Back to Journals » Journal of Inflammation Research » Volume 15
Oral Lycopene Administration Attenuates Inflammation and Oxidative Stress by Regulating Plasma Lipids in Rats with Lipopolysaccharide-Induced Epididymitis
Authors Li Y , Zhu J, Zhao X, Sun Y , Xu F, Xu S, Shang X
Received 12 July 2022
Accepted for publication 23 November 2022
Published 30 November 2022 Volume 2022:15 Pages 6517—6531
DOI https://doi.org/10.2147/JIR.S380785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Yu Li,1 Jinde Zhu,1 Xiaodong Zhao,2 Yi Sun,2,3 Feng Xu,1,2 Song Xu,1,2 Xuejun Shang1,2
1Department of Urology, Jinling Hospital, The First School of Clinical Medicine, Southern Medical University, Nanjing, People’s Republic of China; 2Department of Urology, Jinling Clinical Medical College of Nanjing Medical University, Nanjing, People’s Republic of China; 3Department of Pathology, Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Xuejun Shang, Department of Urology, Jinling Hospital, The First School of Clinical Medicine, Southern Medical University, No. 305 East Zhongshan Road, Nanjing, 210002, People’s Republic of China, Tel +8613813905418, Email [email protected]
Purpose: Epididymitis histological alterations and related long-term reproductive issues cannot be cured by antibiotics alone. Few studies have been done on the effect of lycopene on epididymitis, despite the fact that it is an efficient antioxidant. The objective of this study was to assess the impact of lycopene on Lipopolysaccharide (LPS)-induced epididymis and lipid metabolism.
Methods: Thirty-one 260– 290g rats were separated into the blank control group (n=10), the oil-control group (n=10), the single intraperitoneal injection of 5 mg/kg LPS (n=5), and the continuous intragastric of 5 mg/kg lycopene (n=6). The animals were euthanized after four weeks, and blood and the epididymis were removed for analysis.
Results: Lycopene significantly decreased IL-1α, IL-1β, TNF-α, MCP-1, IL-6 and lipid peroxidation product Malondialdehyde in serum and epididymis. It significantly increased the epididymis’s antioxidant enzyme and total antioxidant capacity. According to LC-MS plasma lipidomics, lycopene increased phosphatidylcholine, lysophosphatidylcholine, decreased phosphatidylethanolamine, triacylglycerol, and diacylglycerol levels, changed the composition of lipids, altered metabolic pathways, and these changes were related to the mechanism of anti-inflammatory and oxidative stress. 20 lipids, including PC (20:5e) and LPC (14:0), were identified through additional Spearman correlation analysis as being related to cytokines and oxidation indices. They served as possible lipid markers that may be utilized to gauge the severity of inflammation.
Conclusion: Lycopene has anti-inflammatory and antioxidant properties that improve histopathological and functional damage in LPS-induced epididymitis and is an alternate supplement for treating epididymitis. Lipidomics provide new perspectives on the possible mechanism of lycopene in protecting against LPS-induced epididymitis by integrating lipid metabolism and inflammation.
Keywords: lycopene, lipidomics, inflammatory, antioxidant, lipids regulation, correlation
Introduction
Epididymitis, a more frequent infection of the male reproductive system than orchitis, can occur alone or with orchitis.1 Young and middle-aged males which are the main demographic for reproduction, frequently get epididymitis. As known, sperms mature, are protected and are stored in the epididymis. So epididymitis dysfunction caused by infection and inflammation will lead to temporary or persistent infertility,2 posing a severe risk to the health of males and creating significant issues for the general public.
Lipopolysaccharide (LPS) is the main component of Escherichia coli (Gram-negative bacteria), the main pathogenic bacteria of epididymitis,3 which binds to LPS-binding protein, protein CD14, and protein MD2 to create a complex. The TLR4 expressed in epididymis epithelial cells is activated by the complex and causes intracellular signal cascades that activate nuclear factor B (NF-κB) and inflame the epididymis.4 Recent research has indicated that plasma membrane lipids are crucial controllers of LPS-induced inflammatory signaling pathways.5 In addition, epididymal immune and non-immune cells secrete cytokines and produce reactive oxygen species (ROS) through TLR4-dependent pathways, which adversely influence epididymal sperm parameters by direct cytotoxicity and/or indirect damage. Instead of being a result of spermatogenesis and testicular steroidogenesis abnormalities, the LPS-induced adverse effects on the epididymis micro-environment are caused by its interference with epididymis-self.6 Moreover, epididymitis can result in remodelling fibrosis and blockage of the epididymal duct, both impair fertility.7–9
Acute epididymitis is caused mainly by microbial infection, and the most common pathogenic organism is Escherichia coli. Chronic epididymitis can often result from acute infections treatment is not timely or thorough, and inflammation remains. Due to its subtle nature, chronic epididymitis is frequently disregarded, but it needs enough attention because it will continue to cause harm.2 Antibiotics are still the most widely used treatment for epididymitis, but they are not always practicable. Due to the fact that antibiotic intervention can eradicate epididymitis-related infections but that antibiotic usage alone cannot reverse histopathological alterations and their associated long-term reproductive issues.10,11 In addition to using potent broad-spectrum antibiotics, finding alternative supplements is essential for treating epididymitis.
