Back to Journals » International Journal of Nanomedicine » Volume 17
Optimization of Lipid Nanoformulations for Effective mRNA Delivery
Authors Chen H , Ren X, Xu S, Zhang D, Han T
Received 7 March 2022
Accepted for publication 24 June 2022
Published 2 July 2022 Volume 2022:17 Pages 2893—2905
DOI https://doi.org/10.2147/IJN.S363990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Huiling Chen,1 Xuan Ren,2 Shi Xu,3 Dekui Zhang,4,* TiYun Han4,*
1Department of Hematology, Lanzhou University Second Hospital, Lanzhou, People’s Republic of China; 2Department of Endocrinology and Metabolism, Lanzhou University Second Hospital, Lanzhou, People’s Republic of China; 3Therarna. Co. Ltd., Nanjing, Jiangsu, People’s Republic of China; 4Department of Gastroenterology, Key Laboratory of Digestive Diseases, Lanzhou University Second Hospital, Lanzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: TiYun Han; Dekui Zhang, Department of Gastroenterology, Key Laboratory of Digestive Diseases, Lanzhou University Second Hospital, No. 82, Cuiying Road, Chengguan District, Lanzhou, 730030, People’s Republic of China, Tel +86-13893384179 ; +86-13919788616, Email [email protected]; [email protected]
Introduction: Since the coronavirus disease 2019 (COVID-19) pandemic, the value of mRNA vaccine has been widely recognized worldwide. Messenger RNA (mRNA) therapy platform provides a promising alternative to DNA delivery in non-viral gene therapy. Lipid nanoparticles (LNPs), as effective mRNA delivery carriers, have been highly valued by the pharmaceutical industry, and many LNPs have entered clinical trials.
Methods: We developed an ideal lipid nanoformulation, named LNP3, composed of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cholesterol, and observed its release efficiency, sustained release, organ specific targeting and thermal stability.
Results: In vitro studies showed that the transfection efficiency of LNP3 was higher than that of LNPs composed of DOTAP-DOPE and DOTAP-cholesterol. The positive to negative charge ratio of LNPs is a determinant of mRNA transfer efficiency in different cell lines. We noted that the buffer affected the packaging of mRNA LNPs and identified sodium potassium magnesium calcium and glucose solution (SPMCG) as a favorable buffer formulation. LNP3 suspension can be lyophilized into a thermally stable formulation to maintain activity after rehydration both in vitro and in vivo. Finally, LNP3 showed sustained release and organ specific targeting.
Conclusion: We have developed an ideal lipid nanoformulation composed of DOTAP, DOPE and cholesterol for effective mRNA delivery.
Keywords: lipid nanoparticles, mRNA delivery, 1,2-dioleoyl-3-trimethylammonium-propane, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, cholesterol
Introduction
Vaccines play an important role in safe-guarding world health. New technologies are needed to meet the changing demand for vaccines, so as to treat emerging infections and improve life expectancy.1 Nucleic acid vaccine may meet these needs and show great promise for the future. DNA vaccine has been proved to be a flexible platform and has a wide range of roles in animal models, but the lack of transgenic expression or immune stimulation limits its wide application in clinical trials.2,3 It has been proved that the vaccine based on recombinant virus vector technology has the advantage of efficient delivery of nucleic acid materials, but its immunogenicity, production limitations and security hamper its wide application.4,5 As a substitute for DNA vaccine, mRNA mediated nucleic acid therapy has been widely used in the fields of genetic diseases, cancer immunotherapy and stem cell biomedical research.6–8 As a direct source of gene products, mRNA has several advantages, including no need for nuclear entry,9 little chance of integration into the host genome,10 and avoiding insertion mutations that cause abnormal transcription and expression of oncogenes. Furthermore, genes expressed through mRNA are relatively short-lived and can be completely biodegradable through metabolic pathways, making them safer to use than DNA vaccines.11 Last but not least, for specific gene expression, mRNA-mediated expression platform requires fewer elements than DNA-mediated expression platform, thus reducing the delivery difficulty and risks of side effects. In addition, the cytoplasmic transmission of mRNA bypasses the nuclear membrane, resulting in a higher level of gene expression in vivo than in DNA for a shorter duration.12 These properties make mRNA a safe and attractive genetic material based on gene therapy.11
However, due to perceived instability and fragility, easy degradation by universally expressed nucleases, insufficient translatability and immune stimulation effect, these will significantly inhibit the expression efficiency and cause in vivo immunotoxicity, making it difficult for mRNA to be widely used as a therapeutic tool for gene therapy.13 On the contrary, chemically modified mRNA synthesized in vitro combined with naturally modified ribonucleotides overcomes the above shortcomings, improves intracellular stability and translation efficiency, and thus shows the great prospect of using mRNA as a powerful therapeutic tool.14 At present, extensive preclinical or clinical studies have shown that mRNA has great potential for in vitro transcription (IVT).15,16 Besides, another reason for extensive inhibition of mRNA application is that it can provide antigen stimulation and activate the innate immune system in an antigen-independent manner.17 It has been proved that free mRNA can be used as antigen material for vaccination of mice, and antigen-specific immune response can be induced after vaccination with mRNA.18,19 However, naked mRNA did not result in high levels of protein expression because it was immediately degraded by tissue nucleases, and further mRNA was immunostimulatory and functioned as a pathogen related molecular model.20 Therefore, unprotected mRNA delivered by itself is not suitable for a wide range of therapeutic applications.
