Back to Journals » Infection and Drug Resistance » Volume 13
Oral Infection Caused by Non-O1/Non-O139 Vibrio cholerae in a Patient with Esophageal Cancer Undergoing Esophagectomy and Chemoradiotherapy: A Case Report
Authors Xie H, Wu Y, Liu C, Guo J, Ma J, Li X, Sun X
Received 26 July 2020
Accepted for publication 6 September 2020
Published 3 November 2020 Volume 2020:13 Pages 3923—3927
DOI https://doi.org/10.2147/IDR.S274077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hongxiang Xie,1,2,* Yuanyuan Wu,3,* Cuiping Liu,4 Jianzhuang Guo,2 Jianping Ma,2 Xiaofeng Li,2 Xiaojun Sun2
1Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Mainland China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Shandong First Medical University, Jinan, Mainland China; 3Department of Shungen Conservative and Endodontic Dentistry, Jinan Stomatology Hospital, Jinan, Mainland China; 4Department of General Medicine, The First Affiliated Hospital of Shandong First Medical University, Jinan, Mainland China
*These authors contributed equally to this work
Correspondence: Xiaojun Sun Department of Clinical Laboratory
The First Affiliated Hospital of Shandong First Medical University, 16766 Jingshi Road, Jinan, Shandong 250014, Mainland China
Tel +86-571-87666666
Fax +86-571-85335800
Email [email protected]
Abstract: Non-O1/non-O139 Vibrio cholerae (NOVC) are increasingly being recognized as causes of sporadic cases of gastroenteritis and extra-intestinal invasive infections, such as bacteremia as well as skin and wound infections in immunosuppressed hosts. However, oral infections caused by these microorganisms have rarely been reported. We present a case of oral infection caused by NOVC in a patient undergoing chemoradiotherapy after esophagectomy for esophageal cancer. The patient recovered well after antibiotic treatment. The isolate from the patient was screened for phenotypic and genetic characteristics with reference to their major virulence genes. Our report provides supporting evidence for oral infection due to NOVC in a patient with esophageal cancer and suggests that some putative accessory virulence factors may be crucial in the pathogenicity of this strain. To the best of our knowledge, this is the first documented case of oral infection due to NOVC.
Keywords: non-O1/non-O139 Vibrio cholerae, oral infection, esophageal cancer, chemoradiotherapy
Introduction
Vibrio species are halophilic, facultative, anaerobic gram-negative bacilli, which are ubiquitously distributed in estuarine and marine environments. Based on the surface O antigen of the lipopolysaccharide, Vibrio cholerae are divided into over 200 serogroups. Historically, only toxigenic serogroups O1 and O139 are responsible for widespread cholera epidemics.1 Serogroups apart from O1 and O139 are known as non-O1/non-O139 Vibrio cholerae (NOVC). Most NOVC strains lack the cholera toxin, and therefore, do not cause cholera. The clinical significance of these microorganisms has not been a major topic of discussion and oral infection due to NOVC has rarely been documented. However, in recent decades, there has been an increasing trend of infection with these species.2 Here, we describe a previously unreported case of oral infection caused by NOVC and investigate the genomic and phenotypic characterization of the isolate.
Case
A 51-year-old Chinese man was admitted to our hospital on October 8, 2019 with thrombocytopenia that persisted for more than 10 days. The patient reported eating fish and shrimp regularly. He had been admitted to a local hospital 10 days prior because of fever of 38.5°C, abdominal pain, and diarrhea after eating prawns. At the time, he had watery diarrhea with over ten bowel movements per day. He developed multiple blood blisters on his lips and inside his cheeks that ruptured easily. Erosions covered with a pseudomembrane were widespread on the inner mucosa of the upper and lower lips as well as on the palate. Large, painful areas of ulcerations were located under the tongue. Blood tests revealed anemia, leukopenia, thrombocytopenia, and elevated procalcitonin levels. After anti-infective and symptomatic treatments at the local hospital, the patient showed normal body temperature and improvement in diarrhea, but the platelet count remained low.
The patient had a medical history of coronary heart disease and esophageal cancer (pT3N1M0, stage IIIa) with postoperative metastasis to the mediastinal lymph nodes (cT3N2M0, stage IIIb). He had received 12 sessions of chemotherapy and 27 sessions of radiotherapy. At admission to our hospital, his body temperature was 36.8°C and vital signs were normal. Blood blisters were visible on the lips, some of which had developed black-purple blood crusts. Widespread erosions persisted on the inner mucosa of the upper and lower lips as well as the mucosa of the palate with a pseudomembrane; ulcerations were observed under the tongue (Figure 1). Breath sounds were coarse in both lungs; a 10-cm horizontal scar was found in the upper abdominal region. The bilateral lower limbs showed mild edema. Laboratory tests revealed that the white blood cell count (WBC) was 3.34×109/L with 81% neutrophils; erythrocyte count, 2.03×1012/L; hemoglobin, 75.0 g/L; platelet count, 13×109/L; and albumin, 34.80 g/L. The remainder of his initial laboratory findings were normal. Supportive symptomatic treatment for bleeding prevention, anemia correction, and hematopoiesis through bone marrow stimulation were provided after admission.
