Back to Journals » Cancer Management and Research » Volume 12
Oridonin Induces Apoptosis of Laryngeal Carcinoma via Endoplasmic Reticulum Stress
Authors Kou B, Yang Y, Bai YE, Shi YH, Gao RX, Yang FL, Zhang SQ, Liu W
Received 11 July 2020
Accepted for publication 21 August 2020
Published 11 September 2020 Volume 2020:12 Pages 8387—8396
DOI https://doi.org/10.2147/CMAR.S271759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Bo Kou,1,* Yang Yang,2,* Yin-E Bai,3 Yu-Han Shi,4 Rui-Xia Gao,5 Fang-Li Yang,1 Shao-Qiang Zhang,1 Wei Liu6
1Department of Otorhinolaryngology-Head & Neck Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, People’s Republic of China; 2Department of Cardiovascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, People’s Republic of China; 3Department of ENT, Yichuanxian Renmin Hospital, Yan’an 716200, Shaanxi Province, People’s Republic of China; 4Department of Thyroid Breast Surgery, The First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong 515000, People’s Republic of China; 5School of Science, Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 6Department of Vascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shao-Qiang Zhang; Wei Liu Email [email protected]; [email protected]
Purpose: Oridonin, a bioactive diterpenoid derived from Rabdosia rubescens, has been widely reported to exhibit anticancer activity in multiple types of cancer. However, the molecular mechanism of oridonin in human laryngeal carcinoma has not been clearly elucidated. This study investigated the function of oridonin in laryngeal carcinoma to provide a research basis for laryngeal carcinoma therapy.
Methods: The proliferation of laryngeal carcinoma Hep-2 and TU212 cells treated with oridonin was determined by MTT assay. The apoptotic induction effect of oridonin on Hep-2 and TU212 cells was analyzed by flow cytometry, Western blot analysis and caspase3 activity assay. In addition, the caspase inhibitor, Z-VAD-fmk, was synergistically treated with oridonin to detect the function of caspase cascade in oridonin-mediated apoptosis. Then, the expressions of endoplasmic reticulum (ER) stress-related proteins (GRP78, phosphorylated-PERK, phosphorylated-eIF2α and CHOP) were measured in Hep-2 and TU212 cells by Western blotting. The cells were treated with 4-PBA (an ER stress inhibitor) or knockdown of CHOP to explore the role of ER stress in oridonin-mediated apoptosis in laryngeal carcinoma. Subsequently, a nude mouse xenograft model was constructed to confirm the function of oridonin in laryngeal carcinoma in vivo.
Results: Oridonin was found to significantly inhibit the proliferation of laryngeal carcinoma Hep-2 and TU212 cells in a concentration-dependent manner. Then, we confirmed that oridonin could induce apoptosis in human laryngeal carcinoma cells. The caspase inhibitor, Z-VAD-fmk, could partially reverse the pro-apoptotic effect of oridonin on human laryngeal carcinoma cells. Subsequently, Western blotting analysis demonstrated that endoplasmic reticulum (ER) stress-related proteins (GRP78, phosphorylated-PERK, phosphorylated-eIF2α and CHOP) were up-regulated in Hep-2 and TU212 cells exposed to oridonin. In addition, 4-PBA (an ER stress inhibitor) or knockdown of CHOP could antagonize oridonin-induced apoptosis. Oridonin significantly decreased the tumorigenicity of Hep-2 cells in a nude mouse xenograft model.
Conclusion: Oridonin-induced apoptosis of human laryngeal carcinoma through the activation of ER stress.
Keywords: oridonin, laryngeal carcinoma, ER stress, apoptosis
Introduction
Laryngeal carcinoma is one of the most common malignant cancer worldwide with a remarkable increase in new cases and deaths.1 There are various optional ways to antagonize laryngeal carcinoma. Currently, the total laryngotomy is still recognized as the most effective method for the treatment of laryngeal carcinoma, while brings some severe side effects, such as swallowing and voice problems.2 Although early-stage laryngeal carcinoma could be cured by surgery or radiotherapy, for the majority of patients with the advanced stage, there is still a limited improvement in the past two decades.3 In order to improve the therapeutic effect and survival rate, it is of great necessity to better understand the pathogenesis. Despite the tremendous efforts made in recent years, the molecular mechanisms involved in the occurrence and progression of laryngeal carcinoma still need investigation. Hence, to find out the appropriate therapeutic agents against laryngeal carcinoma has turned into a top priority.