So far, many non-viral systems have been developed to effectively deliver mRNA. These delivery systems involve cationic nano emulsions (CNE),21 polyplexes,22 and in particular, lipid nanoparticles (LNPs).23 Previously, various studies have confirmed that ionizable amino lipids are the most effective.24,25 Dlin-MC3-DMA, an ionizable cationic lipid, was approved by the FDA in 2018 to be used for the preparation of an ionizable LNP (iLNP). Therapeutic siRNA was enclosed by the iLNP for the treatment of hereditary transthyretin mediated amyloidosis. The iLNP formulation based on Dlin-MC3-DMA and its derivatives is not only optimized for intravenous delivery of siRNA but also an effective mRNA delivery system.26 Another iLNP formulation based on the ionizable lipid C12-200 was previously used to deliver siRNA and further optimized and developed to deliver mRNA encoding erythropoietin intravenously.27 Although ionized lipids have obvious properties of transferring mRNA, they are more expensive than cationic lipids such as DOTMA (1, 2-di-O-octadecenyl-3-trimethylammonium propane) and DOTAP (1,2-dioleoyl-3-trimethylammonium-propane). In contrast, cationic lipid-based LNPS have been widely proven to deliver antigens, DNA and mRNA, and further studies have shown that they have acceptable safety.28–30 Among these cationic lipids, DOTAP is one of the most widely used cationic lipids.31,32 DOTAP can maintain positive charge at all physiological pH and can easily concentrate anionic mRNA. Biontech has used a combination of DOTAP and fusion assisted lipid coating in its cancer vaccine platform to form lipid complexes.33 In addition, the ratio of cationic lipids to DOPE can be adjusted to selectively target splenic antigen-presenting cells for mRNA vaccine delivery.34 Promising therapeutic outcome has been seen in several ongoing clinical trials treating metastatic melanoma. Besides DOPE as a helper lipid for taking part in mRNA delivery, cholesterol is also incorporated to improve lipid bilayer stability and aid membrane fusion and endosomal escape.35 In addition, the lipid-anchored PEG such as DSPE-PEG2000 is involved in reducing macrophage-mediated clearance. More importantly, lipid-anchored PEG helps to prevent particle aggregation and improve storage stability.36
Buffer is an important component that must be carefully selected when developing mRNA delivery systems, especially in clinical applications. Ringer’s solution and Ringer’s lactic acid are two common buffers for dissolving and diluting mRNA LNP complex before injection.37,38 Other commonly used buffers include phosphate buffered saline (PBS), normal saline, and isotonic sodium bicarbonate solution.39 The buffer used under the condition of mRNA transmission plays an important role in influencing the assembly efficiency of mRNA LNP, the transfection ability in vitro and in vivo, and further affecting the expression level of related genes. In addition, the buffer also regulated the biological distribution of LNP and the nonspecific accumulation of nanoparticles. Therefore, it is very important to study the properties of buffers and better understand the nano particle/biological interaction, so it can be used as a guide for better assembly and delivery of mRNA LNP.
Another possible limitation of mRNA LNP development is usually related to LNP hydrolysis, mRNA leakage, aggregation formation and other phenomena, thus changing the biological distribution in vivo, thus affecting the efficacy and safety.40 One option to address instability is to dry the mRNA LNP formulation. Among the possible methods, freeze-drying is still the main technology studied, even though the examples of freeze-drying drugs on the market are limited.41 The freeze-drying process is complex because it is a challenge to select excipients and process parameters to protect LNP integrity from stresses caused by freezing and dehydration. mRNA LNP contains an aqueous solution and can be assembled spontaneously in the presence of water. However, when the aqueous solution is removed, it may cause significant and irreversible changes in the structure of mRNA LNP.42
Here, we optimized the formulation of LNPs to achieve efficient mRNA delivery, focusing on the components of cationic lipid DOTAP, auxiliary lipid DOPE and cholesterol as the main driving factors for optimizing mRNA expression and tolerance. In vivo biodistribution of mRNA LNP and the subcutaneous and muscular expression of mRNA were studied using an in vivo imaging system. Our study confirmed that the optimized formula has significant effects in vivo and in vitro. In addition, the buffer used to dilute and stabilize mRNA LNP suspension also had a significant effect on mRNA LNP uptake and protein synthesis. In addition, the protective agent has a significant effect on the integrity of mRNA LNP, so as to achieve an appropriate freeze-drying formula and protect mRNA LNP from changes in mRNA transmission. In conclusion, these results suggest that understanding the characteristics of lipid components, the effects of buffer and the relationship between LNP and lyophilized preparations is expected to optimize lipid nanoparticles to achieve efficient mRNA delivery.