Lycopene (LYC) is a natural carotenoid with the molecular formula C40H56, which is a lipid-soluble substance. LYC possesses potent antioxidant, anti-inflammatory, and antidiabetic potential.12 Due to its many conjugated double bonds, LYC shows two and ten times higher singlet oxygen quenching rates than β-carotene and α-tocopherol.13,14 According to the study, LYC reduces ROS and inhibits LPS-induced IL-6 expression in pancreatic acinar cells, preventing the occurrence of alcohol/LPS-related pancreatitis.14 In another study of LPS-induced uveitis in SD rats, LYC injection significantly reduced inflammatory cell infiltration and the levels of pro-inflammatory cytokines, indicating the inhibitory effect of LYC on inflammation.15 In addition, for Escherichia coli-induced chronic bacterial prostatitis, the combination of LYC and ciprofloxacin was superior to ciprofloxacin alone in improving bacterial growth and prostate inflammation.16 However, there is limited data on the preventive role of LYC against LPS-induced epididymis damage. Furthermore, the underlying molecular mechanisms by which LYC exerts its protective effects are complex. Recent researches revealed a connection between serum metabolome and the LYC intervention mechanism. For example, a diet high in fat and sugar increases the production of LPS, promoting systemic inflammation and leading to cognitive impairing cognitive function via the gut-liver-brain axis. LYC ameliorated this injury by improving gut-liver-brain lipid metabolism and inflammatory response.17 It appears that LYC exerts dual regulatory effects on LPS-induced inflammatory response and lipid metabolism disorders.
Liquid chromatography-mass spectrometry- (LC-MS-) based lipidomics often used to swiftly and accurately isolate and identify single molecules, which has particular advantages in mechanism research.18 Therefore, the present study aimed to evaluate the protective effect of LYC against epididymitis. LC-MS was used to explain the possible mechanism of the protective effect of LYC from the perspective of lipid metabolism and to screen potential lipid markers.
Materials and Methods
Animals
The Jinling Hospital provided 31 sexually mature male Sprague Dawley rats, aged eight weeks and weighing 280±10g. During the study, the animals were kept at a constant temperature (24±2°C) and relative humidity (60±5%) with a 12-hour light: dark cycle and free access to food, water, and libitum.
Chemicals and Experimental Design
LPS (L2630-10MG) was purchased from Sigma-Aldrich, Co., (USA). LYC was obtained from the Nanjing Yuanjian Biotechnology Co., LTD (Nanjing, China).
After two weeks of acclimatization, the rats were split into four groups. The first was the blank control group, and the second was given oil (5 mL/kg/d, 4 weeks) as the vehicle control group. The third group received LPS (5 mg/kg), dissolved in 0.9% sodium chloride injection, intravenously. The epididymal inflammatory response was induced by intraperitoneal injection of LPS in SD rats. Intragastric administration of LYC was started 24 h after the LPS injection. LPS (5 mg/kg, dissolved in 0.9% sodium chloride injection, i.p.) and LYC (5 mg/kg/d, dissolved in oil, i.g., 4 weeks) were administered to the fourth group. This study was approved by the Experimental Animal Ethics Committee of East Region Military Command General Hospital (Jinling Hospital, 2022JLHSXJDWLS-0021) and carried out according to the National Institutes of Health protocol to laboratory animal care and use.
Epididymal Sperm Analysis
The cauda epididymis, being scissored, was placed in the 1mL saline-filled Eppendorf tube. Then the Eppendorf tube was placed in a 37°C warm water tank for 30 minutes to make the sperm swim out fully. After that, 10 μL of the suspension were aspirated, transferred into a new Eppendorf tube with 990 μL saline, and mixed. Then 10 μL of sperm suspension was transferred into the counting grids of the modified Neubauer blood cell counting plate. A light microscope (×400) was used to evaluate the sperm specimens’ concentration and motility. Sperm parameters were identified using the WHO laboratory guidelines for analyzing and processing human sperm (WHO, 2010). Sperm count and motility were observed and assessed by one researcher, and at least 200 sperm were observed.
