Back to Journals » Infection and Drug Resistance » Volume 15
Oral Bacteria Dysbiosis in Patients with Linezolid-Induced Black Hairy Tongue: A Case Series
Authors Shangguan Y, Ji Z, Guo W, Hu W, Li X, Xu K
Received 3 May 2022
Accepted for publication 15 August 2022
Published 14 September 2022 Volume 2022:15 Pages 5449—5454
DOI https://doi.org/10.2147/IDR.S373266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Yanwan Shangguan,* Zhongkang Ji,* Wanru Guo, Wenjuan Hu, Xiaomeng Li, Kaijin Xu
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kaijin Xu, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China, Tel/Fax +86-0571-87236440, Email [email protected]
Abstract: Linezolid-induced black hairy tongue is a self-limiting benign disease that is rare. Here, we report three patients who developed black hairy tongue after linezolid treatment. The severe dysbiosis of oral bacterial communities was observed in all these patients. Proteobacteria was the most prevalent phylum (over 90%) at the black tongue stage. Furthermore, the dramatic oral bacterial alteration took a long time to reverse after the BHT resolved.
Keywords: tuberculosis infection, linezolid, treatment, side-effect, black-hairy tongue, microorganism metabolism
Introduction
Linezolid is a synthetic antibiotic, belonging to oxazolidinones, with unique bacteriostatic activity against antibiotic-resistant Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci.1 Linezolid has been proved to be effective against M. tuberculosis isolates2 and is currently recommended as Group A core agent for treating multidrug-resistant tuberculosis (MDR-TB) based on the World Health Organization (WHO) guidelines.3 Linezolid has extremely high oral bioavailability closing to 100% in human. It inhibits the early steps of protein synthesis in Gram-positive bacteria by binding to the 23S site of ribosomal RNA on the 50S subunit and preventing the formation of the 70S translation initiation complex.1 The common linezolid-induced adverse events include myelosuppression, lactic acidosis, peripheral neuropathy and optic neuropathy, which are closely associated with prolonged treatment duration.4,5 Black hairy tongue (BHT) is also a rare adverse effect associated with Linezolid.6,7 BHT is characterized by pigmentation of the dorsal tongue which appears black, or green or yellow, and may be accompanied by hypertrophy of the filiform papillae.8–10 The prevalence of black hairy tongue varies highly in the general population ranging from 0.6% to 11.3% and is significantly higher in males.11,12 Here, we report three male patients with BHT induced by linezolid during the anti-tuberculosis treatment, and the dynamic changes of the oral bacterial profiles during discoloration and recovery stages were investigated by 16S rRNA sequencing.
Case Presentations
Case Report 1
A 74-year-old man was admitted with fever, severe cough, and yellow sputum for 1 month. He was diagnosed with lung adenocarcinoma (cT4N2M0, IIIB) 3 months ago and received 2 cycles of pemetrexed-cisplatin adjuvant chemotherapy. He had a history of tuberculosis and a 10-year history of smoking but had quit for 4 years. He maintains a good oral hygiene. Chest Computed tomography (CT) scan showed: (1) multiple lesions in bilateral lobe of the lung; (2) enlarged hilar and mediastinal lymphadenopathy; (3) infection lesions in the lower lobe of the left lung. Repeated sputum acid-fast bacteria (AFB) smear staining was positive. Sputum GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA) and culture were positive and sensitive to rifampicin. Considering the retreatment of pulmonary tuberculosis, isoniazid (H), rifampin (R), ethambutol (E), pyrazinamide (Z), moxifloxacin were used as the anti-tuberculosis regimen, and linezolid 600 mg once daily was added to strengthen the regimen. The patient’s temperature returned to normal after 5 days of treatment, and cough was significantly resolved after 2 weeks of treatment. However, 12 days after the initiation of linezolid treatment, the patient complained of a black tongue without any other symptoms (Figure 1A). Tongue swab culture showed Enterobacter kobei. Considering that this side effect was related to linezolid, we discontinued linezolid and the tongue discoloration returned to normal within 17 days.