Materials and Methods
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-amino(polyethylene glycol)-2000 ammonium salt (DSPE-PEG2000), DOTAP and DOPE were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol was purchased from Sigma-Aldrich (St Louis, MO). Unless otherwise specified, all the other chemicals were purchased from Sigma-Aldrich. pTK194 was a gift from Ron Weiss (Addgene plasmid #59563).43 HiScribe T7 ARCA mRNA kit and the restriction endonuclease used in the experiment were purchased from New England Biolabs (Beijing) Ltd. mMessage mMachine T7 Transcription Kit, Opti-MEM, Dulbecco’s modified Eagle’s medium (DMEM) and serums were purchased from Thermo Fisher Scientific. HEK293T human embryonic kidney cell line and the DC2.4 murine dendritic cell line were purchased from ATCC. THP-1 and Jurkat cell were gifts from Gao Daxing, USTC. All cell lines were cultured according to vendor instructions (37°C, 5% CO2). Female C57BL/6J mice aged 6–8 weeks were raised in the animal facility at Lanzhou University Second Hospital. All animals were manipulated according to protocols approved by the Animal Ethics Committee of Lanzhou University Second Hospital. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
The Preparation of Plasmid and mRNA Synthesis
IVT template was constructed with commercial plasmid pUC57 as vector, which contained a T7 promoter, a minimal 5`-UTR and a human beta globin 3`-UTR, and a ploy(A100) followed.44 Construction details are as follows. A restriction endonuclease site was attached at the end of the polyA to linearize the IVT template (the constructed plasmid named pUC57IVT). The gene of interest was inserted between the 5` and 3` UTR. The plasmid DNA was obtained by growing Escherichia coli culture overnight in LB medium and antibiotics (50 μg/mL of ampicillin). Then, the plasmids were purified by means of EndoFree plasmid purification. The purified plasmids were diluted by deionized water and stored at −20°C. The integrity of the plasmids was confirmed by agarose gel electrophoresis. The purity and concentration of the plasmids were determined by NanoDrop2000 Spectrophotometer.
Plasmid DNA was immediately linearized at the 3 ′ end of polyA sequence by restriction digestion. Then, the enzyme was degraded with protease K and sodium dodecyl sulfate, extracted with phenol/chloroform/indoleacetic acid, and precipitated with ethanol. Reuse of granular DNA in RNase free water. According to the manufacturing instructions, the linearized DNA template was transcribed into RNA using the hiscribe T7 ARCA mRNA Kit (New England BioLabs) and purified by lithium chloride precipitation. The linearized pTK194 template was transcribed in vitro using the mMessage mMachine T7 transcription kit. The integrity of mRNA was confirmed by agarose gel electrophoresis. The purity and concentration of mRNA were determined by UV absorption at 260/280 nm.
The Preparation of Lipid Nanoformulations
Cationic lipid transfection agents were prepared according to previous reports.45 DOTAP and cholesterol (2:3 molar ratio) were co-dissolved in chloroform, the solvent was evaporated, and the resulting lipid membrane was dried for 1 h at 37°C using rotary evaporation. The lipid film was hydrated by gently shaking to obtain a raw colloid with a total lipid concentration of approximately 1 mg/mL, which was left overnight at 4°C for equilibration. The lipid mixture of DOTAP and cholesterol was named LNP2-Chol. Using the same strategy, the lipid mixtures of DOTAP and DOPE (1:1 molar ratio, called LNP2-DOPE), and DOTAP blended with DOPE and cholesterol (DOTAP: DOPE:cholesterol 40:10:48 molar ratio, called LNP3) were prepared, separately. For size adjustment, the dispersion was then sequentially extruded through 450, 200 and 100 nm polycarbonate membranes (Millipore, Billerica, MA) to form liposomes of appropriate size.
The Preparation of Lipid Nanoformulations and RNA Formulation
The formation of lipid nanoparticle mRNA was performed with proprietary protocols, derived from extensive internal formulation development activities. General procedures were derived from protocols described elsewhere.33 A diversity of formulations complexed with the green fluorescent protein (EGFP)-encoding RNA was assembled, with lipid nanoparticles containing different cationic and helper lipids to obtain various N/P ratios, which defined the charge ratio and overall RNA-LNP net charge. The charge ratios were calculated according to the number of positive charges represented by lipid-specific head groups (one positive charge per head group) and the number of negative charges represented by RNA nucleotides, that is, the number of negative charges represented by RNA phosphodiester groups (one negative charge per phosphodiester). In order to calculate the molar ratio between RNA and cationic lipids, the average molar mass of each nucleotide was assumed to be 330 Da. RNA was provided in the form of sodium citrate (1 mm, pH 6.4) buffer solution, and the RNA concentration was 1 mg/mL. N/P ratios are achieved by diluting RNA with buffer, and then adding an appropriate amount of lipid nanoparticles to achieve the selected charge ratio. PEGylation was performed by adding the assigned amount of DSPE-PEG based on the molar ratio with DOTAP to the mRNA-LNP and incubating the mixture for 15 minutes. The size and surface charge of the nanoparticles were determined by Malvern ZS90 (Malvern, Worcestershire, UK).