With a long history of cancer treatment, natural products are considered as the potential bioactive anticancer compounds. Oridonin, a bioactive diterpenoid derived from Rabdosia rubescens, has been reported to exert anti-cancer effect in recent years.4 Studies reported that oridonin exhibited remarkable suppressive activity against various cancers, such as breast cancer, pancreatic cancer, lung cancer.5–7 In laryngeal cancer Hep-2 cells, oridonin is found to induce apoptosis via the inhibition of EGFR signaling and enhancement of oxidative stress.8 However, the underlying mechanism of oridonin against laryngeal carcinoma has not yet been clearly elucidated.
Presently, endoplasmic reticulum stress (ER stress) is reported to have a close correlation with cell apoptosis. ER is a vital organelle of eukaryotic cells and is responsible for the correct folding and modification of proteins.9 The homeostasis imbalance in the endoplasmic reticulum could trigger ER stress and the unfolded protein response (UPR). Subsequently, the three sensors of ER stress, double-strand RNA-activated protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and the downstream protein of ER stress, C/EBP homologous protein (CHOP), would be activated.10 Finally, ER stress lead to the cell apoptosis by the caspase-cascade reaction. In the current research, we aimed to explore the cytotoxic activity of oridonin and to elucidate the possible relationship between ER stress and apoptosis in human laryngeal carcinoma Hep-2 and TU212 cells.
Materials and Methods
Reagents and Cell Culture
Oridonin (C20H28O6), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 4-phenylbutyric acid (4-PBA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). They were both dissolved with dimethyl sulfoxide (DMSO). FITC Annexin V Apoptosis Detection Kit was purchased from BD (Bioscience, San Jose, CA, USA). Z-VAD-fmk (a caspase-3 inhibitor) was obtained from Selleckchem. Caspase 3 detection kit was purchased from Sigma-Aldrich. Antibodies against cleaved caspase-3, cleaved PARP, GRP 78, phosphorylated-PERK (p-PERK), PERK, phosphorylated-eIF2α (p- eIF2α), eIF2α, CHOP and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA).
Human laryngeal carcinoma cell lines Hep-2, TU212 were purchased from the American Type Culture Collection (Manassas, VA, USA). All these cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and maintained at 37°C in a humidified incubator with 5% CO2. As descript previously.
Cell Proliferation Assay
An MTT assay was used to detect the cytotoxicity of oridonin in human laryngeal carcinoma cells. Briefly, Hep-2 and TU212 cells were seeded per well in 96-well plates at a density of 0.8× 104. Then, the cells were treated with the increasing concentrations of oridonin (0, 10, 20, 40, 60, 80, 100, 120 μmol/L) for various durations (0, 24, 48, 72 h). After incubation with 0.5 mg mL−1 MTT for another 4 h, cells were lysed with dimethyl sulfoxide (DMSO). Subsequently, the optical density (OD) was measured by a microplate reader (Bio-Rad, Hercules, CA, USA). The experiment was performed in triplicate.
Colony Formation Assay
A colony formation assay was performed to detect the clonogenicity capability. The cells were planted on the 6-well plate for 48 h and then exposed to different concentrations of oridonin for one week. After washed with ice-cold phosphate-buffered saline (PBS), the cells per dish were fixed with 4% paraformaldehyde and stained with 1% crystal violet solution. Then, the cells in five random fields were then counted and visualized using a microscope at 100 × magnification.
Cell Morphology
Human laryngeal carcinoma Hep-2 and TU212 cells were seeded onto 6-well plates and treated with various concentrations (0, 20, 60 μmol L−1) of oridonin for 24 h. Then, the cell morphology per dish was visualized by a phase-contrast microscopy at 100 × magnification.
