Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Oral Health Status of Young People Infected with HIV in High Epidemic Area of China
Authors Chen F, Cheng Y, Xie T
Received 9 January 2021
Accepted for publication 19 March 2021
Published 20 April 2021 Volume 2021:14 Pages 831—837
DOI https://doi.org/10.2147/JMDH.S301236
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fei Chen1
Yuewu Cheng2
Tiansheng Xie3,4
1The Affiliated Hospital of Stomatology, School of Stomatology, Zhejiang University School of Medicine, and Key Laboratory of Oral Biomedical Research of Zhejiang Province, Hangzhou, Zhejiang, 310006, People’s Republic of China; 2Shangcai Center for Disease Control and Prevention, Zhumadian, Henan, 463800, People’s Republic of China; 3Zhejiang Sino-German Institute of Life Science and Healthcare, School of Biological and Chemical Engineering, Zhejiang University of Science and Technology, Hangzhou, Zhejiang, 310023, People’s Republic of China; 4State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Zhejiang University, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, 310003, People’s Republic of China
Correspondence: Tiansheng Xie
Zhejiang Sino-German Institute of Life Science and Healthcare, School of Biological and Chemical Engineering, Zhejiang University of Science and Technology, 318 Liuhe Road, Hangzhou, Zhejiang Province, 310023, People’s Republic of China
Tel +86 571 86021350
Email [email protected]
Purpose: This study aimed to understand the oral hygiene habits and oral health status of young people infected with human immunodeficiency virus (HIV) in Henan province of China.
Methods: Randomized stratified cluster sampling strategy was used to select young people who were receiving highly active anti-retroviral therapy (HARRT) from 6 towns. A total of 104 participants were enrolled and divided into 2 groups, adolescence group and young adult group. By face-to-face oral interview and examination, well-trained dentists collected general information, oral hygiene habits and oral health status of the participants.
Results: Fifty-eight of them were adolescence group and 46 of them were young adult group. In two groups, most of them brushed their teeth once a day (55.20%, 67.40%), and half of them basically brushed their teeth for 2 minutes (51.7%, 50.0%). Majority of participants did not use dental floss (93.1%,91.3%) and also never visited a dentist (81%,78.3%). One-third of participants had spontaneous bleeding, and about half of them had gingival bleeding when brushed their teeth. The most frequent mucosal disease was oral ulcers. Moreover, the prevalence of caries remained very high in both groups, which was 82.76% and 84.8%, respectively. Most of the participants in both groups had low education level and received less than 9 years of education (65.5%, 63%).
Conclusion: The participants had poor oral hygiene habits. The economic and education
level may associate with the awareness of oral health and care.
Keywords: oral hygiene, oral health, mouth mucosa, HIV infections, adolescent, young adult
Introduction
In 2019, there were 38,000,000 HIV-infected people worldwide, which included 1,800,000 children under 15 years of age, and 1,700,000 adolescents (10–19 years).1,2 Although the epidemic trend of global HIV infection was declining, children represented a growing share of people living with HIV worldwide and China had no exception.2 Shangcai County of Henan province is one of the counties with the highest rate of HIV infection in China. In late 1980s and early 1990s, a large number of rural residents were infected with HIV due to unhygienic paid blood donation, and a large number of children were perinatally infected from their mothers.3 The district government carried out the Highly Active Anti-Retroviral Therapy (HAART), social care, psychological counseling and so on for these HIV-infected children for more than 10 years.4
Standardized and effective HAART altered perceptions on HIV/AIDS from an epidemic to a manageable chronic illness, and reduced the probability of opportunistic infections including oral infections in adults as well as children.5 Despite dramatic declines in their incidence, opportunistic infections remained important causes of morbidity after HAART initiation in this regional cohort of HIV-infected children in Asia.6 Some studies revealed that the increased incidence of dental caries in children with HIV infection was due to intake of sugar-containing drugs, sugar-rich diet, Anti-Retroviral Therapy(ART), poor oral hygiene and reduced saliva flow rate.7,8 Nevertheless, developing countries still have a high prevalence of the main HIV-related oral manifestations because of the persistence of many risk factors, such as the difficulty to access treatment, poor oral hygiene, low socioeconomic status and delay in diagnosis.9
Oral diseases are commonly described to have an impact on quality of life because they may cause pain and compromise nutrition, speech and appearance.10 Children’s oral health‐related quality of life (OHRQoL) is related to parental socio‐economic status, dental anxiety, childhood dental anxiety, oral health behaviours.11 The high caries experiences have significant negative impacts on the children’s QoL, especially during the primary dentition period.12 Frequency of tooth brushing and dental visits, intervals between dental visits, negative dental experiences and dental anxiety are associated with dental caries.13 Brushing the teeth less than two times a day and viral load exceeding 10,000 HIV-RNA copies per millilitre of plasma were directly associated (p < 0.05) with a poorer oral health-related quality of life.14 Recognizing the factors that were associated with poorer OHR-QoL in children with AIDS may contribute to the planning of dental services for this population.