Detection of Transfection Efficiency in vitro
In the in vitro transfection study, 1 mL cells in the complete medium supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin were directly inoculated into the well of a 24-well plate (5 × 104 cells per well) and incubated for 24 h. Then, the nanoparticles containing solution (50 μL with 1ug of mRNA) were added to each well, and the cells were incubated at 37°C. The detailed protocol of mRNA-LNP preparation is described below. First, the appropriate volume of LNP (based on the specified charge ratio) is diluted with buffer (such as Opti-MEM, 0.9% NaCl, sodium bicarbonate injection, Ringer solution, sodium potassium magnesium calcium and glucose injection, and the buffers developed based on these injections) to 25μL and incubated at room temperature for 5 minutes. During the waiting period, 1μg mRNA is diluted with the same buffer to 25 μL 5 minutes later, mixing the two buffers and incubated at room temperature for 0.5 h. Then, the mixture was added to one well of the 24-well plate. The gene expression was evaluated after 24, 48 and 72 h. For the expression of fluorescent proteins, the cells were observed using an inverted fluorescence microscope (Mshot MF53). For the expression of firefly luciferase, the cells were added to D-fluorescein sodium salt with the final concentration of 0.15–0.3 mg/mL, and incubated 5–10 min at 37°C, then image analysis with Kodak in vivo imaging system FX Pro (Kodak, Rochester, NY).
The Lyophilization of Lipid Nanoformulations and RNA Formulation
LNP-mRNA complexes for lyophilization were prepared with 40% (w/v) sucrose or trehalose. The detailed scheme of lyophilized complex preparation was described below. After LNP-mRNA preparation was completed, mixing with 40% sucrose or trehalose to the final concentration of 1%, 5%, 10%, 15% and 20%. The lyophilized complex was prepared using Virtis AdVantage LGJ-10F bench-top freeze dryer controlled by the microprocessor-based software. The lyophilization cycle consisted of a freezing step at −40°C, a primary drying step at −30°C and 50 mTorr, and a secondary drying step at 20°C and 50 mTorr. At the completion of the cycle, samples were brought to atmospheric pressure and stored at a suitable temperature before use. Lyophilized material was reconstituted using nuclease-free water and gently swirled, then added to cells for in vitro transfection.
Detection of Transfection Efficiency in vivo
Nanoparticles equivalent to 10 μg of firefly luciferase mRNA prepared as aforementioned were injected intravenously, intramuscularly and subcutaneously, respectively. The level of uptake and translation of Luc-mRNA were evaluated by in vivo bioluminescence imaging using the Spectrum CT Biophotonic Imager (PerkinElmer, Boston, MA). Several time nodes post injection were adopted to measure the expression and bio-distribution of Luc-mRNA. Mice were injected intraperitoneally with a dosage of 150 mg/kg D-luciferin and then image acquisition and analysis. Emitted photons from live animals or extracted tissue were quantified 10 min later with an exposure time of 1 min. The region of interest was quantified as average radiance (photons−1 cm−2 sr−1, represented by color bars). To study transfection in vivo and the properties of organ-targeting, the mice were killed 24 hours after tail vein injection, and the organs (liver, spleen, lung, kidney and skin) were collected and imaged.
Statistical Analyses
Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by the Tukey-Kramer multiple comparison test for more than two groups. Comparisons between the two groups were performed Students’ test by Prism (v.8, GraphPad Software, La Jolla, CA). Values of p< 0.05 were considered to be significant.
Results
The New Lipid Nanoformulations Has Strong Cell Transfection Efficiency
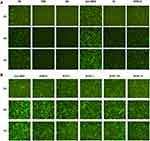
We initially evaluated mRNA lipoplexes with a different lipid composition. The mRNA lipoplexes were composed of a cationic lipid DOTAP, and a helper lipid, either DOPE or cholesterol. Meanwhile, we also constructed an mRNA lipoplex involving DOTAP, DOPE and Cholesterol, and named it as lipoplex LNP3. Previously, it was reported that the mRNA LNP complex assembled based on DOTAP cholesterol and DOTAP-DOPE showed precise and effective in vivo targeting. It is only needed to optimally adjust the net charge without functionalizing the particles through molecular ligands.33 In vitro, RNA nanoparticles encoding EGFP mRNA were used to evaluate the efficiency of mRNA delivery to cells by changing the N/P ratio. In order to systematically evaluate the effect of total particle charge on mRNA transmission in vitro, we changed the N/P ratio, which has not been explored so far. First, the effect of charge ratio on mRNA transfection was studied using LNP3. We found that positive and negative charges had opposite effects on mRNA transfection, and positive and negative charges mRNA LNPS significantly reduced transfection level and EGFP mRNA expression. In contrast, slightly negatively charged and near-neutral mRNA-LNPs showed the better mRNA delivery and gene expression (Figure 1A). It was by changing the positive/negative charge ratio from 3:1 to 1:3 that unexpected differences occurred in mRNA transmission and expression, which prompted us to test whether LNP3 was consistent and universal with other cells. Therefore, we selected mouse dendritic cell-line DC2.4 to study the mRNA transfection by changing the charge ratio. Similarly, LNP3 mediated mRNA transfections with slight negative and near neutral charges showed significant mRNA delivery efficiency and significant gene expression regardless of the cell type (Figure 1B).
