Back to Journals » International Journal of Nanomedicine » Volume 13 » T-NANO 2014 Abstracts
Oral bioavailability enhancement of agomelatine by loading into nanostructured lipid carriers: Peyer’s patch targeting approach
Authors Prajapati JB, Verma SD, Patel AA
Received 14 October 2016
Accepted for publication 21 November 2016
Published 15 March 2018 Volume 2018:13(T-NANO 2014 Abstracts) Pages 35—38
DOI https://doi.org/10.2147/IJN.S124703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jagruti B Prajapati, Sneha D Verma, Amit A Patel
Department of Ppharmaceutics and Ppharmaceutical Technology, Rramanbhai Patel College of Ppharmacy, Charotar University of Science and Technology, Changa, Gujarat, India
Abstract: Agomelatine (AGM) is a new antidepressant drug with a novel mechanism of action and fewer side effects compared with older antidepressants. AGM is a melatonin receptor (MT1 and MT2) agonist and 5-hydroxytryptamine receptor (5-HT2C) antagonist. In the present study, the enhancement of the oral bioavailability of AGM was formulated and loaded into nanostructured lipid carriers (NLCs), using ultrasonication method. In vitro and ex vivo drug release was performed using a dialysis bag and rat duodenum, respectively. Our pharmacodynamic study showed that AGM–NLCs are more efficacious than a pure drug and marketed product, and confocal microscopy revealed lymphatic uptake of AGM–NLCs. The present study concluded that the NLCs enhanced the oral bioavailability of AGM (6.5-fold) by avoiding its first-pass metabolism by way of lymphatic uptake.
Keywords: agomelatine, nanostructured lipid carriers, antidepressant drug
Introduction
Agomelatine (AGM) is a new antidepressant drug with a novel mechanism of action and fewer side effects compared with older antidepressants, such as selective serotonin reuptake inhibitors and tricyclic antidepressants. AGM is a melatonin receptor (MT1 and MT2) agonist and 5-hydroxytryptamine receptor (5-HT2C) antagonist. It undergoes extensive first-pass metabolism showing poor oral bioavailability (<5%). The purpose of this study was to develop a delivery system capable of improving its oral bioavailability. It was hypothesized that by formulating AGM loaded into nanostructured lipid carriers (NLCs) and targeting Peyer’s patch, the oral bioavailability could be increased and hence the therapeutic effect also increased. It can also be effective long term by having a controlled release of the drug from NLCs. NLCs are second-generation lipidic nanoparticles, which have gained the interest of scientists for the oral delivery of drugs. By developing a system like NLCs, one can enhance the solubility, and as the system is lipidic in nature and nano size, NLCs can directly and easily get absorbed by the M cells of Peyer’s patches. It can also avoid first pass metabolism due to their nano size.
Materials and methods
Materials
The materials used were AGM (Servier, Suresnes, France), Dynasan 118 (Sasol Germany GmbH, Hamburg, Germany), Labrafil M 2125 CS (Gattefosse, Mumbai, India), Tween-80 (Loba Chemicals, Gujarat, India), trehalose (Sigma Aldrich, Gujarat, India), Rhodamine dye (Sigma Aldrich, Gujarat, India), methanol (Loba Chemicals, Gujarat, India), acetonitrile (Loba Chemicals, Gujarat, India), and chloroform (Loba Chemicals, Gujarat, India).
Methods
Preformulation study
Preformulation studies, such as melting point determination, Fourier transform infrared (FT-IR), X-ray diffraction (XRD), differential scanning calorimetry (DSC), solubility study, and wavelength determination were performed for the identification of AGM and excipients and for checking drug–excipient compatibility.
Preliminary optimization study
Selection of solid lipid was done based on solubility and partition coefficient studies. Screening of oil was done by saturation solubility study. Selection of surfactants was done by comparing mean particle size (MPS) and percentage entrapment efficiency (%EE) of batches prepared using different surfactants.
Optimization of AGM–NLCs
AGM–NLCs were prepared by ultrasonication method. The AGM–NLCs were optimized using Box–Behnken design by considering independent variables: 1) formulation variables – total lipid, amount of oil, and surfactant concentration; and 2) process variables – sonication time and dependent variables: MPS and %EE.1 Check point analysis was also done. Batch was optimized by desirability function.
Lyophilization of AGM–NLCs
AGM–NLCs were lyophilized using trehalose (a cryoprotectant) to increase the stability. Ratio of AGM–NLCs to trehalose was optimized on the basis of MPS and %EE.
Characterization of AGM–NLCs
The optimized AGM–NLCs were characterized for MPS and zeta potential (Zeta Sizer), %EE, and morphology (transmission electron microscopy).2
Drug release study
In vitro and ex vivo drug release (protocol no: RPCP/IAEC/2013-14/MPH-PT-33) was performed using a dialysis bag and rat duodenum, respectively.2 Samples were taken at predetermined time intervals and the drug was analyzed by ultra-violet–spectrophotometer.
Ex vivo cellular uptake study
Permeability of AGM–NLCs using rat duodenum was done by confocal laser scanning microscopy (CLSM) of rhodamine-labeled AGM–NLCs.