Histopathological Examination
The other side epididymis tissue was preserved in 4% paraformaldehyde for 24 hours. Gradient dehydration was conducted using ethanol in various percentages (80%, 90%, 95%, and 100%). After 1.5 hours in a solution of ethanol and xylene, the tissue was transferred to xylene and impregnated to make it transparent. It was embedded in paraffin, made into wax blocks, and cut into 5μm slices. The slices were deparaffinized with xylene, then hydrated by immersing with ethanol at various percentages (100%, 90%, 80%, 70%) and water for 2 minutes. Then the slices were immersed in a hematoxylin staining solution. After washing off the staining solution and differentiation with hydrochloric acid ethanol, the slices were immersed in ammonia water to return to blue. The next step was to dip the slices in an eosin staining solution. The stained sections were dehydrated and sealed, then observed using a microscope.
Measurement of Cytokines in Serum and Epididymis
Cytokine levels in serum and epididymis were measured using ELISA kits according to the manufacturer’s instructions. Interleukin1alpha (IL-1α, Cat num: ELK1148), interleukin 1 beta (IL-1β, Cat num: ELK1272), tumor necrosis factor-alpha (TNF-α, Cat num: ELK1396), monocyte chemotactic protein 1 (MCP-1, Cat num: ELK5504) and interleukin 6(IL-6, Cat num: ELK1158) were assessed in serum and the epididymis. The manufacturer’s recommendations state that those cytokines were found using commercial assays (ELK Biotechnology CO., LTD, Wuhan, Hubei, China).
Assay for Redox Status in the Epididymis
According to the manufacturer’s instructions, commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) were used to detect catalase (CAT, A007-1), glutathione peroxidase (GSH-pX, A005-1), and superoxide dismutase (SOD, A001-1) in the epididymis. The total antioxidant capacity (TAOC) assay kit (A015-1) and the malondialdehyde (MDA) assay kit (A003-1) were also bought from Nanjing Jiancheng Bioengineering Institute.
Plasma Lipidomics Analysis by LC-MS
Blood samples were taken from the −80°C refrigerator and thawed. Take a 100μL sample, add 1.5mL chloroform/methanol solution and 0.5mL pure water, and scroll for 1 min. Then the mixture was centrifuged at 3000 rpm for 10 min at room temperature. The organic phase was transferred into a new tube, and 200μL isopropanol/methanol was added to redissolve. Then the vortex was performed with adding 5μL internal standard to mix. Next, centrifugation was performed at 12000 rpm at 4°C for 10 min. After that, the supernatant was transferred into the sample vial.
The plasma in the sample vial was tested on the apparatus using LC-MS (Waters, UPLC; Thermo, Q Exactive). On the ACQUITY UPLC BEH C18 column (2.1*100 mm, 1.7μm) operating at 40 °C with a flow rate of 0.3mL/min, the separation was carried out. The mobile phase A was formed of Acetonitrile/water (6:4, v/v, with 10mM ammonium formate), and the mobile phase B was composed of Iso-Propyl alcohol/Acetonitrile (9:1, v/v, with 10mM ammonium formate). The injection volume is 1μL (positive mode) and 3μL (negative mode) with the injector temperature held at 4°C to test. After that, the technique for gradient elution was followed. Always insert quality control during samples testing.
Lipidomics Data Processing
After acquiring the pre-processed Data, the multivariate variable pattern recognition studies were carried out using SIMCA (V14.1, Sartorius Stedim Data Analytics AB, Umea, Sweden). Principal component analysis (PCA) is used to first exclude outlier samples and outlier samples (samples outside the Hotelling T2 ellipse in confidence interval). R2Y and Q2 were obtained after cross-validation was used to assess the model’s validity. Orthogonal projections to Latent Structures-discriminant analysis (OPLS-DA) was used for modeling and analysis. The permutation test is used to confirm the model’s stability once more.
Differential Lipid Screening and Pathway Analysis
To identify the different lipids between the LPS group and the LPS+LYC group, the OPLS-DA score plot and univariate statistical analysis of Student′s t-test were utilized. P<0.05 and Variable Importance in the Projection (VIP)>1 were the requirements for differentiating groups. Following the discovery of the differential lipids, matching data was obtained by mapping the differential lipids in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database using enrichment analysis and topological analysis. And a metabolic pathway analysis was carried out to identify the vital route that had the significant association with the lipid difference.
Correlation Analysis
Spearman correlation analysis was used to evaluate the correlation between two variables. The correlations between serum cytokines, epididymal cytokines, oxidation indices and different lipids were analyzed.
Statistical Analysis
Then the data were examined using the statistical program SPSS 26.0 and were diagramed using the GraphPad Prism v8.0 (GraphPad Software Inc., San Diego, CA, USA). Comparisons were done using the Student′s t-test or Analysis of Variance (ANOVA), and the Spearman rank correlation analysis was carried out using Origin 2021. The significance criteria were P<0.05.