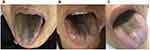 |
Figure 1 The black tongue coat on the patient tongues. (A) Patient 1; (B) Patient 2; (C) Patient 3. |
Case Report 2
A 67-year-old man was admitted with dry cough and shortness of breath. He had no smoking history and oral hygiene was good. Chest CT revealed massive bilateral pleural effusion. Meanwhile, abdominal ultrasound and echocardiography showed moderate ascites and pericardial effusion. Pleural fluid T-SPOT.TB result was positive. The patient was considered to have tuberculous polyserositis and started HRZE diagnostic anti-TB treatment. However, the patient then developed weakness, loss of appetite, oliguria, decreased pulse pressure. Considering the signs of pericardial tamponade, an emergency pericardiectomy was performed. Thickened pericardium adhesions were seen during the operation, some of which were obviously calcified and narrowed, and some were cheese-like lesions. Histopathology of the pericardium showed fibrous tissue hyperplasia, collagenization with exudation of inflammatory necrosis, and a positive TB FISH result. A diagnosis of tuberculous constrictive pericarditis was confirmed after surgery. We adjusted the anti-tuberculosis regimen to meropenem, moxifloxacin, isoniazid, rifampin, he complained of worsening chest tightness, and large extensive pleural fluid was seen in chest CT after one month of treatment. Linezolid 600mg once daily along with steroids was then added to the anti-TB regimen. Thirteen days after intake of linezolid, asymptomatic black tongue was observed (Figure 1B), and tongue swab detected Escherichia coli. After completing 2 months of the anti-TB regimen containing linezolid, his tongue discoloration disappeared 10 days after discontinuation of linezolid.
Case Report 3
A 37-year-old man presented with low-grade fever, persistent headache, and night sweats for a month duration. He had no smoking history and oral hygiene was good. Chest CT showed multiple nodules. Magnetic resonance imaging (MRI) of the brain showed multiple low-density lesions and small abscesses with surrounding edema. The sputum and cerebrospinal fluid GeneXpert MTB/RIF assay indicated positive and further culture proved TB infection. The patient was diagnosed with pulmonary tuberculosis with tuberculous meningitis. He was treated with isoniazid, rifampicin, pyrazinamide, ethambutol, and linezolid 600 mg daily in combination with steroids, and the patient’s headache relieved within two weeks. Brain MRI was repeated after 4 weeks, which showed decreased abscesses and absorbed edema. However, after 28 days of the above anti-tuberculosis treatment, the patient experienced abnormal taste and tongue black discoloration (Figure 1C). Tongue swab culture showed Klebsiella pneumoniae. Upon cessation of linezolid for 15 days, his tongue returned to normal. The patient later met the criteria to be cured of tuberculosis.
Methods
Oral Sample Collection and Assay
To investigate the possible role of oral microbiota alteration in development at the black tongue stage and the recovery stage. 10mL Saliva samples were collected was collected into a 50 mL EDTA tube from three patients by gently rolling a swab under the tongue and between the gum and the cheek. Patients were asked not to drink or eat for at least 8 h before sampling. Tongue coat swabs were taken by swabbing the tongue coat surface by tenth rotational movements.
0.5 mL trypsin was added to samples that were centrifuged for 10 min at 4500g. Then discard the supernatant, add 0.5mL of DNA stabilizer to the precipitate, mixed it well with a disposable pipette, transferred it all into 2mL hard-walled grinding tube. The samples were put into the specimen storage box and stored at −80 ◦C until further analysis.
Bacterial DNA was extracted with the CTAB/SDS method following the manufacturer’s recommendations. 16S rRNA genes of V3-V4 region were amplified with the barcode. All PCRs were performed with Phusion High-Fidelity PCR Master Mix (New England Biolabs). After purification of the PCR products, sequencing libraries were constructed using a TruSeq DNA PCR-free Sample Preparation Kit. The constructed library was quantified by Qubit and Q-PCR, and after the library was qualified, the library was sequenced using NovaSeq6000.
Results
The results of the 16s rRNA sequencing revealed that the patients suffered dramatic dysbiosis of normal oral microbiota. The analysis of the saliva sample at black tongue stage showed that a total of 167 operational taxonomic units (OUTs) were found in Patient 1, 627 OUTs were found in Patient 2, and 249 OUTs were found in Patient 3. Whereas, in the black tongue coat sample, a total of 197 OUTs were found in Patient 1, 281 OUTs were found in Patient 2 and Patient 3, respectively (Figure 2). Overall, we observed that the diversity of bacteria in saliva and tongue coat was similar, Proteobacteria was the most prevalent phylum level (over 90%) at the black tongue stage in all three patients (Figure 3). The differences were found in genus level among the three patients. At the black tongue stage, Haemophilus (44.39%) was the most prevalent genus in Patient 1 and 3. Lautropia (52.00%) was the most prevalent genus in Patient 2. At the recovery stage, despite Proteobacteria phylum remained dominant, its abundance decreased in these patients, and Frimicutes phylum and Fusobacteria phylum increased as the discoloration resolved.