This study reported an observation on oral health status in HIV-infected young people in poor county of China to analyze the possible factors that affect their oral health status, and tried to provide references for HIV-related oral health prevention in young people.
Methods
Study Design, Settings and Participants
This study was conducted in Shangcai county of the Henan Province, one of the highest incidences of HIV-infection in China. There are totally 26 towns in this county. Using randomized stratified cluster sampling strategy, 6 towns were selected. Young people receiving HARRT who were 12–24 years old were invited into study, those who had used antibiotics in the past three months or suffer from systemic diseases were excluded.104 participants finished this observation study finally Participants who were 12–18 years old were defined as adolescences, and those who were19–24 years were defined as the young adults.
Survey Procedure
The doctor who was in charge of AIDS health care in the Shangcai county called the participants together with the help of the Center for Disease Control and the Prevention officer. All participants were received interviews and oral examinations individually by the same well-trained dentist.
The survey was conducted in line with the basic methods of oral health survey formulated by World Health Organization (WHO,2013, the fifth edition). The contents of interviews were as follows: (1) general information, such as education level, whether working or not, etc. (2) information on oral hygiene habits, such as brushing frequency, using dental floss and mouthwash or not, performing regular oral inspections or not, etc. (3) dental conditions, such as dental caries, loss, repair, biofilm index (BI), modified gingival index (MGI), calculus index (CI) and soft tissue diseases, etc. HIV transmission routes were obtained by checking the medical records of the participants. They could get a toothpaste set each as an appreciation gift for participation after finishing the interview.
The study was approved by the Ethics Committee of The First Affiliated Hospital at the School of Medicine of Zhejiang University, and conducted according to the Declaration of Helsinki principles. Written informed consents were obtained from participants or their guardians (<18 years old).
Data Collection
Paper questionnaires of General information and oral hygiene habits were obtained by face-to-face interviews. The questions were read one by one followed by the optional answers for every participant.
Oral examinations were implemented to assess Decayed, Missing, and Filled Teeth (DMFT), Biofilm Index (BI), Modified Gingival Index (MGI), Calculus Index (CI) and soft tissue diseases. The participants were examined with a headlamp, disposable oral examination trays, disposable gloves and masks while they were sitting on a chair in a comfortable position. The examiner used an electronic recorder to record the participant’s examinations, which would later be reorganized to paper and electronic versions.
Definition of Index
The BI was expressed in terms of Ribeiro index.15 BI was ranged from 0 to 5 grades. The definitions of the grades were described as follows: 0: No visible biofilm; 1: thin scattered biofilm on anterior or posterior teeth; 2: thin scattered biofilm on anterior and posterior teeth; 3: thick and firmly attached biofilm on anterior or posterior teeth; 4: thick and firmly attached biofilm and thin scattered biofilm on posterior teeth or thick and firmly attached biofilm on posterior teeth and thin scattered biofilm on anterior teeth; 5: thick and firmly attached biofilm on anterior and posterior teeth.