Cell Apoptosis Analysis
Cells were seeded on 6-well plates and exposed to different concentrations of oridonin (0, 20, 40, 60 μmol/L) for 24 h. Then, the cells were harvested and washed with PBS buffer. The collected cells were incubated with binding buffer and treated with Annexin V and PI staining solution for 15 min in the dark. The cell apoptosis was analyzed by FACS Calibur flow cytometry following the manufacturer’s protocol. The experiment was performed in triplicate.
Western Blotting Assay
It was performed as previously mentioned.11 Briefly, laryngeal carcinoma Hep-2 and TU212 cells were harvested after oridonin treatment, and the protein lysates were extracted by RIPA Cell Lysis Buffer. Then, the cell lysates were centrifuged at 12,000 g at 4°C for 15 min. After denaturation, the lysates (about 30–60 µg) were separated by 10–15% SDS-polyacrylamide gel electrophoresis (10% or 15%) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Membranes were then incubated with corresponding antibodies against cleaved caspase-3, cleaved PARP, GRP 78, phosphorylated-PERK (p-PERK), PERK, phosphorylated-eIF2α (p- eIF2α), eIF2α, CHOP and β-actin at 4°C overnight. Subsequently, the membranes were washed with TBST three times and incubated with horseradish peroxidase (HRP)-conjugated IgG antibody for 1 h at room temperature. Ultimately, the membranes were visualized with an ECL Substrate and exposed to X-ray film.
Caspase-3 Activity Assay
Human laryngeal carcinoma Hep-2 and TU212 cells were seeded on 6-well plates and exposed to a certain concentration of oridonin. After resuspension and centrifugation, supernatant and AcDEVD-pNA were mixed with the reaction system at 37°C for 2 h. Subsequently, the caspase-3 activity was evaluated by using a colorimetric assay at the wavelength of 405 nm (Beyotime, China).
Plasmid Transfection
It was conducted as previously mentioned.11 CHOP cDNA was cloned into pcDNA3.1 vector. After laryngeal carcinoma Hep-2 or TU212 cells reached 80% confluency for plasmid transfection, then the cells were transiently transfected with X-tremeGENE HP DNA Transfection Reagent (Roche, Switzerland) following the manufacturer’s instructions, and prepared for the subsequent experiments.
Animal Experiments
Four-week male BALB/C nude mice were obtained from and maintained in the Animal Care and Use Committee of Xi’an Jiaotong University, Xi’an, China. All procedures involving animal treatment were approved by the Ethics Committee of Animal care and use of Xi’an Jiaotong University (No.XJTULAC 2019–1268). All animals were handled according to the guidelines of the Institutional Animal Care and Use Committee of Xi’an Jiaotong University. 5×106 Hep-2 cells were subcutaneously injected into the right flank of nude mice. When the tumor volumes of the xenografts reached approximately 90 mm3, the mice were randomly divided into two groups: the vehicle group and oridonin group (10 mg/kg via intraperitoneal injection, 4 mice per group). The body weights of the nude mice were weighed every 3 days. The tumor volume was measured with a caliper and estimated by the following formula: 1/2 × (length) × (width)2. Finally, the nude mice were euthanized and tumors were weighed.
Statistical Analysis
All of the results were represented as mean ± standard deviation of triplicate experiments. All statistical analyses were implemented with GraphPad Prism (version 6.0) software, and Student’s t-test (two-sided) and ANOVA test were used to analyze the differences between groups. A value of P < 0.05 was considered to have a statistically significant difference.
Results
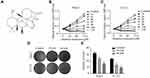
Oridonin Suppressed the Proliferation and Colony Formation of Human Laryngeal Carcinoma Cells in vitro
First of all, the molecular structure of oridonin is shown in Figure 1A. The MTT assay was conducted to explore the cytotoxic effect of oridonin on the human laryngeal carcinoma cell lines. The results demonstrated that oridonin dramatically decreased the viability of Hep-2 and TU212 cells in a concentration-dependent manner (Figure 1B and C). The subsequent colony formation assay revealed that oridonin treatment repressed the number of colonies in a dose-dependent manner (Figure 1D and E). These findings indicated that oridonin exerted anti-proliferative activity in the two laryngeal carcinoma cells.