Discussion
BHT is a rare benign disorder which is more common in men, elderly, smokers, HIV-positive patients, and cancer patients.11 We performed the drug causality assessment (Naranjo) in our three patients with a final score of 8, which supported a probable adverse drug reaction of linezolid on BHT. The underlying predisposing factors of tongue discoloration include smoking, poor oral hygiene, psychotropics (eg, olanzapine), antibiotics (eg, tetracyclines, metronidazole).12–14 Differential diagnoses include oral hairy leukoplakia, acanthosis nigricans, pigmented fungiform papillae, melanocytic nevi, and pseudo BHT (like food coloring).9 Dermoscopy can help to confirm.15
Cases of linezolid-induced BHT commonly suffer from poor appetite and loss of taste. Tongue discoloration occurs in nearly two week or long-term use of linezolid and can be resolved 2–3 weeks after linezolid discontinuation,6 but the BHT may recur after reintroduction of linezolid.16 However, little is known about the alterations in oral bacteria on the black tongue and the correlation between these changes and the occurrence of the black tongue. To our knowledge, this is the first study to report the dysbiosis of oral microbiota composition in patients with BHT.
In the healthy people, oral bacteria are predominantly composed of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, accounting for 96% of total oral bacteria.17 And diet and environment have little effect on the composition on gut microbiome. The physiological activities of oral symbiotic flora and its metabolites may have beneficial effects. Our study found in these BHT patients Proteobacteria was dominant in oral bacteria, contain above 90% of the taxa, which overwhelmingly disturbed the oral microenvironment. Proteobacteria were found to be significantly enriched in the feces of TB patients, which contain numerous gram-negative bacteria and opportunistic pathogenic species.18 Enterobacter kobei, Escherichia coli, and Klebsiella pneumoniae were detected in patient’s tongue swabs, respectively, which were considered to be microbial colonization due to dysbiosis of the oral bacteria.
The microbial community of human respiratory tract is continuous.19 Although the respiratory tract microbial communities varied in terms of diversity, oropharynx samples can be used to analyze the alteration of community structure associated with tuberculosis.20 Firmicutes, Actinobacteria and Proteobacteria are significantly higher in sputum samples from TB patients when compared to healthy controls,21 but the extreme imbalances have not been seen. The diversity indices of samples from healthy patients were significantly higher than that of TB patients. The dynamics of oral microorganisms and the balance of their composition and richness may be disrupted, leading to disorders of oral microorganism metabolism in TB patients. Antibiotics have a great impaction microbiota alters, resulting in continuously reducing microbial community diversity.22 Linezolid largely exacerbated the oral bacteria dysbiosis in TB patient, which may associate with BHT. Furthermore, the dramatic oral bacterial alteration needs a long time to reverse after the BHT resolved.
Only three patients were included in this study, the data may be hard to be generalized. However, our findings could provide the basis for subsequent studies and the data provide a complete spectrum of changes in microbiota related to linezolid which can fill in the knowledge gaps. We are willing to future conduct multicenter studies to include more linezolid-induced black hairy tongue patients for analysis and to do further research to reveal the relationship between black hairy tongue and oral bacterial alteration and strive to explore solutions to balance the alteration of the oral microbiota possibly responsible for the hairy tongue.
BHT is a self-limiting benign disease; however, it may raise aesthetic concerns,11 which can cause non-compliance. It has been shown that merely minor degrees of non-adherence or missed doses significantly increased the risk of unfavorable outcomes of TB treatment.23 Physicians need more patience and communication skill to address the patient’s tensions to make them benefit from anti-TB treatment. BHT caused by linezolid will resolve within 1–2 weeks after discontinuation of linezolid. During the period of tongue discoloration, patients should maintain good oral hygiene, brush the dorsum of tongue avoid predisposing factors.
Conclusion
Linezolid induced black hairy tongue is a rare and self-limiting benign disease. The intense oral bacteria dysbiosis induced by linezolid was observed in these patients and may be related to BHT. Proteobacteria was the most prevalent phylum (over 90%) at the black tongue stage. Furthermore, the dramatic oral bacterial alteration took a long time to reverse after the BHT resolved.
Abbreviations
BHT, black hairy tongue; MDR-TB, multidrug-resistant tuberculosis; WHO, World Health Organization; CT, Computed tomography; H, isoniazid; R, rifampin; E ethambutol, Z pyrazinamide; MRI, Magnetic resonance imaging; OUTs, operational taxonomic units.
Data Sharing Statement
The raw datasets presented in this study are available from the corresponding author on reasonable request.