Gingival inflammation was evaluated using a simplified modified gingival index (MGI) that was based on that described by Lobene et al.16 The MGI was rated: 0: healthy gingiva, 1: minor inflammation of the gingiva, slight change in gingiva color and mild edema, 2: moderate inflammation of the gingiva, red color, and bright edema, and 3: severe inflammation, significantly swollen or ulcerated.
The supragingival calculus was visually assessed and rated using a 4-point ordinal scale that were based on the Löe and Silness calculus index. The CI was rated: 0: no supragingival calculus, 1: less than 1/3 of the tooth surface was covered by supragingival calculus, 2: 1/3 – 2/3 of the tooth surface was covered by supragingival calculus, 3: more than 2/3 of the tooth surface was covered by supragingival calculus.
DMFT index examination was performed using a dental probe and a dental mirror, followed by examining of decayed teeth due to caries (decay), missing/removed teeth due to caries (missing), teeth that were patched or filled with caries were examined. DMFT was the sum of the number of Decayed, Missing due to caries, and Filled Teeth in the permanent teeth. The mean number of DMFT was the sum of individual DMFT values divided by the sum of the participants.18
Laboratory Tests
CD4+ T cells count was detected by BECKMAN COULTER. The viral load was tested using a commercial HIV-1 monitor (Roche) kit by COBAS AmpliSensor-PCR. The operation was performed according to the kit instructions, and the results were expressed as viral copies per mL of plasma, with a minimum test limit of 50 copies.
Data Analysis
All data collected by paper-and-pencil surveys were input manually into a custom-designed database and analyzed using SPSS for Windows Version 16.0. We organized demographic information and examined whether there were differences between the scale for ages, education levels, working conditions, CD4+ T cells count. All tests were two-tailed and the significance level was set at p < 0.05. Differences between the groups were tested by Chi square test.
Results
General Information
A total of 104 participants were divided into two groups based on their ages: adolescence (12–18 years) group and young adult (19–24 years) group. The adolescence group consisted of 58 participants (33 males, 56.9%), with an average age of 16.29±1.79 years old. The young adult group consisted of 46 participants (27 males, 58.7%), with an average age of 21.89±1.82 years old. Most of the participants in both groups had low education level and received less than 9 years of education (65.5%, 63%). In the adolescence group, 28 (48.27%) participants already left the school and began to work. Meanwhile, only 2 participants went to universities in the young adult group, the rest of them 44 (95.65%) all started to work. Fifty-three participants (91.4%) in the adolescence group were infected due to mother-child transmission, and 31 (67.4%) participants in the young adult group were infected due to mother-child transmission. The average CD4+ T cells count in both groups was more than 500 (571 and 548), which indicated a good disease control. The average duration of HAART treatment of participants was over 10 years (10.28±2.89 and 11.10±2.72 years). See Table 1.
 |
Table 1 General Information of Participants |
Oral Hygiene Habits of Different Groups
Participants in both groups had poor oral hygiene habits. Most of the participants brushed their teeth once a day (55.20%, 67.40%). The participants who brushed their teeth twice a day were only 43.1% and 32.6%. Half of the participants brushed their teeth for 2 minutes (51.7%, 50.0%), while participants who brushed for more than 3 minutes were only 29.3% and 39.1%. The proportion of participants who used mouthwash in the young adult group (41.3%) was significantly higher than that in the adolescence group (24.1%). In addition, the participants in both groups hardly ever used dental floss after meals, and the majority of them never visited a dentist even if they had dental health problems (81%, 78.3%). See Table 2.
