232596712110171. doi:10.1177/23259671211017135
88. Cameron JI, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation of the femoral trochlea. Am J Sports Med. 2016;44(3):633–638. doi:10.1177/0363546515620193
89. Knapik DM, Harris JD, Pangrazzi G, et al. The basic science of continuous passive motion in promoting knee health: a systematic review of studies in a rabbit model. Arthrosc - J Arthrosc Relat Surg. 2013;29(10):1722–1731. doi:10.1016/J.ARTHRO.2013.05.028
90. Shimizu T, Videman T, Shimazaki K, Mooney V. Experimental study on the repair of full thickness articular cartilage defects: effects of varying periods of continuous passive motion, cage activity, and immobilization. J Orthop Res. 1987;5(2):187–197. doi:10.1002/JOR.1100050205
91. Howard JS, Mattacola CG, Romine SE, Lattermann C. Continuous passive motion, early weight bearing, and active motion following knee articular cartilage repair: evidence for clinical practice. Cartilage. 2010;1(4):276–286. doi:10.1177/1947603510368055
92. Patel S, Amirhekmat A, Le R, Williams RJ, Wang D. Osteochondral allograft transplantation in professional athletes: rehabilitation and return to play. Int J Sports Phys Ther. 2021;16(3):941–958. doi:10.26603/001C.22085
93. Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521.
94. Haapala J, Arokoski J, Pirttimäki J, et al. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21(1):76–81. doi:10.1055/S-2000-8860
95. Vanwanseele B, Lucchinetti E, Stüssi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthr Cartil. 2002;10(5):408–419. doi:10.1053/JOCA.2002.0529
96. Behrens F, Kraft EL, Oegema TR. Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7(3):335–343. doi:10.1002/JOR.1100070305
97. Spak RT, Teitge RA. Fresh osteochondral allografts for patellofemoral arthritis: long-term followup. Clin Orthop Relat Res. 2006;444:193–200. doi:10.1097/01.BLO.0000201152.98830.ED
98. Wang D, Chiaia T, Cavanaugh JT, Rodeo SA. Team approach: return to play after anterior cruciate ligament reconstruction. JBJS Rev. 2019;7(1):E1. doi:10.2106/JBJS.RVW.18.00003
99. Giorgini A, Donati D, Cevolani L, Frisoni T, Zambianchi F, Catani F. Fresh osteochondral allograft is a suitable alternative for wide cartilage defect in the knee. Injury. 2013;44:S16–S20. doi:10.1016/S0020-1383(13)70005-6
100. Krych AJ, Robertson CM, Williams RJ. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053–1059. doi:10.1177/0363546511435780
101. Nielsen ES, McCauley JC, Pulido PA, Bugbee WD. Return to sport and recreational activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2017;45(7):1608–1614. doi:10.1177/0363546517694857
102. Balazs GC, Wang D, Burge AJ, Sinatro AL, Wong AC, Williams RJ. Return to play among elite basketball players after osteochondral allograft transplantation of full-thickness cartilage lesions. Orthop J Sport Med. 2018;6(7):232596711878694. doi:10.1177/2325967118786941
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.