Back to Journals » Journal of Pain Research » Volume 11
Oral methylnaltrexone is efficacious and well tolerated for the treatment of opioid-induced constipation in patients with chronic noncancer pain receiving concomitant methadone
Authors Webster LR , Israel RJ
Received 22 December 2017
Accepted for publication 21 September 2018
Published 23 October 2018 Volume 2018:11 Pages 2509—2516
DOI https://doi.org/10.2147/JPR.S160625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Lynn R Webster,1 Robert J Israel2
1Scientific Affairs, PRA Health Sciences, Salt Lake City, UT, USA; 2Clinical and Medical Affairs, Salix Pharmaceuticals, Bridgewater, NJ, USA
Purpose: To evaluate the safety and efficacy of oral methylnaltrexone for opioid-induced constipation (OIC).
Patients and methods: This was a post hoc analysis of patients receiving methadone in a randomized, double-blind, placebo-controlled, Phase 3 trial. The trial included adults with chronic noncancer pain for ≥2 months receiving opioid doses ≥50 mg/day of oral morphine equivalents for ≥14 days and with a history of OIC. Patients were assigned to oral methylnaltrexone (150, 300, or 450 mg) or placebo once daily (QD) for 4 weeks followed by 8 weeks as needed. Percentage of dosing days that resulted in a rescue-free bowel movement (RFBM) within 4 hours of dosing was assessed during QD dosing (primary efficacy endpoint). Other endpoints included percentage of responders (ie, ≥3 RFBMs/week, with an increase of ≥1 RFBM/week from baseline for ≥3 of the 4 weeks) during QD dosing and change in weekly number of RFBMs. Adverse events were assessed.
Results: Concomitant methadone was reported in 120 patients (oral methylnaltrexone: 150 mg [n=33], 300 mg [n=30], and 450 mg [n=31]; placebo [n=26]). Oral methylnaltrexone-treated patients had significant increases in mean percentage of dosing days with RFBMs within 4 hours of dosing during weeks 1–4 with 300 mg (33.6%; P<0.01) and 450 mg (38.2%; P<0.001) vs placebo; improvements with 150 mg (20.0%) vs placebo (15.1%) did not reach statistical significance. The percentage of responders was greater vs placebo, but not significant, for the higher doses during the QD period (150 mg [39.4%], 300 mg [60.0%], 450 mg [67.7%], and placebo [38.5%]). Change from baseline in the mean number of weekly RFBMs (weeks 1–4) was significantly greater with oral methylnaltrexone 450 mg vs placebo (least-squares mean difference vs placebo, 1.2; P=0.04); no significant differences were found for 300 or 150 mg. Oral methylnaltrexone was well tolerated at all doses; few patients discontinued treatment.
Conclusion: Oral methylnaltrexone, particularly 450 mg, was efficacious and safe for treating OIC in these patients.
Keywords: methylnaltrexone, methadone, opioid-induced constipation, µ-opioid receptor antagonist, chronic pain
Introduction
Opioid analgesic drugs activate receptors in the gastrointestinal (GI) tract, slowing GI transit and leading to opioid-induced constipation (OIC).1,2 OIC is caused largely by activation of enteric µ-opioid receptors resulting in decreased neurotransmitter release that alters GI function leading to impairment of motility and water resorption.3–6 It is consistently reported to be the most common and undesirable GI side effect in patients receiving opioids for chronic noncancer pain, with treatment duration being a major risk factor for development.7–9 While over-the-counter laxatives are usually the first-line treatment for OIC, they do not address the underlying pathophysiology and may themselves cause side effects.5,7,8,10,11
Methylnaltrexone (Relistor®; Salix Pharmaceuticals, a division of Valeant Pharmaceuticals North America LLC, Bridgewater, NJ, USA) tablets and subcutaneous (SC) injection are approved for the treatment of OIC in adults with chronic noncancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (eg, weekly) opioid dosage escalation.12 The efficacy and safety of SC-administered methylnaltrexone for the treatment of OIC have previously been demonstrated in patients with advanced illness receiving palliative care and in patients with chronic noncancer pain.13–16 The efficacy and safety of the oral formulation were demonstrated for the treatment of OIC in patients with chronic noncancer pain,17 and this formulation received US Food and Drug Administration approval for this indication in July 2016.12
Several oral pharmacologic therapies are currently available for the treatment of OIC in patients with chronic noncancer pain that act specifically on GI receptors including lubiprostone (a chloride channel activator) and naloxegol and methylnaltrexone (both µ-opioid receptor antagonists).12,18,19 Naloxegol is a specific µ-opioid receptor antagonist that, when administered at recommended dosing, antagonizes the μ-receptor in the GI tract, thereby decreasing the constipating effects of opioids.20 Methylnaltrexone is also a µ-opioid receptor antagonist; however, it has been developed as a quaternary amine, which restricts its ability to cross the blood–brain barrier based on its chemical structure, allowing this agent to effectively reduce the symptoms of OIC without affecting the analgesic effects of opioid medications.12,21,22
Because of the variable effects of pharmacologic therapies for the treatment of OIC in patients receiving methadone,18,19 the present study reports on a post hoc analysis from the pivotal Phase 3 clinical trial of oral methylnaltrexone in patients with chronic noncancer pain and OIC to determine the efficacy and safety of methylnaltrexone in a subset of patients concomitantly receiving methadone.