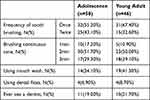 |
Table 2 Oral Health Behaviors in Two Groups |
Oral Health Status of Different Groups
As shown in Table 3, approximately 1/3 of the participants in the two groups had spontaneous bleeding. About 1/2 of the participants had gingival bleeding when brushing their teeth. The MGI of the adolescence group (0.98±0.09) was significantly lower than that of the young adult group (1.41±0.11)(P<0.05). However, the two groups had comparable Biofilm index (4.05±0.14, 4.02±0.18) and CI (1.71±0.11, 1.92±0.15).
 |
Table 3 Oral Health Hygiene in Two Groups |
Oral ulcers were the most common soft tissue diseases (15.52%, 24.14%) in both groups. One subject from young adult group suffered from mucosal fibrosis due to long-term chewing of areca. No characteristic linear gingival erythema and no obvious symptoms of candidiasis were observed in all the participants.
The prevalence of dental caries remained very high in both groups (82.76%, 84.8%). And there was no significant difference between the two groups (3.17±0.42, 3.87±0.58) concerning the decay-missing-filled index(DMFT). Few participants regularly visited the dentists. There were only 5 and 3 teeth were filled in the adolescence group and young adult group, respectively. There were 27 and 24 teeth that should be removed due to dental caries, and 19 and 12 teeth were lost in the adolescence and young adult, respectively. See Table 4.
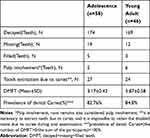 |
Table 4 Conditions of Permanent Teeth |
Discussion
The World Health Organization (WHO) has recently recognized the need to address the growing problem of oral health epidemics and also acknowledged poverty, inequality and systemic diseases as indicators.19 To prevent oral health in high-risk populations, oral health care should be paid more attention to prevent oral diseases.20 As it is known to all that the plaque should be carefully cleared with a toothbrush twice a day. The plaque between the teeth should be removed by using dental floss every 24 hours to prevent gingivitis and dental caries. The American Dental Association recommends brushing teeth twice a day for duration of at least 2 minutes.21 Evidence had shown that the oral hygiene efficacy increased with the time of brushing.22 Therefore, it is encouraged to brush the teeth for 2 minutes or more while the choice of toothbrush is less important. Increasing the brushing time might be the easiest way to control daily brushing effectively. Adherence to the recommended oral hygiene regimen is also regarded as a basic factor for the prevention and treatment of periodontal diseases and related tooth loss.23 In the present study, most of the participants lived in the rural areas and had low education level. After receiving 9 years of compulsory education, most of them started working instead of further education. Only two participants were studying in the university. They had poor consciousness to control dental plaques and maintain good oral hygiene habits. Only a small proportion of participants brushed their teeth twice a day for more than 3 minutes. They hardly ever used dental floss after the meal. Study showed that dental caries was also 1.5 times more among adolescents living with HIV who brush their teeth less frequently.24 Caries was lowest in HIV-positive children who brushed their teeth at least twice a day.25
Different articles have reported varied prevalence of caries in children with HIV infection. The highest prevalence of dental caries (86%) was observed in children with HIV infection in western Africa, while those in Nigeria had the lowest prevalence of caries (12.1%).26,27 In other countries, the prevalence rates of dental caries were all very high and the differences between them were not big.15,28–31 The journal of AIDS revealed that PHIV youth who received combination ART(cART)containing an integrase inhibitor had a significantly higher number of untreated active caries than those on cART without an integrase inhibitor in 2018.32 The prevalence of untreated caries was significant associated with low socioeconomic status eating habits (high frequency of sugar consumption) and poor clinical conditions (HIV viral load and severity of symptoms).33 The increase in the number of dental caries was also related to the reduction of salivary flow rate, high-sugar and high-carbohydrate diet, deficient oral hygiene and parental irresponsibility.34 DMFT scores were negatively associated with the CD4+ cell count in male children with HIV infected in Phnom, Cambodia.35 However, one study had pointed out that there was no statistical significance between the CD4+ cell count and caries in HIV-positive children treated with ART in Mangaluru, India.36 In this study, the prevalence of caries remained very high in both groups, which was 82.75% in the adolescence group and 84.8% in the young adult group. Participants in this study had received HAART for a long period, and achieved good therapeutic effect. The high prevalence of caries might be related to poor oral hygiene habits and low education level. At the same time, further research should pay attention to the participants’ salivary flow rate, dietary preferences and parents’ oral health awareness as well.