Results
Weight Gain and Sperm Quality of Caudal Epididymis
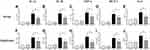
The weight gain of the LPS-treated rats was considerably higher than that of the Control and Oil groups, demonstrating a significant difference. After the LYC intervention, the rise in weight gain was reduced when compared to the LPS group, although the difference was not statistically significant (Figure 1A). Rats treated with LPS had considerably lower sperm motility thereafter. The sperm motility increased after LYC intervention compared to the LPS group, however the difference was not statistically significant (Figure 1B). Sperm count decreased significantly when LPS and Control were compared and increased in the LPS+LYC group, but it was not statistically significant compared with the LPS group (Figure 1C).
Epididymis Histopathological Evaluation
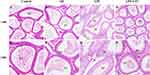
The pathogenic effects of LYC on the epididymal inflammation caused by LPS were assessed using epididymal histology. Control and Oil group rats had closely spaced epididymal tubules (Figure 2A and B). Many sperm cells could be seen in the lumens, and epithelial cells were grouped in a clean manner with distinct features (Figure 2E and F). Contrarily, rats given LPS had atrophied epididymal lumens that displayed severe tissue edema and increased voids (Figure 2C). The lumens were chaotic and unequal in shape, and contained little to no sperm (Figure 2G). There was a significant improvement in LYC following the intervention, as well as a partial restoration of tissue morphology, a reduction in tissue space edema, and a tendency for epididymal epithelial cells to be arranged in a certain order (Figure 2D, H).
Serum and Epididymis Cytokines Determination
Cytokines concentrations in serum and epididymal homogenates were measured to determine the effects of LYC on systemic and epididymal inflammation induced by LPS. Following LPS treatment, pro-inflammatory cytokines in serum levels of IL-1α, IL-1β and TNF-α increased significantly (Figure 3A–C). MCP-1, a byproduct of the NF-κB inflammatory pathway, was considerably more significant than the control groups (Figure 3D). IL-6 involves in many biological processes. In addition to being a pro-inflammatory cytokine, it can control spermatogenesis and sperm maturation. Compared to the control groups, the levels of IL-6 dramatically increased after LPS treatment (Figure 3E). Following LYC intervention, the serum levels of IL-1α, IL-1β, TNF-α, MCP-1 and IL-6 dramatically decreased. In line with those in serum, these cytokines’ changes in concentration in the epididymal homogenate were also constant (Figure 3F–J).
Oxidative Stress Index Measurement of the Epididymis
The levels of antioxidant stress indicators and the lipid peroxidation degradation product MDA were tested to ascertain the LYC affected LPS-induced epididymis oxidative stress. GSH-pX can break down active compounds while preserving the structural and functional integrity of cell membranes. The antioxidant enzymes SOD and CAT are markers of the antioxidant defence system. Compared to the control groups, the GSH-pX, SOD, and CAT levels in the LPS group were considerably lower. GSH-pX and SOD levels in the LYC+LPS group considerably increased when compared to the LPS group, while CAT concentration increased but did not differ significantly (Figure 4A–C). The MDA levels in the LYC group reduced, which had a significant difference from the LPS group. Moreover, The MDA levels comparison between the LYC and control groups is not significant, indicating that it had reverted to the normal level (Figure 4E). Comparing the LPS group to the control groups, the TAOC decreased; however, it considerably increased in the LPS+LYC group (Figure 4D).
Multivariate Statistical Analysis Results
After preprocessing the data obtained in positive ion mode, multivariate statistical analysis was carried out. Figure 5A displays the results of the PCA analysis of the data from the LPS and LPS+LYC groups. According to a significant separate trend between the two groups (R2X=0.48), LYC exhibited a controlling impact on the inflammation caused by LPS. The OPLS-DA model score diagram was created in this study to determine the differences in lipid metabolites between the LPS+LYC group and the LPS group (Figure 5B). The phenotypic explanatory power R2Y=0.988 and phenotypic predictive power Q2=0.79 of this model demonstrated its dependability and demonstrated its good classification predictability and repeatability. The model was confirmed by the permutation test, and the outcomes revealed that R2 and Q2 on the left were less inconsequential than the initial R2 and Q2 on the right, further demonstrating the model’s resilience (Figure 5C). Then putative differential lipid metabolites were evaluated based on the VIP>1 of the LYC group and LPS group, along with P<0.05.
Differential Lipid Screening and Pathway Analysis
The variables with weight values VIP>1 of the OPLS-DA were eliminated, and the lipid metabolites with a significant difference were determined in conjunction with the P-value of the t-test (P<0.05) and the VIP of the OPLS-DA. A total of 135 differential lipids, including PC (Lecithin), PE, and LPC, were discovered. All the discovered difference lipids are visualized in Figure 6A.