Patients and methods
Study description
This post hoc analysis of a Phase 3, randomized, placebo-controlled, double-blind trial was conducted at 117 sites in the US between September 2010 and November 2011 to evaluate the efficacy and safety of oral methylnaltrexone for OIC in a subgroup of patients receiving methadone for the treatment of their chronic noncancer pain (ClinicalTrials.gov identifier: NCT01186770).17 The study protocol and informed consent form were reviewed and approved by applicable institutional review boards (Meritus Medical Center Institutional Review Board, Hagerstown, MD; Partners Human Research Committee, Boston, MA; Quorum Review IRB, Seattle, WA; Schulman Associates IRB, Inc., Cincinnati, OH; SDHIPM IRB, San Diego, CA; University of Utah IRB, Salt Lake City, UT). This trial was conducted in full accordance with the ethical principles in the Declaration of Helsinki; the International Council for Harmonization Good Clinical Practice (ICH GCP) guidelines, Code of Federal Regulations 50, 56, and 312; and all other applicable laws and regulations. All patients who participated in this trial provided written informed consent prior to study enrollment.
Patients
Key inclusion criteria
Male and female outpatients ≥18 years of age with a documented history of chronic nonmalignant pain for ≥2 months and receiving ≥50 mg/day of oral morphine-equivalent doses for at least 14 days and a history of OIC were eligible for inclusion in the study. Patients were required to have had no history of chronic constipation before starting opioid therapy. OIC was confirmed during the 2-week screening period, defined as less than three rescue-free bowel movements (RFBMs) plus ≥1 of the following: ≥25% of RFBMs categorized as type 1 or type 2 on the Bristol Stool Form Scale;23 straining during ≥25% of RFBMs or ≥25% of RFBMs with a sensation of incomplete evacuation. Patients were required to be taking laxative therapy for ≥30 days and willing to discontinue all laxative therapy at the start of the screening period, except study-permitted rescue laxatives throughout the study. Patients who were not surgically sterile or were not postmenopausal were required to commit to the use of a medically acceptable method of birth control or sexual abstinence for the duration of the study, including 30 days after the last dose of study treatment.
Key exclusion criteria
Patients were excluded from the study if they had ever been treated with oral methylnaltrexone or SC methylnaltrexone in the previous 30 days. Additional criteria for exclusion included patients with a WHO performance status >2 and women who were pregnant or breastfeeding; a history of mechanical bowel obstruction or megacolon; fecal incontinence, rectal prolapse, fecal ostomy, inflammatory bowel disease, or other clinically significant GI disorders; rectal bleeding within 60 days of providing consent, which was not associated with hemorrhoids or fissures; the need for manual disimpaction or pelvic floor support techniques, including manual maneuvers, within 14 days before the screening visit; or the presence of rectal outlet obstruction or fecal impaction at screening visit. Additional exclusion criteria included a history of substance abuse in the past year; any unstable hepatic, renal, pulmonary, cardiovascular, ophthalmologic, neurologic, psychiatric, or any other medical condition that might have compromised the study or increased the risk of participation; a history or presence of symptomatic orthostatic hypotension or any other clinically significant abnormalities on screening physical examination, electrocardiogram, or laboratory tests; planned surgery during the study; a known allergy or other contraindication to opioids, opioid derivatives, or opioid antagonists; use of investigational treatments within 30 days before the screening visit; current treatment with partial opioid agonists or combination agonists/antagonists; or a urine drug screen negative for the presence of opioids.