Previous studies have shown that when the charge ratio is 1.7:2, the lipid complex constructed by DOTAP and DOPE or DOTAP and cholesterol can independently mediate the mRNA transmission targeting the spleen in vivo.33 Therefore, we chose a charge ratio of 1.7:2 to study the transfection difference between LNP3 and the two. We found that all three had high transfection efficiency in HEK293T cells; however, the mRNA transfer efficiency of LNP3 was better than that of DOTAP-DOPE and DOTAP-cholesterol in DC2.4 cells (Figure 1C). In addition, we used LNP3 to deliver EGFP mRNA to suspension cells, such as THP-1 and Jurkat cells. LNP3 mediated EGFP mRNA transfection into THP-1 showed significant efficiency. In contrast, only a few EGFP were expressed when mRNA was transferred to Jurkat (Supplementary Figure 1A). In addition, in order to verify that LNP3 can mediate mRNA of different lengths, we selected firefly luciferase mRNA (~1700nt) and self-amplifying replicon RNA for expressing mKate protein (~12000nt) as testing materials. Fluorescent signals were detected in HEK293T cells transfected with two different lengths of mRNAs. Therein, LNP3 mediated long RNA (>10000nt) transfection shows slightly poorer efficiency (Supplementary Figure 1B and C). In the study, the actual electric potential values for LNPs in the presence and absence of mRNA payload was shown in Supplementary Figure 2.
DSPE-PEG (1,2-distearoyl-sn-glycerol-3-phosphate ethanolamine polyethylene glycol), a biocompatible, biodegradable and amphiphilic material, was widely used as a phospholipid polymer conjugate in drug delivery applications.46 In the process of our experiment, DSPE-PEG2000 amino was added with different molar ratio to DOTAP. We found that the molar ratio of DESPE-PEG2000: DOTAP changed from 1:20 to 1:4, it had almost no effect on mRNA delivery and gene expression. In contrast, higher molar ratio of DSPE-PEG2000 could lead to lower transfection effect (Figure 1D).
In addition, we observed and sampled all the above experimental results for 72 hours, and found that the number of positive cells (ie the number of cells expressing related genes) and the expression level gradually increased in the initial period after transfection. Therefore, LNP3 mediated mRNA transfection has sustained-release properties.
Effect of Delivery Buffer on Transfection Efficiency of mRNA Lipoplexes
We found that the transfection buffer affects the transfection efficiency. In previous experiments, we used Opti-MEM as diluent, but the application of Opti-MEM cannot be used in clinic. Therefore, we selected a variety of clinical injections as candidate injections, such as normal saline (NS), sodium bicarbonate solution (SB), ringer lactate solution (RL), phosphate buffer solution (PBS), and sodium potassium magnesium calcium and glucose solution (SPMCG). When NS and SPMCG were used as buffers, the transfection efficiency was better than those mediated by PBS, SB and RL, and PBS-mediated shows the worst effect on mRNA delivery (Figure 2A).
Besides, we also studied the effect of these buffers for transfection in vitro when LNP3 was amino modified by DSPE-PEG2000 (the liposome assembled with DSPE-PEG2000 was called LNP4, here we choose the molar ratio of DOTAP: DSPE-PEG2000 is 20:3). We found that, except for SPMCG, the effects of all the above buffers on EGFP mRNA transfected cells were negligible. Although the effect of transfection of mRNA-LNP4 by SPMCG was better, it was also worse than that of Opti- MEM (Supplementary Figure 3). In general, we preliminarily explored and found that injection can be used as a substitute for Opti-MEM to mediate mRNA transmission in vitro, especially SPMCG.
In addition, we speculate that the transfection buffer may be optimized based on SPMCG. Therefore, we diluted SPMCG with three other injections, NS, SB and RL, and evaluated the transfection effect of mRNA-LNP3. After continuous observation for 72 hours, we found that the four optimized buffers had similar transfection effects as SPMCG (Figure 2B). Among these buffers, buffer I diluted SPMCG with NS, diluted SPMCG with RL to obtain buffer II, diluted SPMCG with PBS to obtain buffer VIII, and diluted SPMCG with NS and RL to obtain buffer IX. However, SPMCG diluted with SB had a negligible effect on mRNA transmission (data not shown). By analyzing the pH of these injections, we found that NS, PBS and SPMCG were near neutral pH solution, RL was acid-deficient solution, and SB was alkaline solution. Therefore, these results suggest that SPMCG diluted with neutral or acid deficient solution has little effect on the transfection of mRNA-LNP3 complex, and alkaline conditions may lead to the opposite results.