The pathways of differential lipid mapping discovered by the aforementioned investigation were sorted using KEGG. Through enrichment analysis and topological analysis of the pathways, the key pathways with the strongest association to lipid differences were eliminated. Glycerophospholipid metabolism, Linoleic acid metabolism, alpha-Linolenic acid metabolism, Glycosylphosphatidylinostol (GPI)-anchor biosynthesis, Glycerolipid metabolism and Arachidonic acid metabolism are mediated (Figure 6B). The plasma concentrations of PC and LPC dramatically rose while those of PE, TG, and DG significantly decreased following LYC therapy with LPS-induced inflammation. It is clearly demonstrate changes in lipid concentrations and their impact on metabolic pathways by grouping the important metabolic routes and displaying them notably distinct lipids (Figure 6C).
Correlation Between Different Types of Lipids and Biochemical Indices
Different types of differential lipids were subjected to cluster analysis, and the results were shown as a differential heat map, highlighting differences in lipid metabolism between the two groups (Figures 7–10A). Then, correlation heat maps were created using Spearman correlation analysis on lipids from various categories along with serum cytokines (Figures 7–10B), epididymal cytokines (Figures 7–10C), and oxidation markers (Figures 7–10D). Correlation results are shown in Color and Significant Mark. PC (10:0e/6:0), PC (8:1e/11:0), PC (8:1e/12:4), PC (20:5e), PC (32:2), PC (35:2), PE (12:0e/8:0), PE (17:1/23:1), LPC (14:0), LPC (19:1) and LPC (20:5) were simultaneously correlated with all cytokines in serum and epididymis, indicating that cytokine levels in serum and epididymis increased with the increase of these lipid levels. Meanwhile, PC (10:0e/12:2). PE (10:0e/11:2), LPC (18:2), LPC (18:2e), and LPC (22:2) were positively correlated with TAOC and negatively correlated with MDA. PC (8:0e/6:0), PC (8:0e/9:0), PC (20:5e) and LPC (14:0) were negatively correlated with TAOC and positively correlated with MDA.
Discussion
In this study, the biochemical indicators and pathological characteristics were evaluated to determine the protective effect of LYC in LPS-induced on epididymitis. Meanwhile, LC-MS was used to examine the changes in plasma lipid metabolites oral LYC in rats with LPS-induced epididymitis, to identify the lipid metabolites and metabolic pathways affected by LYC, and to explain the possible mechanism of LYC in reducing epididymitis inflammation.
By Decreasing Inflammation and Oxidative Stress, LYC Protects Against LPS-Induced Epididymitis
The cytokine levels and inflammatory response in the epididymitis are decreased by LYC. LPS-induced systemic inflammation causes increases levels of pro-inflammatory cytokines, particularly TNF-α,19 and leukocyte infiltration and fibrosis in the caudal epididymis to result in infertility. At present study, LYC decreased LPS-induced pro-inflammatory cytokines IL-1α, IL-1β and TNF-α levels. LPS can induce and work in conjunction with the rise in saturated fatty acids, such as palmitic acid to exacerbate the inflammatory response.20,21 The previous study has found that, LYC dramatically lowers the gene expression of the adipokines IL-6 and MPC-1 in epididymal adipose tissue while not affecting body weight or obesity. This alleviates the chronic low-grade inflammation brought on by obesity.22 The present study also demonstrated that weight gain in LPS-treated rats was significantly increased. LYC did not significantly reduce weight gain but did reduce IL-6 and MCP-1 levels. In addition, by resuming the activities of the antioxidant enzymes SOD, GSH-pX, and CAT, LYC improved the LPS toxicity to sperm parameters of infertile men with oligospermia.23 The current study has come to the same conclusion, these antioxidant enzymes may be activated in the epididymis tissue by LYC, according to our research. As measures of antioxidant defense, they can decrease lipid peroxidation and lower MDA levels. In this way, LYC raises the TAOC while reducing over-produced ROS’s adverse effects on the plasma membrane of the sperm.