Most parents were unaware of their children’s oral hygiene. For this reason, educational interventions should be provided to increase the parents’ knowledge and skill. They should be educated to realize their children’s dental needs, especially for those low-socio-economy parents and whose children with poor oral hygiene.37 It was reported that teenagers whose parents had high educational achievement used flossing regularly.38 Previous studies have revealed that educational pattern videos, lectures and pamphlets for the parents and children exerted similar effect on reducing the plaque index.39 To improve children’s oral hygiene, parents should encourage them to brush their teeth from their young age, and supervise brushing until they get used to the coordination of muscle movements for effective cleaning.40 It’s true that children’s oral health prevention needs the attention and guidance of parents.
However, this study has some limitations. All participants were only received oral consultations and examinations, and did not undergo X-ray examinations, adjacent caries, periodontal indexes such as periodontal exploration and bleeding on probing were not examined. At the same time, the comparison between the control group of similar age in the same area was not included.
Conclusion
In conclusion, the participants in this study had a high prevalence of caries, and a high demand for oral treatment, at the same time they had low education level, poor oral hygiene habits, and most of them did not have consciousness to visit dentists. The level of economic and education affected the awareness of oral health and care. It is strongly recommended to encourage good oral hygiene habits (correct brushing and flossing) and timely dental clinic, so as to keep oral health for these particular group of young people.
Acknowledgments
We would like to thank Mrs. Xin Zhang for the help in language polishing for this publication. We also wanted to thank all the staffs in Shangcai Center for Disease Control and Prevention for their support and endeavor for this study.
Funding
This study was supported by the Medical and Health Science and Technology Project of Zhejiang(2014KYA243), and the Zhejiang Provincial Natural Science Foundation of China (LQ19H280007), and the National Natural Science Foundation of China (No.81602943), and the Mega-Project for National Science and Technology Development (2017ZX10105001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Latest HIV estimates and updates on HIV policies uptake. World Health Organization; November 2020. Available from: https://www.who.int/docs/default-source/hiv-hq/latest-hiv-estimates-and-updates-on-hiv-policies-uptake-november2020.pdf?sfvrsn=10a0043d_12.
2. Reimagining a resilient HIV response for children, adolescents and pregnant women living with HIV. UNICEF; 2020. Available from: https://data.unicef.org/resources/world-aids-day-report-2020/.
3. Cheng Y, Lou C-H, Mueller LM, et al. Effectiveness of a school-based AIDS education program among rural students in HIV high epidemic area of China. J Adolesc Health. 2008;42(2):184–191. doi:10.1016/j.jadohealth.2007.07.016
4. Zhou E, Qiao Z, Cheng Y, et al. Factors associated with depression among HIV/AIDS children in China. Int J Ment Health Syst. 2019;13:10. doi:10.1186/s13033-019-0263-1
5. Gaitán-Cepeda LA, Sánchez-Vargas O, Castillo N. Prevalence of oral candidiasis in HIV/AIDS children in highly active antiretroviral therapy era. A literature analysis. Int J STD AIDS. 2015;26(9):625–632. doi:10.1177/0956462414548906
6. Prasitsuebsai W, Kariminia A, Puthanakit T, et al. Impact of antiretroviral therapy on opportunistic infections of HIV-infected children in the therapeutic research, education and AIDS training asia pediatric HIV observational database. Pediatr Infect Dis J. 2014;33(7):747–752. doi:10.1097/INF.0000000000000226
7. dos Santos Pinheiro R, França TT, Ribeiro CMB, Leão JC, de Souza IPR, Castro GF. Oral manifestations in human immunodeficiency virus infected children in highly active antiretroviral therapy era. J Oral Pathol Med. 2009;38(8):613–622. doi:10.1111/j.1600-0714.2009.00789.x
8. Ramos-Gomez FJ, Folayan MO. Oral health considerations in HIV-infected children. Curr HIV/AIDS Rep. 2013;10(3):283–293. doi:10.1007/s11904-013-0163-y
9. Ottria L, Lauritano D, Oberti L, et al. Prevalence of HIV-related oral manifestations and their association with HAART and CD4+ T cell count: a review. J Biol Regul Homeost Agents. 2018;32(2Suppl. 1):51–59.