mRNA Lipoplexes Still Has Strong Transfection Efficiency After Lyophilization and Rehydration
The stability of lipoplexes in dispersion form during storage is a major challenge in the development of mRNA-delivered formulations. Lyophilization of nanoparticles prior to administration provides a solution for stabling lipoplexes during storage. Lyophilized lipoplexes can be stored for years without losing efficacy and can be administered more easily, as users simply need to add water and inject.47 The amount of solutes added to the lipoplexes during formulation is easily adjustable to ensure that the resulting aqueous suspension is isotonic after adding water. We adopted sucrose and trehalose as lyoprotectants to investigate the effect on mRNA delivery in vitro mediated by LNP3. Originally, we selected Opti-MEM as diluted buffer and sucrose as lyoprotectant, the fluorescence activity of EGFP mRNA-LNP3 complexes rehydrated immediately after lyophilization, found that approximately no signal could be detected when no sucrose was used as lyoprotectant, in contrast, when sucrose added to mRNA-LNP3 complex, the fluorescence signal could be detected, and higher concentration of sucrose has better effect (Figure 3A). Afterwards, to further confirm the generality and universality, we chose SPMCG as diluted buffer and sucrose or trehalose as lyoprotectant. The results verified that sucrose as lyoprotectant has a significant effect on stabilizing mRNA delivery after lyophilization and rehydration, and trehalose as one alternative of lyoprotectants, also shows the characteristic of ensuring lipoplexes stabilization during the process of lyophilization and rehydration (Supplementary Figure 4A and B). Furthermore, we also found that the freeze-dried powder still had the activity of mRNA delivery and gene expression. After mRNA-LNP3 was lyophilized, we stored mRNA-LNP3 at room temperature, 4°C, −20°C and −80°C, and found that the four mRNA-LNP3 all have the properties of mRNA delivery and expression, although the activity is significantly lower than the mRNA-LNP3 prepared freshly (Figure 3B).
Effect of mRNA Lipoplexes on Transfection Efficiency and Biodistribution in vivo
First, we evaluated the in vivo transfection efficiency after subcutaneous and intramuscular injection of LNP3 lipoplex preparation in Balb/c nude mice. In order to track the localization of mRNA lipid complexes in vivo, we used Fluc mRNA as a reporter gene to evaluate mRNA expression in vivo. Subcutaneous and intramuscular injection in mice 10 μg mRNA, the in vivo transfection efficiency of mRNA lipid complex was evaluated after 12, 24, 48, 72 and 96 hours. Figure 4A shows a representative whole-body image of injected mice. The in vivo transfection efficiency of the two routes of administration was similar, and the fluorescence signal could last for more than two days.
Furthermore, we explored the biodistribution of the lipoplex formulations of LNP3 and LNP4 after systemic administration in Balb/c nude mice. Mice were injected intravenously via tail vein at a dose of 10 μg mRNA, and 24 and 48 hours later, the biodistribution of the mRNA lipoplexes was measured, respectively. Figure 4B shows representative whole images of the injected mice. LNP3-mediated mRNA expression mainly accumulates in the lungs, spleen and liver, roughly. However, no signal was detected in LNP4 mediated mRNA expression in vivo. Moreover, to quantify the administered accumulation of the mRNA lipoplexes in specific organs, lungs, spleen, liver, kidney and skin were isolated and imaged ex vivo 48 hours later. Comparing the expression of two lipid plexus mediated Fluc mRNA in isolated organs, we observed that LNP3 mediated mRNA expression mainly accumulated in the spleen, and no signal was detected in other organs. In contrast, no LNP4 mediated accumulation of mRNA expression was found in the above organs (Figure 4C). The results showed that LNP3 had significant in vivo transfection efficiency and good organ targeting, especially when the positive and negative charge ratio of mRNA to LNP3 was 1.7:2. In addition, DSPE-PEG2000 modified LNP3 had no effect on mRNA transmission in vitro in the appropriate concentration range. However, PEGylation of LNP3 has the opposite effect in vivo, which leads to no organ targeting of LNP3.
Discussion
mRNA has great potential to express proteins or peptides with therapeutic purposes in a safe and effective manner without the risk of random integration into the genome. Although considerable progress has been made in the previous studies on the introduction of mRNA into cells or in vivo by non-viral methods, the clinical application of mRNA has been limited due to various reasons. mRNA is considered to be unstable, unable to obtain sufficient protein expression and induce a strong immune response, which limits the wide application of mRNA.48,49 Therefore, it is necessary to study an effective non-viral mRNA delivery system to overcome the instability of mRNA, so as to achieve efficient in vitro and in vivo mRNA delivery. In this work, we observed the effects of helper lipids and cholesterol on mRNA expression in vitro. Our working hypothesis is that the inclusion of both auxiliary lipids and cholesterol in LNP will lead to the improvement of mRNA tolerance and the optimization of transmission effect. Interestingly, in our initial screening process, we noticed that there was a significant correlation between the charge ratio and in vitro mRNA transfection, and the use of auxiliary lipids and cholesterol had better effects than the use of both. In addition, we also observed that the diluted buffer used to prepare mRNA LNP plays an important role in regulating the transmission and expression of mRNA, and mRNA LNP is relatively stable after freeze-drying and rehydration. In addition, the intramuscular and subcutaneous drug delivery routes showed significant gene expression and persistence, and intravenous drug delivery had the characteristics of organ targeting.