Study design and treatment
The study consisted of a 14-day screening period, a 28-day (4-week) period during which patients received treatment once daily (QD), a 56-day (8-week) period during which patients were administered treatment as needed (PRN), and a 14-day follow-up period. OIC was confirmed during the screening period. Patients were randomized to receive one of four treatment arms: placebo or oral methylnaltrexone at doses of 150, 300, or 450 mg QD. Patient treatment status was blinded to both the patient and the study staff. Oral bisacodyl tablets, up to three per day, were provided as rescue laxative treatment for patients who did not respond to active or placebo treatment, defined as no bowel movement for three consecutive days during the screening and treatment periods.
Study assessments
The primary efficacy endpoint of the study was the mean percentage of dosing days that resulted in an RFBM within 4 hours of dosing during weeks 1–4 (QD period). Secondary endpoints included the time to first RFBM after the first dose of study treatment, the percentage of responders (defined as those patients who had ≥3 RFBMs/week, with an increase of ≥1 RFBM/week from baseline for at least 3 of the 4 weeks) during weeks 1–4 (QD period), and change in the weekly number of RFBMs from baseline during weeks 1–4 (QD period). Patients reported the date and time of all bowel movements, OIC symptom ratings, and rescue laxative use daily via telephone using an interactive voice response system.
Safety assessments
Adverse events (AEs) were assessed during the entire treatment period, including the QD and PRN phases of the study.
Statistical analysis
This post hoc analysis included patients in the intent-to-treat and safety populations who received concomitant methadone for the treatment of their chronic pain during the study. The intent-to-treat population included all randomized patients who took ≥1 dose of the study drug. The safety population included all randomized patients who took ≥1 dose of the study drug.
The percentage of dosing days that resulted in RFBMs was compared between the four treatment groups using an analysis of covariance model with randomized dose group as a fixed effect and analysis center (analysis center refers to pooled or clustered study centers) as a covariate. Time-to-event endpoints were estimated using the Kaplan–Meier method and compared using the log-rank test stratified by center. Analyses of responders were based on a logistic regression model with treatment as an effect and geographic region as a covariate. During the QD period, the change in weekly number of RFBMs from baseline was analyzed using an analysis of covariance model with treatment as an effect and geographic region and baseline values as covariates. AEs were coded using the Medical Dictionary for Regulatory Activities version 13.0.
Results
Patients
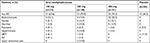
A total of 120 patients reported concomitant use of methadone, with approximately equal amounts of patients using methadone in each treatment group (oral methylnaltrexone: 150 mg [n=33], 300 mg [n=30], and 450 mg [n=31]; placebo [n=26]). A similar percentage of patients who were on concomitant methadone completed the 12-week trial in all study groups (oral methylnaltrexone: 150 mg [13.4%], 300 mg [15.8%], 450 mg [12.7%]; placebo [14.1%]). The subgroup of patients using methadone included 68 women (57%), and averaged from 1.3 to 1.5 RFBMs per week at baseline. Demographic information and baseline characteristics are summarized in Table 1.
  | Table 1 Demographic and baseline characteristics Abbreviations: MED, morphine-equivalent dose; RFBMs, rescue-free bowel movements. |
Efficacy
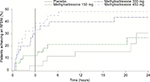
A significantly greater percentage of patients in the oral methylaltrexone 300 mg (33.6%; P<0.01) and 450 mg (38.2%; P<0.001) groups met the primary endpoint (mean percentage of dosing days that resulted in an RFMB within 4 hours of dosing during weeks 1–4 during the QD period) compared with patients receiving placebo (15.1%). Patients in the oral methylnaltrexone 150 mg group also demonstrated improvements over placebo (19.9%); however, this improvement was not significant. Significant improvements were noted in the treatment groups as early as week 1 and were maintained through the PRN period (Figure 1).
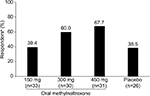
Figure 2 summarizes the time to achieve a first RFBM for each treatment group and the placebo group. Although the time to achieve a first RFBM was shorter for patients treated with both oral methylnaltrexone 300 and 450 mg, only the 300 mg dose produced a statistically significant response compared with placebo (P=0.02). During the QD period, there was a greater percentage of responders in the oral methylnaltrexone 300 mg (60.0%) and 450 mg (67.7%) groups compared with the placebo (38.5%) group; however, these differences were not statistically significant (Figure 3). Patients treated with oral methylnaltrexone 450 mg/day experienced a significant increase in the mean number of weekly RFBMs during the QD period of the study (least-squares mean difference [95% CI], 1.2 [0.1–2.3]; P=0.04) compared with patients receiving placebo; no significant increases in weekly RFBMs were observed for oral methylnaltrexone 300 or 150 mg compared with placebo.