10. Coogan MM, Greenspan J, Challacombe SJ. Oral lesions in infection with human immunodeficiency virus. Bull World Health Organ. 2005;83(9):700–706. doi:10.1590/S0042-96862005000900016
11. Buldur B, Güvendi ON. Conceptual modelling of the factors affecting oral health-related quality of life in children: a path analysis. Int J Paediatr Dent. 2020;30(2):181–192. doi:10.1111/ipd.12583
12. Masiga MA, M’Imunya JM. Prevalence of dental caries and its impact on quality of life (QoL) among HIV-infected children in Kenya. J Clin Pediatr Dent. 2013;38(1):83–87. doi:10.17796/jcpd.38.1.62l1q94650j5l815
13. Buldur B. Pathways between parental and individual determinants of dental caries and dental visit behaviours among children: validation of a new conceptual model. Community Dent Oral Epidemiol. 2020;48(4):280–287. doi:10.1111/cdoe.12530
14. Massarente DB, Domaneschi C, Marques HHS, Andrade SB, Goursand D, Antunes JLF. Oral health-related quality of life of paediatric patients with AIDS. BMC Oral Health. 2011;11:2. doi:10.1186/1472-6831-11-2
15. de Aguiar Ribeiro A, Portela MB, de Souza IPR. The oral health of HIV-infected Brazilian children. Int J Paediatr Dent. 2013;23(5):359–365. doi:10.1111/ipd.12008
16. Lobene RR, Mankodi SM, Ciancio SG, Lamm RA, Charles CH, Ross NM. Correlations among gingival indices: a methodology study. J Periodontol. 1989;60(3):159-162. doi:10.1902/jop.1989.60.3.159
17. J Periodontol.Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi:10.3109/00016356408993968
18. Klein H, Palmer CE, Knutson JW. Studies on dental caries: I. Dental status and dental needs of elementary school children. Public Health Rep. 1938;53(19):751. doi:10.2307/4582532
19. Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl 1):3–23. doi:10.1046/j..2003.com122.x
20. Holmes HK, Stephen LXG. Oral lesions of HIV infection in developing countries. Oral Dis. 2002;8(Suppl 2):40–43. doi:10.1034/j.1601-0825.2002.00010.x
21. Van der Weijden GA, Timmerman MF, Nijboer A, Lie MA, Van der Velden U. A comparative study of electric toothbrushes for the effectiveness of plaque removal in relation to toothbrushing duration. Timerstudy. J Clin Periodontol. 1993;20(7):476–481. doi:10.1111/j.1600-051x.1993.tb00394.x
22. van der Weijden GA, Timmerman MF, Danser MM, Piscaer M, Ijzerman Y, van der Velden U. Approximal brush head used on a powered toothbrush. J Clin Periodontol. 2005;32(3):317–322. doi:10.1111/j.1600-051X.2005.00700.x
23. Eickholz P, Kaltschmitt J, Berbig J, Reitmeir P, Pretzl B. Tooth loss after active periodontal therapy. 1: patient-related factors for risk, prognosis, and quality of outcome. J Clin Periodontol. 2008;35(2):165–174. doi:10.1111/j.1600-051X.2007.01184.x
24. Malele Kolisa Y, Yengopal V, Shumba K, Igumbor J, Bohlius J. The burden of oral conditions among adolescents living with HIV at a clinic in Johannesburg, South Africa. PLoS One. 2019;14(10):e0222568. doi:10.1371/journal.pone.0222568
25. Masiga MA, Machoki JM. Correlation of oral health home-care practices, snacking habits and dental caries experience among HIV-positive children in nairobi, Kenya. East Afr Med J. 2012;89(7):217–223.