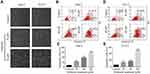
Oridonin-Induced Apoptosis of Human Laryngeal Carcinoma Cells via Activation of Caspase
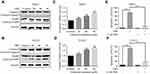
Hep-2 and TU212 cells were exposed to different concentrations of oridonin and examined for the cell morphological changes. The results showed that oridonin could induce cell shrinkage and cell floating in laryngeal carcinoma cells (Figure 2A). To investigate whether oridonin could have a pro-apoptotic effect on human laryngeal carcinoma cells, Hep-2 and TU212 cells were treated with oridonin and detected with flow cytometry. The findings revealed that oridonin induced the apoptosis of human laryngeal carcinoma Hep-2 cells in a concentration-dependent manner (Figure 2B and C). Similar with the above findings, oridonin could also dose-dependently induce apoptosis of TU212 cells (Figure 2D and E). In order to further explore the mechanisms of apoptosis induced by oridonin in Hep-2 and TU212 cells, we examined the expressions of cleaved caspase-3 and cleaved PARP using Western blotting assay. The results of Western blotting analysis indicated that the levels of cleaved subunits of caspase-3 and PARP in laryngeal carcinoma Hep-2 and TU212 cells were increased by oridonin in a dose-dependent manner (Figure 3A and B). Then, we focused on the function of oridonin on caspase-3 activity, and as we expected, the caspase-3 activity was also increased upon oridonin treatment in these two cell lines (Figure 3C and D). In addition, co-treatment with a caspase inhibitor, Z-VAD-fmk, could partially reverse the pro-apoptotic effect of oridonin on human laryngeal carcinoma cells as evidenced by flow cytometry (Figure 3E and F, Supplementary Figure 1A and B). These results suggested that oridonin could induce apoptosis in laryngeal carcinoma cells in a caspase-dependent pathway.
Oridonin Activated Endoplasmic Reticulum Stress in Human Laryngeal Carcinoma Cells
ER stress is reported to participate in the initiation of agent-induced apoptosis.12 And glucose regulatory protein 78 (GRP78) is a vital effector molecule induced by endoplasmic reticulum stress.13 Hence, the expression of GRP78 was investigated. As shown in Figure 4A and B, oridonin dose-dependently increased the protein level of GRP78 in Hep-2 and TU212 cells. Next, we detected the expression of ER stress-related proteins. Western blot analysis showed that the protein levels of phosphorylated-PERK, phosphorylated-eIF2α were significantly upregulated in human laryngeal carcinoma cells upon oridonin treatment (Figure 4A and B). To further confirm whether ER stress pathway participated in the apoptotic induction effect of oridonin on laryngeal carcinoma, cells were pre-treated with 1 mmol/L 4-PBA (an ER stress inhibitor) for 2 h and then exposed to oridonin for 24 h. Subsequently, the percentage of apoptosis and caspase-3 activity were examined by flow cytometry and colorimetric assay. The results of the present study revealed that 4-PBA could significantly weaken the pro-apoptotic effect of oridonin in laryngeal carcinoma (Figure 4C and D, Supplementary Figure 1C and D). Additionally, 4-PBA could partially reverse the up-regulation of caspase-3 activity induced by oridonin in laryngeal carcinoma Hep-2 and TU212 cells (Figure 4E and F). These results indicated that inhibition of oridonin-induced ER stress can partially protect human laryngeal carcinoma cells from death.
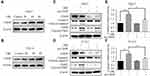
The Activation of CHOP Mediated the Pro-Apoptotic Effect of Oridonin in Human Laryngeal Carcinoma Cells
In the present study, the interaction between oridonin-induced ER stress and apoptosis was explored. Studies reported that CHOP is a crucial pro-apoptotic protein in the downstream of ER stress.14 Western blotting was conducted to detect the expression of CHOP upon oridonin treatment. As we expected, oridonin significantly upregulated CHOP protein level in laryngeal carcinoma cells in a concentration-dependent manner (Figure 5A and B). To further investigate whether CHOP is involved with pro-apoptotic effect of oridonin in human laryngeal carcinoma cells, we knocked down CHOP by transiently transfecting CHOP plasmid. The results demonstrated that knockdown of CHOP could partially reverse the change of cleaved-caspase 3 and cleaved-PARP protein levels induced by oridonin in human laryngeal carcinoma Hep-2 and TU212 cells (Figure 5C and D). Furthermore, knockdowning CHOP could also reverse oridonin-mediated elevated caspase-3 activity in laryngeal carcinoma (Figure 5E and F). These findings strongly suggested that oridonin-induced laryngeal carcinoma cell apoptosis via the activation of CHOP.