Besides, it is reported that PEGylation not only enhances the passive lymphatic targeting of DOTAP liposome vaccines but also regulates their biological distribution.50 Hence, PEGylation should be a promising strategy to improve the efficiency of cationic lipid DOTAP-formulated vaccines.51 Based on the results of screening charge ratio, we selected the charge ratio of 1.7:2 as the best transfection condition. In addition, it was verified in vitro that the mole ratio of DSPE-PEG2000 to DOTAP was in the range of 1:40–1:4, which had no significant effect on mRNA transmission and gene expression. A higher mole of DSPE-PEG2000 would interfere with LNP3 mediated mRNA transfection and expression. Based on the results of modifying LNP3 with DSPE-PEG2000, we selected the mole ratio of DSPE-PEG2000 to DOTAP as 3:20, so as to obtain LNP4 as an optimized polyethylene glycol lipid complex to study the effect of LNP3 on mRNA transmission in vivo. When the net charge of the lipid complex composed of DOTAP-DOPE and DOTAP cholesterol was negative, the transferred mRNA could accumulate in the spleen and show accurate DC targeting.33 We also found that when LNP3 assembled mRNA at a charge ratio of 1.7:2, it was spleen targeted when administered through the tail vein. Nevertheless, PEGylation led to the opposite effect. LNP4 mediated mRNA transmission did not find signal enrichment in organs. Moreover, we also investigated LNP3-mediated mRNA expression by subcutaneous and intramuscular injection. There was no significant difference in mRNA expression between the two methods of administration, and the duration of LNP3 mediated gene expression was longer in the two methods.
In addition, the buffer of mRNA-LNP formulation also plays an important role in regulating mRNA delivery and gene expression. However, early studies have shown that buffer-formulated mRNA can lead to measurable levels of immunogenicity administration by intramuscular route in rodents. However, a recent Phase I trial of a rabies mRNA vaccine administered in Ringer’s buffer yielded no immunogenicity.52,53 Hence, the diluted buffer still plays a role in influencing the assembled effect of mRNA-LNP and further mRNA delivery, such as pH and ionic concentration. In our work, the ultimate purpose of developing mRNA non-viral delivery system is to achieve pre-clinical and clinical applications; therefore, we selected several clinical injections and developed a few buffers based on the injections to investigate the buffer function during mRNA-LNP formulation. We found that SPMCG has a relatively better effect in mediating mRNA transmission and expression. In addition, we also found that SPMCG has significant buffering properties. The buffer with close pH value can be used to dilute SPMCG, and the effect on mRNA transmission can be ignored. Compared with Opti-MEM-formula mRNA transfection, the transfection effect of clinical injection is slightly poor. However, it provides an optimization strategy to improve the formulation of mRNA LNP with weak immunogenicity.
Lyophilization is an effective method for preserving non-viral mRNA vaccines. Since mRNA-LNP complex is prone to physical and chemical degradations when stored as a ready-to-use solution, it is necessary to determine the correct formulation conditions, the optimal amount of the correct excipients and the correct dosage form to maximize the stability, biological activity and safety.54 Disaccharides form an amorphous sugar glass, which is the most stable product during lyophilization, especially sucrose and trehalose.55 Further, the choice of buffer is critical in the development of lyophilized formulations, and the buffer used should undergo minimal pH change during freezing. Combined sucrose or trehalose with Opti-MEM or SPMCG, we found that the mRNA-LNP complex had the activity of transfection after lyophilization and rehydration. Furthermore, the freeze-dried powder of mRNA-LNP complex still has the function of mRNA delivery and expression after long-term storage, regardless of the effect of temperature difference.
Conclusion
In conclusion, we have developed an ideal lipid nanoformulation composed of DOTAP, DOPE and cholesterol, with 4 favorable characteristics: high delivery efficiency in vivo, extended release, organ specific targeting and thermal stability, which may be a valuable tool for vaccination.
Abbreviations
LNPs, lipid nanoparticles; iLNP, ionizable LNP; IVT, in vitro transcription; CNE, cationic nano emulsions; DOTMA, 1, 2-di-O-octadecenyl-3-trimethylammonium propane; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; PBS, phosphate-buffered saline; DMEM, Dulbecco’s modified Eagle’s medium; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG2000, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-amino(polyethylene glycol)-2000 ammonium salt; EGFP, enhanced green fluorescent protein; NS, normal saline; SB, sodium bicarbonate solution; RL, ringer lactate solution; SPMCG, sodium potassium magnesium calcium and glucose solution.