26. Meless D, Ba B, Faye M, et al. Oral lesions among HIV-infected children on antiretroviral treatment in West Africa. Trop Med Int Health. 2014;19(3):246–255. doi:10.1111/tmi.12253
27. Oyedeji OA, Gbolahan OO, Abe EO, Agelebe E. Oral and dental lesions in HIV infected Nigerian children. Pan Afr Med J. 2015;20:287. doi:10.11604/pamj.2015.20.287.5273
28. Burnett D, Aronson J, Asgary R. Oral health status, knowledge, attitudes and behaviours among marginalized children in Addis Ababa, Ethiopia. J Child Health Care. 2016;20(2):252–261. doi:10.1177/1367493515569328
29. Rovaris N, Galato D, Schuelter-Trevisol F, et al. Oral health status and its impact on the quality of life of children and adolescents living with HIV-1. BMC Res Notes. 2014;7(1):478. doi:10.1186/1756-0500-7-478
30. Coker M, El-Kamary SS, Enwonwu C, et al. Perinatal HIV infection and exposure and their association with dental caries in Nigerian children. Pediatr Infect Dis J. 2018;37(1):59–65. doi:10.1097/INF.0000000000001702
31. Starr JR, Huang Y, Lee KH, et al; for the Pediatric HIV/AIDS Cohort Study. Oral microbiota in youth with perinatally acquired HIV infection. Microbiome. 2018;6(1):100. doi:10.1186/s40168-018-0484-6
32. Shiboski CH, Yao T-J, Russell JS, et al. The association between oral disease and type of antiretroviral therapy among perinatally HIV-infected youth. AIDS. 2018;32(17):2497–2505. doi:10.1097/QAD.0000000000001965
33. Massarente DB, Domaneschi C, Antunes JLF. Untreated dental caries in a Brazilian paediatric AIDS patient population. Oral Health Prev Dent. 2009;7(4):403–410.
34. Soares LF, de araújo castro GFB, de Souza IPR, Pinheiro M. Pediatric HIV-related oral manifestations: a five-year retrospective study. Braz Oral Res. 2004;18(1):6–11. doi:10.1590/s1806-83242004000100002
35. Kikuchi K, Furukawa Y, Tuot S, Pal K, Huot C, Yi S. Association of oral health status with the CD4+ cell count in children living with HIV in Phnom Penh, Cambodia. Sci Rep. 2019;9(1):14610. doi:10.1038/s41598-019-51077-0
36. Muraleedharan S, Panchmal GS, Shenoy RP, Jodalli P, Sonde L, Pasha I. Correlation of CD4 count with cariogenic oral flora indicators and dental caries in HIV-seropositive children undergoing antiretroviral therapy in Mangaluru, South India. J Investig Clin Dent. 2018;9(2):e12292. doi:10.1111/jicd.12292
37. Shaghaghian S, Savadi N, Amin M. Evaluation of parental awareness regarding their child’s oral hygiene. Int J Dent Hyg. 2017;15(4):e149–e155. doi:10.1111/idh.12221
38. Wigen TI, Wang NJ. Characteristics of teenagers who use dental floss. Community Dent Health. 2021;38(1):10–14. doi:10.1922/CDH_00006Wigen05
39. Ramezaninia J, Naghibi Sistani MM, Ahangari Z, Gholinia H, Jahanian I, Gharekhani S. Comparison of the effect of toothbrushing education via video, lecture and pamphlet on the dental plaque index of 12-year-old children. Children. 2018;5(4). doi:10.3390/children5040050
40. Pujar P, Subbareddy VV. Evaluation of the tooth brushing skills in children aged 6–12 years. Eur Arch Paediatr Dent. 2013;14(4):213–219. doi:10.1007/s40368-013-0046-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