Oridonin-Inhibited Tumorigenicity of Laryngeal Carcinoma in vivo
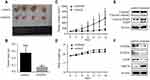
The above in vitro results verified that oridonin had the pro-apoptotic effect in human laryngeal carcinoma cells. Then, we explored that therapeutic effect of oridonin in nude mice. The results showed that the tumor mass and volume were dramatically reduced upon oridonin treatment, compared with the vehicle controls (Figure 6A–C), while the body weights in the two groups were similar (Figure 6D). Then, Western blot was conducted to detect the apoptosis- and ER stress-related proteins. The results revealed that cleaved-caspase-3, cleaved-PARP, phosphorylated-PERK, phosphorylated-eIF2α, GRP78 and CHOP protein levels were higher in tumor tissues exposed to oridonin (Figure 6E and F). These data suggested that oridonin could suppress laryngeal carcinoma tumorigenesis in vivo.
Discussion
In recent years, natural products gained more and more attention because of their low toxicity in cancers.15–18 Previous studies have demonstrated that oridonin had effective antitumor effects against a variety of cancers. Oridonin could inhibit colon cancer cells via the inactivation of TGF-β/smads-PAI-1 signaling pathway.19 Oridonin can also inhibit migration in gallbladder cancer by HIF-1α/MMP9 signal pathway.20 In human oral cancer, oridonin exerted anti-proliferative effect by PI3K/AKT signaling pathway.21 Furthermore, Oridonin has been reported to induce apoptosis by activating multiple signaling pathways, including ROS/JNK/c-Jun axis, p53, PI3K/AKT/mTOR, Ras/Raf.22–24 Previous studies showed that inhibition of EGFR signaling enhanced oridonin-induced apoptosis in human laryngeal cancer Hep-2 cells.8 And studies reported that the apoptotic induction by oridonin was mediated by the inhibition of caspase 9 in Hep-2 cells.25 Nevertheless, whether ER stress is involved in the apoptotic induction of oridonin in laryngeal carcinoma cells is still unclear. Based on these results, we aim to explore the inhibition effect of oridonin and to investigate the relationship between ER stress and cell apoptosis in human laryngeal carcinoma Hep-2 and TU212 cells. The results of cell viability and colony formation assay indicated that oridonin had a cytotoxic effect on laryngeal carcinoma Hep-2 and TU212 cells. The results of nude mice xenograft model further confirmed that oridonin played an anticancer role to inhibit growth of laryngeal carcinoma xenografts in vivo. Furthermore, oridonin led to the significant morphological changes by a phase-contrast microscopy. Subsequently, Annexin V/PI staining demonstrated that oridonin-induced apoptosis in these two laryngeal carcinoma cells in a concentration-dependent manner. The expressions of cleaved caspase-3 and cleaved-PARP, and the caspase-3 activity were dose-dependently increased upon oridonin treatment. The data above suggested that caspase cascade reaction played a vital role in oridonin-induced laryngeal carcinoma cells. When we pretreated laryngeal carcinoma cells with Z-VAD-fmk to disturb the caspase cascade, it is interesting to observe that the apoptotic induction effect was not fully reversed. Therefore, it strongly suggested that the caspase-dependent and -independent pathway were concomitant in the apoptotic process induced by oridonin.