The Potential Lipid-Regulating Mechanisms of LYC Effect on LPS-Induced Epididymis Injury
LYC altered the concentration and composition of different lipids in plasma, such as a significant increase in PC and LPC and a decrease in PE, DG, and TG. These changes could attenuate the epididymal injury induced by LPS in variety of approaches. For example, PC inhibits multiple proinflammatory mediators neutrophils produce to attenuate LPS-induced inflammatory and oxidative damage in multiple organs and tissues.24 In addition, the higher PC concentration improved LYC bioavailability, enhancing antioxidant capacity and oxidative metabolism.25 On the other hand, LPS results in hypertriglyceridemia and weight gain by causing the accumulation of triglycerides, and promoting systemic inflammatory responses.26 Similarly, the LPS group rats in the present study observed a significant increase in weight gain. Although the weight gain of rats in the LYC group did not decrease significantly, the TG concentration was reduced, and the inflammation caused by LPS was alleviated. Meanwhile, lipid metabolism disorders such as excessive saturated fatty acids cause mitochondrial dysfunction and produce large amounts of ROS.27 LYC can inhibit the oxidative stress caused by excessive saturated fatty acids and increase the activities of antioxidant enzymes.28 Furthermore, LYC may interfere with LPS proinflammatory signalling pathway by impacting the lipid metabolism pathway. Among several KEGG metabolic pathways enriched in this study, GPI-anchor synthesis was noted. It is established that an inflammatory response to be induced by LPS binding to CD14 protein and TLR4/MD2 receptor. CD14 known as a GPI-anchor protein is mainly found in rafts, which are areas of the plasma membrane that are abundant in sphingolipids and cholesterol.5 Lipid rafts are essential to LPS-induced inflammatory signalling pathways. It has been found that lipid rafts formed by CD14 aggregation are the sites of the generation and turnover of essential regulators of lipid-dependent steps of LPS, such as PI(4,5)P2, controlling two pathways of TLR4 signalling inflammatory response.29,30 LYC has been shown to impede the translocation of TLR4 to lipid rafts induced by LPS, and inhibit the LPS/TLR4-induced inflammatory response.31 According to the findings of this study, LYC may disrupt the LPS signalling pathway in lipid rafts and prevent the production of inflammatory cytokines through the GPI-anchors synthesis.
Correlation Between Lipids and Biochemical Indices
Curious as to which lipids were significantly associated with improving inflammation, we analyzed the correlations of various lipids with serum cytokines, epididymal cytokines, and oxidation indicators to screen for potential biomarkers. LPC (14:0) was one of the screened lipid molecules in this research. The role of LPC in the development of inflammation mainly depends on the length and saturation of the acyl chain. As an illustration, LPC (16:0) and LPC (18:0) are efficient pro-inflammatory mediators that promote the release of inflammatory factors IL6 and IL8 by endothelial cells. LPC (22:6) suppresses the synthesis of inflammatory mediators as a polyunsaturated acyl, to antagonize inflammation induced by saturated fatty acids.32 Consistent with previous studies, LPC (14:0), as a saturated LPC, was positively correlated with the levels of cytokines IL-α, IL-β, TNF-α, IL-6 and MCP-1 and positively correlated with MDA, reflecting the degree of inflammatory damage. The previous study suggested that LPC (14:0) in preoperative plasma of patients with esophageal squamous cell carcinoma is a marker metabolite for evaluating the prognosis of patients.33 In another study, LPC (14:0) <0.24nmol/L is independently associated with gestational diabetes risk.34 There are few publications on LPC (14:0) in studies pertaining to inflammation, and the potential application value of LPC (14:0) in this field deserves attention. In addition, PC (20:5e) was also found to be significantly positively correlated with all cytokines and MDA levels examined. Lipid metabolites opposite to their effects are LPC (18:2) and LPC (22:2). They were negatively correlated with MDA and positively correlated with TAOC, indicating antioxidant capacity, according to the present study. At present, the evidence for LPC’s importance as the biomarker to inflammation and oxidative stress is further developed.5,32,35 Moreover, LPC also has the benefit of being a marker of fertility in that, in contrast to proteins, it is a non-specific marker that may be useful in both human and animal sperm.36 The lipid compounds identified in this study as potential biomarkers still require comprehensive studies to confirm their therapeutic applicability.
Conclusion
This study demonstrated that LYC prevents inflammation and reduces oxidative stress in LPS-induced epididymitis, and is a substitute supplement for chronic epididymitis. Plasma lipidomics as evaluated by LC-MS demonstrated that LYC could regulate lipids. This new theoretical perspective, combined with established findings, suggests that LYC exerts anti-inflammatory and anti-oxidative effects by integrating lipid metabolism and inflammatory response, which is a possible pathway mechanism for LYC. Furthermore, the potential value of identified lipids as markers in genital tract inflammation deserves attention, and clinical utility will be further verified in future studies.