Safety
Oral methylnaltrexone was generally well tolerated. AEs reported in ≥8.0% of patients in any treatment group during the QD, PRN, and follow-up periods are summarized in Table 2. The incidence of hyperhidrosis, a potential symptom of opioid withdrawal, was higher in the methylnaltrexone 150, 300, and 450 mg/day groups (9.1%, 10.0%, and 3.2%, respectively) compared with the placebo group (0%). Overall, two patients reporting concomitant methadone use discontinued from this study because of AEs: one patient treated with oral methylnaltrexone 300 mg/day discontinued because of upper abdominal pain and another patient treated with oral methylnaltrexone 450 mg/day discontinued because of vertigo.
Discussion
Opioid analgesics, such as methadone, administered to treat moderate-to-severe noncancer pain, bind to μ-opioid receptors in the gut decreasing motility and secretion of electrolytes and water, with the end result being constipation.5 Previous studies have demonstrated that SC-injected methylnaltrexone is effective in relieving the symptoms of OIC in patients with advanced illness receiving palliative care and in patients with chronic noncancer pain. A recent Phase 3 study demonstrated the efficacy and safety of the oral formulation of methylnaltrexone with a significantly greater percentage of patients with chronic noncancer pain treated with oral methylnaltrexone 300 mg/day (24.6%; P=0.002) and 450 mg/day (27.4%; P<0.0001) experiencing an increase in mean percentage of dosing days that resulted in an RFBM within 4 hours of dosing during weeks 1–4 compared with patients receiving placebo (18.2%).17 The present post hoc analysis of that Phase 3 study evaluated the efficacy and safety of oral methylnaltrexone in patients with chronic noncancer pain concomitantly receiving methadone. The results of the present post hoc analysis are similar to the Phase 3 results of the overall population, in that a significantly greater percentage of patients concomitantly receiving methadone and treated with oral methylnaltrexone 300 mg/day (33.6%; P<0.01) and 450 mg/day (38.2%; P<0.001) achieved the primary efficacy endpoint compared with patients receiving placebo (15.1%). The present results are also consistent with the efficacy results of a trial conducted in patients with methadone-induced constipation who experienced a laxation response after administration of intravenous methylnaltrexone and significantly improved oral–cecal transit times compared with patients receiving placebo (P<0.001) with no opioid withdrawals and no significant AEs.24
Overall, in the post hoc analysis, the incidence of AEs was slightly higher in patients treated with oral methylnaltrexone over the entire study period (QD and PRN treatment periods) than in patients receiving placebo. In the primary Phase 3 results of the overall population, the incidence of AEs was assessed separately for the QD and PRN periods and was similar for patients treated with oral methylnaltrexone (range of 42.0%–45.3% of patients for the QD period and 38.8%–44.3% of patients for the PRN period) compared with those receiving placebo (44.3% of patients for the QD period and 43.0% of patients for the PRN period).17
OIC is common in patients receiving opioid analgesics for chronic noncancer pain and can often be treated with oral agents including lubiprostone.18,19 However, previous studies have indicated that lubiprostone may have decreased efficacy in patients taking concomitant methadone.18,25,26 In a post hoc subgroup analysis of two clinical trials, patients with chronic noncancer pain treated with lubiprostone and concomitantly receiving methadone had a lower spontaneous bowel movement response compared with patients treated with other opioid agonists.26
Studies have demonstrated that treatment of OIC with methylnaltrexone does not affect opioid-mediated analgesia.21,24,27 In a study by Webster et al, patients with chronic noncancer pain with OIC treated with SC methylnaltrexone experienced a stable median daily morphine-equivalent dose and pain intensity score and lacked any clinically meaningful central opioid withdrawal, indicating that methylnaltrexone does not reduce opioid-mediated analgesia or precipitate opioid withdrawal.27
The results of this study are encouraging, although they may be limited by the small number of patients in each treatment group. The primary outcome of number of days with an RFBM was correlated with the dose of methylnaltrexone administered, as were the time to achieve a first RFBM and the percentage of responders in each treatment group. A larger study may have been able to differentiate between these doses.
Conclusion
Although the number of patients in this analysis was small, oral methylnaltrexone, particularly the 450 mg dose, was effective at treating OIC in patients taking concomitant methadone. In addition, this agent was well tolerated at all doses and few patients discontinued treatment during the study.
Data sharing statement
The datasets generated and/or analyzed during the current study are not publicly available at this time due to the proprietary nature of this information. Requests for additional information should be made to the corresponding author.