In vivo study (pharmacodynamic and pharmacokinetic)
Pharmacodynamic study (protocol no: RPCP/IAEC/2013-14/MPH-PT-39) was done to check the efficacy of AGM–NLCs compared with a pure drug (dispersion) and marketed product (Agotine tablet; Precise Chemipharma Pvt ltd, Nashik, India). This study was performed by a forced swim test with an animal model.
Pharmacokinetic study (protocol no: RPCP/IAEC/2013-14/MPH-PT-40) was performed to find out the Cmax, Tmax, area under the curve (AUC), and the oral bioavailability of AGM–NLCs, pure drug (dispersion), and marketed product (Agotine tablet). This study was performed using Sprague Dawley rats.3
All animal experiments were approved by the Institutional Animal Ethics Committee of Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, Charusat, and followed the IAEC guidelines for animal research.
Hemolytic study
A hemolytic study was performed to check the toxicity of prepared AGM-NLCs on RBCs. AGM-NLCs were taken as a test and were compared with the standard of triton as it considerably damages RBCs.
Stability study
A stability study of AGM–NLCs was done for 45 days at room temparature (25°C) and refrigerated conditions (2°C–4°C), and MPS and %EE were measured at particular time intervals.
Results and discussion
Preformulation study
Results of melting point and wavelength determination, FT-IR, XRD, DSC, and solubility study showed that the drug and excipients are pure and compatible.
Preliminary optimization study
Dynasan 118, Labrafil M 2125 CS, and Tween-80 were selected as solid lipid, oil, and surfactant, respectively, based on the solubility and partition coefficient study, MPS, and %EE.
Optimization of AGM–NLCs
From all 17 batches of Box–Behnken design, one batch was optimized having the least MPS and highest %EE, which has 1 desirability. Statistical test was also applied. Contour plots and 3D surface response curves were also obtained from the software.3 As a process parameter, a sonication time of 6 minutes was optimized based on MPS and %EE.
Lyophilization of AGM–NLCs
Optimized ratio of AGM–NLCs to trehalose was 1:3.
Characterization of AGM–NLCs
Optimized AGM-NLCs having MPS 169 nm, %EE: 91.64%±2.9%, zeta potential: −21.3 (Figure 1 of zeta potential indicates stable formulation).
  | Figure 1 Zeta potential of agomelatine–nanostructured lipid carriers. |
Results of TEM (Figure 2) show that particles were spherical in shape and had an MPS of 165 nm.
  | Figure 2 Transmission electron microscopic image of agomelatine–nanostructured lipid carriers. |
Drug release study
The percentage cumulative drug release was obtained for 31 hours (Table 1), indicating controlled release, which followed the Higuchi model.
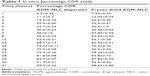  | Table 1 In vitro percentage CDR study |
Ex vivo cellular uptake study (permeability study, protocol no: RPCP/IAEC/2013-14/MPH-PT-33).
The CLSM showed that rhodamine-labeled AGM–NLCs were transported from the lumen of duodenum to the wall to the blood (Figure 3).
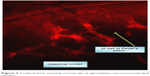  | Figure 3 Confocal laser scanning microscopy of agomelatine–nanostructured lipid carriers. |
In vivo study (pharmacodynamic and pharmacokinetic: protocol no: RPCP/IAEC/2013-14/MPH-PT-39, 40).
Pharmacodynamic study results indicated that the AGM–NLCs were more efficacious than pure drug and marketed product.
Pharmacokinetic study results indicated that Cmax, AUC, and oral bioavailability of AGM–NLCs was higher compared with a pure drug and marketed product (Table 2). Oral bioavailability of AGM–NLCs was increased by 6.5-fold.
  | Table 2 Pharmacokinetic parameters |
Haemolytic study
Results of the percentage hemolysis showed that AGM–NLCs were not toxic.
Stability study
Results of the stability study showed that AGM–NLCs were stable for up to 45 days (Table 3).
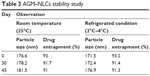  | Table 3 AGM–NLCs stability study |
Conclusion
AGM was loaded into NLCs with the objective of enhancing its oral bioavailability. The in vivo studies indicated enhanced bioavailability of AGM–NLCs compared with AGM suspension and market formulation. The CLSM images revealed lymphatic uptake of AGM–NLCs. Therefore, it can be concluded that the NLCs enhanced the oral bioavailability of AGM (6.5-fold) by avoiding its first-pass metabolism by way of lymphatic uptake.
Acknowledgment
The authors would like to thank the staff from Ramanbhai Patel College of Pharmacy, CHARUSAT University, in particular the principal Dr RH Parikh and Mr Kiran Jani.
Disclosure
The authors report no conflicts of interest in this work.
References
Sanad RA, Abdelmalak NS, Elbayoomy TS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS PharmSciTech. 2010;11(4):1684–1694. | ||
Sahu MK, Soni GC, Prajapati SK. Formulation and characterization of topical nanostructured lipid carrier gel of flurbiprofen and its comparison with micellar gel preparation. World J Pharm Sci. 2012;1(3): 1235–1247. | ||
Zhang XY, Qiao H, Ni JM, Shi YB, Qiang Y. Preparation of isoliquiritigenin-loaded nanostructured lipid carrier and the in vivo evaluation in tumor-bearing mice. Eur J Pharm Sci. 2013;49(3):411–422. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