Abbreviations
LPS, lipopolysaccharide; LYC, lycopene; LC-MS, Liquid chromatography-mass spectrometry; TLR4, Toll-like receptor 4; PCA, principal component analysis; OPLS-DA, Orthogonal projections to Latent Structures- discriminant analysis; IL-1α, interleukin 1alpha; IL-1β, interleukin 1 beta; TNF-α, tumor necrosis factor-alpha; MCP-1, monocyte chemotactic protein 1; IL-6, interleukin 6; LU, lumen; EP, epithelium; IS, interstitial space; GSH-pX, Glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase; TAOC, total antioxidant capacity; MDA, malondialdehyde; NF-κB, nuclear factor kappa B; ROS, reactive oxygen species; VIP, variable influence on projection; KEGG, Kyoto Encyclopedia of Genes and Genomes; PC, phosphatidylcholine; PE, phosphatidylethanolamine; LPC, lysophosphatidylcholine; PI, phosphatidylinositol; DAG/DG, diacylglycerol/diglyceride; TAG/TG, triacylglycerol/triglyceride; PA, phosphatidic acid; LPI, lysophosphatidylinositol; LPA, lysophosphatidic acid; LPE, lysophosphatidylethanolamine; LPS, lysophosphatidylserine; PIP, phosphatidylinositol; ST, Sulfatide; SPH, Sphingosine; CDP-DAG: CDP-diacylglycerol; PGP, Phosphatidylglycerophosphate; PG, Phosphatidylglycerol; CL, Cardiolipin; G3P, Glycerophosphoric acid; GPI, Glycosylphosphatidylinostol.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Consent for Publication
The authors confirm that the details of any images, videos, recordings can be published.
Ethics Approval for Animal Study
The experiment was authorized by the Experimental Animal Ethics Committee of East Region Military Command General Hospital (Jinling Hospital, 2022JLHSXJDWLS-0021) and carried out according to the National Institutes of Health protocol to laboratory animal care and use.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China under Grant 81771646 and Grant 81370750.
Disclosure
All of the authors report no conflicts of interest in this work.
References
1. Michel V, Pilatz A, Hedger MP, Meinhardt A. Epididymitis: revelations at the convergence of clinical and basic sciences. Asian J Androl. 2015;17(5):756–763. doi:10.4103/1008-682X.155770
2. Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia. 2008;40(2):92–96. doi:10.1111/j.1439-0272.2007.00819.x
3. Pilatz A, Hossain H, Kaiser R, et al. Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur Urol. 2015;68(3):428–435. doi:10.1016/j.eururo.2014.12.005
4. Rodrigues A, Queiroz DB, Honda L, Silva EJ, Hall SH, Avellar MC. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod. 2008;79(6):1135–1147. doi:10.1095/biolreprod.108.069930
5. Kwiatkowska K, Ciesielska A. Lipid-mediated regulation of pro-inflammatory responses induced by lipopolysaccharide. Postepy Biochem. 2018;64(3):175–182. doi:10.18388/pb.2018_129
6. Silva EJR, Ribeiro CM, Mirim AFM, et al. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Sci Rep. 2018;8(1):103. doi:10.1038/s41598-017-17944-4
7. Lang T, Dechant M, Sanchez V, et al. Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E. coli-associated epididymitis. Biol Reprod. 2013;89(3):59. doi:10.1095/biolreprod.113.110379
8. Michel V, Duan Y, Stoschek E, et al. Uropathogenic Escherichia coli causes fibrotic remodelling of the epididymis. J Pathol. 2016;240(1):15–24. doi:10.1002/path.4748
9. Lu Y, Bhushan S, Tchatalbachev S, et al. Necrosis is the dominant cell death pathway in uropathogenic Escherichia coli elicited epididymo-orchitis and is responsible for damage of rat testis. PLoS One. 2013;8(1):e52919. doi:10.1371/journal.pone.0052919
10. Ludwig M, Johannes S, Bergmann M, Failing K, Schiefer HG, Weidner W. Experimental Escherichia coli epididymitis in rats: a model to assess the outcome of antibiotic treatment. BJU Int. 2002;90(9):933–938. doi:10.1046/j.1464-410X.2002.03029.x
11. Klein B, Pant S, Bhushan S, et al. Dexamethasone improves therapeutic outcomes in a preclinical bacterial epididymitis mouse model. Hum Reprod. 2019;34(7):1195–1205. doi:10.1093/humrep/dez073
12. Imran M, Ghorat F, Ul-Haq I, et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants. 2020;9:8.
13. Heymann T, Heinz P, Glomb MA. Lycopene inhibits the isomerization of beta-carotene during quenching of singlet oxygen and free radicals. J Agric Food Chem. 2015;63(12):3279–3287. doi:10.1021/acs.jafc.5b00377
14. Lee J, Lim JW, Kim H. Lycopene Inhibits IL-6 Expression by Upregulating NQO1 and HO-1 via Activation of Nrf2 in Ethanol/Lipopolysaccharide-Stimulated Pancreatic Acinar Cells. Antioxidants. 2022;11:3.