Acknowledgments
The study was funded by Salix Pharmaceuticals, Bridgewater, NJ, USA. Progenics Pharmaceuticals, Inc., New York, NY, USA had a role in the study design, implementation of the study, and data collection. Salix had a role in the data collection, data analysis, and the decision to publish. Technical editorial assistance was provided, under the direction of the authors, by Lisa Feder, PhD, of Echelon Brand Communications, Parsippany, NJ, USA. Funding for this assistance was provided by Salix Pharmaceuticals.
Previous presentation
2016 PAINWeek National Conference, September 6–10, 2016, Las Vegas, NV; 15th Annual ASRA Pain Medicine Meeting, November 17–19, 2016, San Diego, CA; and International Conference on Opioids (ICOO 2017), June 11–13, 2017, Boston, MA.
Disclosure
Dr Israel is an employee of Salix (Valeant) Pharmaceuticals. Dr Webster has served as a board member for Charleston Laboratories, Daiichi Sankyo, Depomed, Egalet, Inspirion, Insys, Kaléo, Orexo, Pfizer, Proove Biosciences, Scilex, Signature Therapeutics, Teva, Trevena; has consulted for Acura Pharmaceuticals, Alcobra, AstraZeneca, AcelRX, Egalet, Elysium, Indivior, Insys, Kaléo, KemPharm, Mallinckrodt, Marathon, Merck, Neura, Pain Therapeutics, Pfizer, Proove Biosciences, Shionogi, Vector Pharma, and Zogenix; and is employed by PRA Health Sciences. The authors report no other conflicts of interest in this work.
References
Tavani A, Bianchi G, Ferretti P, Manara L. Morphine is most effective on gastrointestinal propulsion in rats by intraperitoneal route: evidence for local action. Life Sci. 1980;27(23):2211–2217. | ||
Kaufman PN, Krevsky B, Malmud LS, et al. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1988;94(6):1351–1356. | ||
Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26(10):1386–1395. | ||
Pergolizzi JV Jr, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107–119. | ||
Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155(1–3):11–17. | ||
Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2(1):17–21. | ||
Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med. 2009;10(1):35–42. | ||
Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224–1232. | ||
Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22(4):424–430. | ||
Coyne KS, LoCasale RJ, Datto CJ, Sexton CC, Yeomans K, Tack J. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269–281. | ||
Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(Suppl 5A):S11–S18. | ||
Relistor [package insert]. Bridgewater, NJ: Salix Pharmaceuticals; 2017. | ||
Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. | ||
Slatkin N, Thomas J, Lipman AG, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46. | ||
Chamberlain BH, Cross K, Winston JL, et al. Methylnaltrexone treatment of opioid-induced constipation in patients with advanced illness. J Pain Symptom Manage. 2009;38(5):683–690. | ||
Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12(5):554–562. | ||
Rauck R, Slatkin NE, Stambler N, Harper JR, Israel RJ. Randomized, double-blind trial of oral methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Pract. 2017;17(6):820–828. | ||
Amitiza [package insert]. Deerfield, IL: Takeda Pharmaceuticals America; 2016. | ||
Movantik [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017. | ||
Leppert W, Woron J. The role of naloxegol in the management of opioid-induced bowel dysfunction. Therap Adv Gastroenterol. 2016;9(5):736–746. | ||
Yuan CS, Wei G, Foss JF, O’Connor M, Karrison T, Osinski J. Effects of subcutaneous methylnaltrexone on morphine-induced peripherally mediated side effects: a double-blind randomized placebo-controlled trial. J Pharmacol Exp Ther. 2002;300(1):118–123. | ||
Iyer SS, Randazzo BP, Tzanis EL, et al. Effect of subcutaneous methylnaltrexone on patient-reported constipation symptoms. Value Health. 2011;14(1):177–183. | ||
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–924. | ||
Yuan CS, Foss JF, O’Connor M, et al. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. JAMA. 2000;283(3):367–372. | ||
Cuppoletti J, Chakrabarti J, Tewari K, Malinowska DH. Methadone but not morphine inhibits lubiprostone-stimulated Cl- currents in T84 intestinal cells and recombinant human ClC-2, but not CFTR Cl- currents. Cell Biochem Biophys. 2013;66(1):53–63. | ||
Wong BS, Camilleri M. Lubiprostone for the treatment of opioid-induced bowel dysfunction. Expert Opin Pharmacother. 2011;12(6):983–990. | ||
Webster LR, Brenner DM, Barrett AC, Paterson C, Bortey E, Forbes WP. Analysis of opioid-mediated analgesia in Phase III studies of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain. J Pain Res. 2015;8:771–780. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.