 |
Figure 1 Clinical manifestations of the perioral area (A) and the oral mucosa tissue (B) infected due to non-O1/non-O139 V. cholerae. |
On the second day after admission, the patient developed signs of systemic infection (chills and fever of 39.2°C) and was administered 4 g/day intravenous moxifloxacin. Oral swab samples were collected for culture. After 24 h of incubation, β-hemolytic, oxidase-positive colonies grew on blood agar. On thiosulfate-citrate-bile salts-sucrose agar, the organism appeared as large, yellow colonies. V. cholerae was suspected and identified by the Bruker Biotyper MALDI-TOF MS system, which showed identification log scores of 2.151 (>2.0 is considered reliable for species identification). V. cholerae failed to agglutinate with O1 and O139 antisera. The susceptibility of the isolate was tested using standard disk diffusion on Muller Hinton agar plates. It was susceptible to piperacillin/tazobactam, aztreonam, cefoperazone/sulbactam, ceftazidime, cefepime, gentamicin, amikacin, levofloxacin, ciprofloxacin, imipenem, and meropenem. V. cholerae was not isolated from his stool specimen.
The patient developed fever again on October 13, with his temperature peaking at 38.8°C. Chest computed tomography (CT) scan suggested that the patient had pneumonia. Laboratory tests revealed increased levels of C-reactive protein (25 mg/L) and procalcitonin (0.156 ng/mL). Fungal (1-3)-β-D-glucan, Aspergillus galactomannan antigenemia, and virus were not found, and the blood culture was negative. Antimicrobial therapy was subsequently switched to cefoperazone/sulbactam (1.5 g three times daily) for 9 days and a short course of levofloxacin, based on drug susceptibility tests, in addition to symptomatic treatment, was also provided. The patient’s fever subsided one day later without recurrence.
On hospital day 10, an additional oral swab came back negative, and chest CT scan on day 14 showed improvement. After 16 days of antibiotic treatment, the erosions on the inner mucosa of the upper and lower lips visibly improved. White plaques in the mouth and ulceration under the tongue cleared. Hematologic tests before discharge revealed moderate anemia with normal WBC and platelet count. The patient’s final diagnosis was secondary pancytopenia, esophageal cancer after surgery, stomatitis, pneumonia, and coronary heart disease.
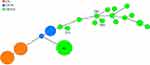
In addition, the strains were examined using whole-genome sequencing for the presence of virulence genes. The isolate was found to be non-toxigenic, as it lacked the ctxAB, tcpA, zot, and ace genes. However, the strain carried other virulence-associated factors, such as toxR, HlyA, nanH, hapA, mshA, ompU, rtxC, and T6SS. Although the specific serotype of this isolate was not identified, multilocus sequence typing (MLST) was performed to determine its genotypic characterization. A novel sequence type (ST986) was assigned to this strain (Figure 2). In the MLST database, the STs most similar to this strain was ST209, which was first discovered from bile samples from the same region. Interestingly, one isolate (ST210), which was obtained from the sputum culture of a patient in Hangzhou, was quite different from this strain in terms of evolutionary divergence. Furthermore, ST986 had six loci different from ST80, the predominant ST in a previous report from China of isolated NOVC in diarrheal stool.3
Discussion
NOVC are recognized as causative agents of sporadic and localized outbreaks of diarrhea and are associated with invasive extra-intestinal disorders, such as bacteremia and skin and wound infections in immunosuppressed hosts.2,4,5 Infections are associated with exposure to aquatic environments or seafood consumption during summer.2 A study showed that chemotherapy can damage epithelial cells of the oral mucosa or directly damage the integrity of the oral mucosa and affect the secretory function of salivary glands, resulting in a change in the microbiota of the mouth and a decrease in its self-cleansing ability; particularly, combined treatment with chemoradiotherapy caused more oral infections.6 Intensive chemoradiotherapy can also suppress the immune system.
The patient was infected during autumn. His medical history suggested that the source of infection was contaminated food. In addition, the patient underwent multiple chemoradiotherapy sessions. Bone marrow suppression, impaired immunity, and damage to the oral mucosa as a result of these treatments could be the main causes of the patient’s NOVC infection.
Following admission, the patient experienced recurrent fever episodes, and after ruling out other possible causes, he was suspected of having pulmonary infection due to NOVC infection. NOVC infection rarely causes pneumonia, and only a few cases have been reported to date.7 However, the patient did not have abdominal pain or diarrhea at the time of admission and his stool was yellow, soft, and formed. Both follow-up blood and stool cultures were negative for NOVC, which may be attributable to the antibiotic treatment administered to the patient before admission. The local hospital had not performed a stool culture, missing the opportunity to identify NOVC in stool specimens.