15. Goncu T, Oguz E, Sezen H, et al. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq Bras Oftalmol. 2016;79(6):357–362. doi:10.5935/0004-2749.20160102
16. Han CH, Yang CH, Sohn DW, Kim SW, Kang SH, Cho YH. Synergistic effect between lycopene and ciprofloxacin on a chronic bacterial prostatitis rat model. Int J Antimicrob Agents. 2008;31(Suppl 1):S102–107. doi:10.1016/j.ijantimicag.2007.07.016
17. Wang J, Wang Z, Li B, et al. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut-liver-brain axis. Int J Obes. 2019;43(9):1735–1746. doi:10.1038/s41366-018-0277-9
18. Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom. 2016;27(12):1897–1905. doi:10.1007/s13361-016-1469-y
19. Wang F, Liu W, Jiang Q, et al. Lipopolysaccharide-induced testicular dysfunction and epididymitis in mice: a critical role of tumor necrosis factor alphadagger. Biol Reprod. 2019;100(3):849–861. doi:10.1093/biolre/ioy235
20. Du L, Lei X, Wang J, et al. Lipopolysaccharides derived from gram-negative bacterial pool of human gut microbiota promote inflammation and obesity development. Int Rev Immunol. 2022;41(1):45–56. doi:10.1080/08830185.2021.1996573
21. Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. 2016;785:24–35. doi:10.1016/j.ejphar.2016.04.024
22. Luvizotto Rde A, Nascimento AF, Imaizumi E, et al. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br J Nutr. 2013;110(10):1803–1809. doi:10.1017/S0007114513001256
23. Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019;33(12):3203–3211. doi:10.1002/ptr.6493
24. Jung YY, Nam Y, Park YS, et al. Protective effect of phosphatidylcholine on lipopolysaccharide-induced acute inflammation in multiple organ injury. Korean J Physiol Pharmacol. 2013;17(3):209–216. doi:10.4196/kjpp.2013.17.3.209
25. Petyaev IM, Chernyshova MP, Pristensky DV, et al. Effect of lycosome-formulated phosphatidylcholine on parameters of biological oxidation after single intake of moderate amount of alcohol. Adv Prev Med. 2018;2018:5840451. doi:10.1155/2018/5840451
26. Alvi SS, Ansari IA, Ahmad MK, Iqbal J, Khan MS. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed Pharmacother. 2017;96:1082–1093. doi:10.1016/j.biopha.2017.11.116
27. Raja AA, Dandare A, Khan MJ, Khan MJ. Free fatty acid overload targets mitochondria: gene expression analysis of palmitic acid-treated endothelial cells. Genes. 2022;13:10. doi:10.3390/genes13101704
28. Ugbaja RN, James AS, Ugwor EI, Akamo AJ, Thomas FC, Kosoko AM. Lycopene suppresses palmitic acid-induced brain oxidative stress, hyperactivity of some neuro-signalling enzymes, and inflammation in female Wistar rat. Sci Rep. 2021;11(1):15038. doi:10.1038/s41598-021-94518-5
29. Plociennikowska A, Hromada-Judycka A, Dembinska J, Roszczenko P, Ciesielska A, Kwiatkowska K. Contribution of CD14 and TLR4 to changes of the PI(4,5)P2 level in LPS-stimulated cells. J Leukoc Biol. 2016;100(6):1363–1373. doi:10.1189/jlb.2VMA1215-577R
30. Plociennikowska A, Zdioruk MI, Traczyk G, Swiatkowska A, Kwiatkowska K. LPS-induced clustering of CD14 triggers generation of PI(4,5)P2. J Cell Sci. 2015;128(22):4096–4111. doi:10.1242/jcs.173104
31. Manabe Y, Hirata T, Sugawara T. Inhibitory effect of carotenoids on ligand-induced lipid raft translocation of immunoreceptors. J Oleo Sci. 2019;68(2):149–158. doi:10.5650/jos.ess18204
32. Engel KM, Schiller J, Galuska CE, Fuchs B. Phospholipases and reactive oxygen species derived lipid biomarkers in healthy and diseased humans and animals - A focus on lysophosphatidylcholine. Front Physiol. 2021;12:732319. doi:10.3389/fphys.2021.732319
33. Chen Z, Dai Y, Huang X, et al. Combined metabolomic analysis of plasma and tissue reveals a prognostic risk score system and metabolic dysregulation in esophageal squamous cell carcinoma. Front Oncol. 2020;10:1545. doi:10.3389/fonc.2020.01545
34. Liu J, Li J, Li S, et al. Circulating lysophosphatidylcholines in early pregnancy and risk of gestational diabetes in Chinese women. J Clin Endocrinol Metab. 2020;105:4. doi:10.1210/clinem/dgaa058
35. Liu P, Zhu W, Chen C, et al. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020;247:117443. doi:10.1016/j.lfs.2020.117443
36. Nimptsch A, Pyttel S, Paasch U, Mohr C, Heinrich JM, Schiller J. A MALDI MS investigation of the lysophosphatidylcholine/phosphatidylcholine ratio in human spermatozoa and erythrocytes as a useful fertility marker. Lipids. 2014;49(3):287–293. doi:10.1007/s11745-013-3870-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.