The endoplasmic reticulum (ER) is responsible for the synthesis and secretion of membrane proteins.26 And the characteristic of ER stress is the aggregation of misfolded and unfolded protein in the lumen of ER, which is known as unfolded protein response (UPR). It is widely reported that UPR has a dual effect on cells, to promote homeostasis or to stimulate apoptosis in cells.27 ER stress could be activated by three signaling pathways: PERK pathway, IRE1 pathway and ATF6 pathway. These three sensors could interact with glucose regulatory protein 78 (GRP78) in an inactive state under physiological condition.28 Once the ER stress is initiated, they could activate the ER-related apoptotic signaling pathway to induce the apoptosis. The results in the current study showed that phosphorylated-PERK, and the downstream phosphorylated-eIF2α, as well as GRP78 protein level were all upregulated upon oridonin treatment in laryngeal carcinoma Hep-2 and TU212 cells. When pretreated with 4-PBA, the percent of apoptotic cells and the activity of caspase 3 were decreased correspondingly. The findings indicated that oridonin-induced apoptosis is mediated by ER stress in human laryngeal carcinoma cells.
CHOP is in a low expression under physiological condition.29 During ER stress, CHOP could be activated by PERK, IRE1 and ATG6 signaling pathways. Studies reported that CHOP is recognized as the crucial pro-apoptotic protein to initiate ER-mediated apoptosis.30 In the present study, we found that oridonin upregulated CHOP expression in these two laryngeal carcinoma cells. Knockdown of CHOP could decrease the protein levels of cleaved caspase3 and cleaved PARP, as well as the caspase3 activity. These results demonstrated that knockdown of CHOP could antagonize the apoptotic induction effect of oridonin in laryngeal carcinoma cells.
Conclusions
In conclusion, our studies revealed that oridonin could inhibit the viability, induce cytotoxicity, and activate ER-related signals of laryngeal carcinoma in vitro and vivo. More importantly, the findings indicated that oridonin induced apoptosis of human laryngeal carcinoma through the activation of ER stress.
Acknowledgments
This research was partially supported by the National Natural Science Foundation of China (81602562, 81802934) and the Fundamental Research Funds for the Central University of Xi’an Jiaotong University (xjj2018120).
Disclosure
The authors declared no conflicts of interest, financial or otherwise.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Arenaz Búa B, Pendleton H, Westin U, Rydell R. Voice and swallowing after total laryngectomy. Acta Otolaryngol. 2018;138(2):170–174. doi:10.1080/00016489.2017.1384056
3. Cata JP, Zafereo M, Villarreal J, et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth. 2015;27(8):672–679. doi:10.1016/j.jclinane.2015.07.012
4. Zhao Y, Xia H. Oridonin elevates sensitivity of ovarian carcinoma cells to cisplatin via suppressing cisplatin-mediated autophagy. Life Sci. 2019;233:116709. doi:10.1016/j.lfs.2019.116709
5. Li J, Wu Y, Wang D, et al. Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol Res. 2019;146:104313. doi:10.1016/j.phrs.2019.104313
6. Lou S, Xu J, Wang B, et al. Downregulation of lncRNA AFAP1-AS1 by oridonin inhibits the epithelial-to-mesenchymal transition and proliferation of pancreatic cancer cells. Acta Biochim Biophys Sin (Shanghai). 2019;51(8):814–825. doi:10.1093/abbs/gmz071
7. Li S, Shi D, Zhang L, Yang F, Cheng G. Oridonin enhances the radiosensitivity of lung cancer cells by upregulating Bax and downregulating Bcl-2. Exp Ther Med. 2018;16(6):4859–4864. doi:10.3892/etm.2018.6803
8. Kang N, Zhang JH, Qiu F, Tashiro S, Onodera S, Ikejima T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Lett. 2010;294(2):147–158. doi:10.1016/j.canlet.2010.01.032
9. Tan J, Jiang X, Yin G, et al. Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling pathway. Oncol Rep. 2017;38(3):1373–1382. doi:10.3892/or.2017.5841
10. Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14(1):20–28. doi:10.1016/j.tcb.2003.11.001
11. Kou B, Kou QS, Ma B, et al. Thymoquinone inhibits metastatic phenotype and epithelial-mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol Rep. 2018;40(3):1443–1450. doi:10.3892/or.2018.6519
12. Li J, Zhang Y, Liu K, et al. Xiaoaiping induces developmental toxicity in zebrafish embryos through activation of ER stress, apoptosis and the Wnt pathway. Front Pharmacol. 2018;9:1250. doi:10.3389/fphar.2018.01250
13. Chern YJ, Wong J, Cheng G, et al. The interaction between SPARC and GRP78 interferes with ER stress signaling and potentiates apoptosis via PERK/eIF2alpha and IRE1alpha/XBP-1 in colorectal cancer. Cell Death Dis. 2019;10(7):504. doi:10.1038/s41419-019-1687-x
14. Chiu CS, Tsai CH, Hsieh MS, et al. Exploiting honokiol-induced ER stress CHOP activation inhibits the growth and metastasis of melanoma by suppressing the MITF and beta-catenin pathways. Cancer Lett. 2019;442:113–125. doi:10.1016/j.canlet.2018.10.026
15. Katebi B, Mahdavimehr M, Meratan AA, Ghasemi A, Nemat-Gorgani M. Protective effects of silibinin on insulin amyloid fibrillation, cytotoxicity and mitochondrial membrane damage. Arch Biochem Biophys. 2018;659:22–32. doi:10.1016/j.abb.2018.09.024
16. Samarghandian S, Azimi‐Nezhad M, Farkhondeh T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J Cell Physiol. 2019;234(7):10421–10431. doi:10.1002/jcp.27710
17. Zhang Y, He W, Zhang S. Seeking for correlative genes and signaling pathways with bone metastasis from breast cancer by integrated analysis. Front Oncol. 2019;9:138. doi:10.3389/fonc.2019.00138
18. Sen Z, Zhan XK, Jing J, Yi Z, Wanqi Z. Chemosensitizing activities of cyclotides from clitoria ternatea in paclitaxel-resistant lung cancer cells. Oncol Lett. 2013;5(2):641–644. doi:10.3892/ol.2012.1042
19. Bu HQ, Shen F, Cui J. The inhibitory effect of oridonin on colon cancer was mediated by deactivation of TGF-beta1/Smads-PAI-1 signaling pathway in vitro and vivo. Onco Targets Ther. 2019;12:7467–7476. doi:10.2147/OTT.S220401
20. Chen K, Ye J, Qi L, et al. Oridonin inhibits hypoxia-induced epithelial-mesenchymal transition and cell migration by the hypoxia-inducible factor-1alpha/matrix metallopeptidase-9 signal pathway in gallbladder cancer. Anticancer Drugs. 2019;30(9):925–932. doi:10.1097/CAD.0000000000000797
21. Yang J, Ren X, Zhang L, Li Y, Cheng B, Xia J. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;100:226–232. doi:10.1016/j.biopha.2018.02.011
22. Zhang D, Zhou Q, Huang D, et al. ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 2019;513(3):594–601. doi:10.1016/j.bbrc.2019.04.011
23. Bi E, Liu D, Li Y, Mao X, Wang A, Wang J. Oridonin induces growth inhibition and apoptosis in human gastric carcinoma cells by enhancement of p53 expression and function. Braz J Med Biol Res. 2018;51(12):e7599. doi:10.1590/1414-431X20187599
24. Jiang JH, Pi J, Jin H, Cai JY. Oridonin-induced mitochondria-dependent apoptosis in esophageal cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J Cell Biochem. 2019;120(3):3736–3746. doi:10.1002/jcb.27654
25. Kang N, Cao SJ, Zhou Y, et al. Inhibition of caspase-9 by oridonin, a diterpenoid isolated from Rabdosia rubescens, augments apoptosis in human laryngeal cancer cells. Int J Oncol. 2015;47(6):2045–2056. doi:10.3892/ijo.2015.3186
26. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168(4):692–706. doi:10.1016/j.cell.2016.12.004
27. Wang WA, Groenendyk J, Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta. 2014;1843(10):2143–2149. doi:10.1016/j.bbamcr.2014.01.012
28. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi:10.1038/sj.embor.7400779
29. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi:10.1038/sj.cdd.4401373
30. Copanaki E, Schurmann T, Eckert A, et al. The amyloid precursor protein potentiates CHOP induction and cell death in response to ER Ca2+ depletion. Biochim Biophys Acta. 2007;1773(2):157–165. doi:10.1016/j.bbamcr.2006.10.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.