This case suggests that oral NOVC infections can occur in any region of the oral mucosa, including the masticatory mucosa and lining mucosa. The infection can also occur outside the vermilion border, even affecting the skin near the corner of the mouth. Clinical symptoms include oral mucosa hyperemia, regional erosion, or ulceration. Ulcers often fuse into patches, and the diameter of lesions can exceed 1 cm. A pseudomembrane with an off-white or yellowish-brown color covers the surface of the erosion or ulceration. The pseudomembrane is fairly thick, with blood and exudate crusts at the edges, accompanied by hyperemia and edema of the surrounding mucus membrane. Symptoms of oral NOVC infections are similar to those of some pemphigoid oral diseases, such as paraneoplastic syndrome or erythema multiforme, which require careful differential diagnosis. Paraneoplastic syndrome and erythema multiforme are autoimmune diseases that affect the skin or mucous membranes of parts of the body, except the oral mucosa. In addition to a pathological diagnosis, a bacterial culture is especially important in the diagnosis and differential diagnosis of oral lesions.
Although oral NOVC infections are not fatal, patients can experience oral pain that affects their food intake and reduces their quality of life. NOVC is sensitive to most antibiotics used clinically. Third-generation cephalosporins, tetracyclines, or fluoroquinolones are the most suitable agents for treating severe Vibrio infections.1 Effective treatment outcomes can be achieved by providing timely and appropriate antibiotic treatment along with treatments for local symptoms, such as using sodium bicarbonate and Kangfuxin oral rinse solution (Periplaneta americana extract) to enhance epithelial cell repair or application of a topical growth factor gel to enhance ulcer healing. For patients with systemic fever, vitamin supplementation and nutrition support therapy can be provided.
The isolates in our case were found to be non-toxigenic because of the lack of major enteric toxin-encoding genes, such as ctxA/B and tcpA, but they harbored other pathogenicity-related genes, which enhanced the pathogenicity of this strain and played synergistic roles in the invasion and seeding of multiple oral sites during infection. The exact pathogenic mechanism of each virulence gene in oral infection requires further research. We investigated the strain using MLST to determine its genotype, and assigned it a novel sequence type (ST986) that did not belong to the dominant clone of NOVC. Perhaps in one country, the sequence type of NOVC has obvious regional characteristics, which requires further investigation.
Although the clinical symptoms of infections caused by Vibrio species differ across cases, skin and soft tissue infections and bullous lesions are almost always associated with V. vulnificus infections, but rarely with NOVC infections.4 Moreover, oral NOVC infection has never been reported before. This case further expands the spectrum of infection caused by V. cholerae and raises the possibility of V. cholerae as a cause of oral infection, especially in patients with low immune function, and suggests that some putative accessory virulence factors may have played an important role in the pathogenicity of this strain.
Ethics and Consent Statement
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images, and our study was approved by the Ethics Committee at The First Affiliated Hospital of Shandong First Medical University to publish the case details.
Funding
This work was supported by the National Natural Science Foundation of China (No: 81600110), Zhejiang Provincial Health Bureau (Nos: 2016KYA010; 2021KY017), and the Outstanding Youth Foundation of Zhejiang Provincial People’s Hospital (No: ZRY2016B008) to Hong-Xiang Xie. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Baker-Austin C, Oliver JD, Alam M, et al. Vibrio spp. infections. Nat Rev Dis Primers. 2018;4(1):8. doi:10.1038/s41572-018-0005-8
2. Engel MF, Muijsken MA, Mooi-Kokenberg E, Kuijper EJ, van Westerloo DJ. Vibrio cholerae non-O1 bacteraemia: description of three cases in the Netherlands and a literature review. Euro Surveill. 2016;21:15. doi:10.2807/1560-7917.ES.2016.21.15.30197
3. Luo Y, Ye J, Jin D, et al. Molecular analysis of non-O1/non-O139 Vibrio cholerae isolated from hospitalised patients in China. BMC Microbiol. 2013;13:52. doi:10.1186/1471-2180-13-52
4. Maraki S, Christidou A, Anastasaki M, Scoulica E. Non-O1, non-O139 Vibrio cholerae bacteremic skin and soft tissue infections. Infect Dis (Lond). 2016;48(3):171–176. doi:10.3109/23744235.2015.1104720
5. Chowdhury G, Joshi S, Bhattacharya S, et al. Extraintestinal infections caused by non-toxigenic vibrio cholerae non-o1/non-o139. Front Microbiol. 2016;7:144. doi:10.3389/fmicb.2016.00144
6. Xu L, Zhang H, Liu J, Chen X. Investigation of the oral infections and manifestations seen in patients with advanced cancer. Pak J Med Sci. 2013;29(5):1112–1115. doi:10.12669/pjms.295.3493
7. Marinello S, Marini G, Parisi G, et al. Vibrio cholerae non-O1, non-O139 bacteraemia associated with pneumonia, Italy 2016. Infection. 2017;45(2):237–240. doi:10.1007/s15010-016-0961-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.