Ethics Statement and Informed Consent
Written informed consent was provided by the patients to allow the case details and any accompanying images to be published, and this report was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Approval number: IIT20220319B).
Acknowledgments
We would like to thank the patients for their support. Thanks to Ming Hu and Xuewen Feng for their contribution in the revision of this article.
Disclosure
The authors declare that they have no conflict of interest in this work.
References
1. Pharmaceutical D. Index M. Linezolid. 2008;88:122–125.
2. Lee JK, Lee JY, Kim DK, et al. Substitution of ethambutol with linezolid during the intensive phase of treatment of pulmonary tuberculosis: a prospective, multicentre, randomised, open-label, Phase 2 trial. Lancet Infect Dis. 2019;19(1):46–55. doi:10.1016/S1473-3099(18)30480-8
3. World Health Organization. Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva: World Health Organization; 2019.
4. Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46(8):2723–2726. doi:10.1128/AAC.46.8.2723-2726.2002
5. Santini A, Ronchi D, Garbellini M, Piga D, Protti A. Linezolid-induced lactic acidosis: the thin line between bacterial and mitochondrial ribosomes. Expert Opin Drug Saf. 2017;16(7):833–843. doi:10.1080/14740338.2017.1335305
6. Luo S, Luo Q, Gao X, Li J. Adverse reaction report and retrospective analysis of black hairy tongue caused by linezolid. Respir Med Case Report. 2020;31:101159. doi:10.1016/j.rmcr.2020.101159
7. Sivaraman S, Gomathi Radhakrishnan G, Manogaran R, Cheriyan BV. An uncommon side effect of linezolid: black hairy tongue. Precision Med Sci. 2021;10(4):167–169. doi:10.1002/prm2
8. Refaat M, Hyle E, Malhotra R, Seidman D, Dey B. Linezolid-induced lingua villosa Nigra. Am J Med. 2008;121(6):9343. doi:10.1016/j.amjmed.2008.02.023
9. Schlager E, St Claire C, Ashack K, Khachemoune A. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18(4):563–569. doi:10.1007/s40257-017-0268-y
10. Manabe M, Lim HW, Winzer M, et al. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch Dermatol. 1999;135(2):177–181. doi:10.1001/archderm.135.2.177
11. Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845–10850. doi:10.3748/wjg.v20.i31.10845
12. Avcu N, Kanli A. The prevalence of tongue lesions in 5150 Turkish dental outpatients. Oral Dis. 2003;9(4):188–195. doi:10.1034/j.1601-0825.2003.02933.x
13. Sil A, Bhanja DB, Chakraborty S. Black carpet over tongue. Postgrad Med J. 2020;96(1138):506. doi:10.1136/postgradmedj-2019-137208
14. Long S, Chen Y, Shi J, Tian J, Ni J. Black hairy tongue associated with olanzapine use in a female dementia patient: a case report. J Clin Psychopharmacol. 2022;42(2):211–214. doi:10.1097/JCP.0000000000001503
15. Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158(5):573. doi:10.1001/jamadermatol.2021.5314
16. Lee J, Chung HS, Roh J, Oh Y, Mok J. Linezolid-induced black hairy tongue in a patient with multidrug-resistant tuberculosis: a case report. Sci Prog. 2021;104(3):368504211042982. doi:10.1177/00368504211042982
17. Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. doi:10.1128/JB.00542-10
18. Luo M, Liu Y, Wu P, et al. Alternation of gut microbiota in patients with pulmonary tuberculosis. Front Physiol. 2017;8:822. doi:10.3389/fphys.2017.00822
19. Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6(2):1–10. doi:10.1128/mBio.00037-15
20. Botero LE, Delgado-Serrano L, Cepeda ML, et al. Respiratory tract clinical sample selection for microbiota analysis in patients with pulmonary tuberculosis. Microbiome. 2014;2:29. doi:10.1186/2049-2618-2-29
21. Hong B-Y, Maulén NP, Adami AJ, Granados H, Balcells ME, Cervantes J. Microbiome changes during tuberculosis and antituberculous therapy. Clin Microbiol Rev. 2016;29(4):915–926. doi:10.1128/CMR.00096-15
22. Abeles SR, Jones MB, Santiago-Rodriguez TM, et al. Microbial diversity in individuals and their household contacts following typical antibiotic courses. Microbiome. 2016;4:1–12. doi:10.1186/s40168-016-0187-9
23. Imperial MZ, Nahid P, Phillips PPJ, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708–1715. doi:10.1038/s41591-018-0224-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.