Acknowledgments
This research was supported by the National Nature Science Foundation of China under Grant 31960212; Fundamental Research Funds for the central Universities under Grant lzujbky-2020-cd05; Higher Education Innovation Fund project and of Gansu Province under Grant 2020B-023 and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital under Grant CY2019-MS12 and CY2019-QN16.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11(12):865–872. doi:10.1038/nri3085
2. Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9(10):776–788. doi:10.1038/nrg2432
3. Fletcher S, Hamilton AD. Targeting protein-protein interactions by rational design: mimicry of protein surfaces. J Royal Soc Interface. 2006;3(7):215–233.
4. Kofler RM, Aberle JH, Aberle SW, Allison SL, Heinz FX, Mandl CW. Mimicking live flavivirus immunization with a noninfectious RNA vaccine. Proc Natl Acad Sci USA. 2004;101(7):1951–1956. doi:10.1073/pnas.0307145101
5. Smerdou C, Liljeström P. Non-viral amplification systems for gene transfer: vectors based on alphaviruses. Curr Opin Mol Ther. 1999;1(2):244–251.
6. Van Tendeloo VF, Ponsaerts P, Berneman ZN. mRNA-based gene transfer as a tool for gene and cell therapy. Curr Opin Mol Ther. 2007;9(5):423–431.
7. Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi:10.1007/3-540-32636-7_2
8. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi:10.1016/j.stem.2010.08.012
9. Van Driessche A, Ponsaerts P, Van Bockstaele DR, Van Tendeloo VF, Berneman ZN. Messenger RNA electroporation: an efficient tool in immunotherapy and stem cell research. Folia Histochemica Et Cytobiologica. 2005;43(4):213–216.
10. Ponsaerts P, Van Tendeloo VF, Berneman ZN. Cancer immunotherapy using RNA-loaded dendritic cells. Clin Exp Immunol. 2003;134(3):378–384. doi:10.1046/j.1365-2249.2003.02286.x
11. Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi:10.1038/nrd4278
12. Probst J, Weide B, Scheel B, et al. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007;14(15):1175–1180. doi:10.1038/sj.gt.3302964
13. Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30(1):1–7.
14. Kormann MS, Hasenpusch G, Aneja MK, et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol. 2011;29(2):154–157. doi:10.1038/nbt.1733
15. Dewitte H, Van Lint S, Heirman C, et al. The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy. J Control Release. 2014;194:28–36. doi:10.1016/j.jconrel.2014.08.011
16. Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep. 2015;5:11315. doi:10.1038/srep11315
17. Fotin-Mleczek M, Duchardt KM, Lorenz C, et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34(1):1–15. doi:10.1097/CJI.0b013e3181f7dbe8
18. Hess PR, Boczkowski D, Nair SK, Snyder D, Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55(6):672–683. doi:10.1007/s00262-005-0064-z
19. Ying B, Campbell RB. Delivery of kinesin spindle protein targeting siRNA in solid lipid nanoparticles to cellular models of tumor vasculature. Biochem Biophys Res Commun. 2014;446(2):441–447. doi:10.1016/j.bbrc.2014.02.120
20. Scheel B, Teufel R, Probst J, et al. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol. 2005;35(5):1557–1566. doi:10.1002/eji.200425656
21. Brito LA, Chan M, Shaw CA, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther. 2014;22(12):2118–2129. doi:10.1038/mt.2014.133
22. Démoulins T, Milona P, Englezou PC, et al. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomed Nanotechnol Biol Med. 2016;12(3):711–722. doi:10.1016/j.nano.2015.11.001
23. Goswami R, Chatzikleanthous D, Lou G, et al. Mannosylation of LNP results in improved potency for self-amplifying RNA (SAM) vaccines. ACS Infect Dis. 2019;5(9):1546–1558. doi:10.1021/acsinfecdis.9b00084
24. Jayaraman M, Ansell SM, Mui BL, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angewandte Chemie. 2012;51(34):8529–8533. doi:10.1002/anie.201203263
25. Vu MN, Kelly HG, Tan HX, et al. Hemagglutinin functionalized liposomal vaccines enhance germinal center and follicular helper T cell immunity. Adv Healthc Mater. 2021;10(10):e2002142. doi:10.1002/adhm.202002142
26. Richner JM, Himansu S, Dowd KA, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168(6):1114–1125.e1110. doi:10.1016/j.cell.2017.02.017
27. Kauffman KJ, Dorkin JR, Yang JH, et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–7306. doi:10.1021/acs.nanolett.5b02497
28. Henriksen-Lacey M, Christensen D, Bramwell VW, et al. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3β-[N-(N’,N’-Dimethylaminoethane)carbomyl] cholesterol (DC-Chol), and 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharm. 2011;8(1):153–161. doi:10.1021/mp100208f
29. McNeil SE, Vangala A, Bramwell VW, Hanson PJ, Perrie Y. Lipoplexes formulation and optimisation: in vitro transfection studies reveal no correlation with in vivo vaccination studies. Curr Drug Deliv. 2010;7(2):175–187. doi:10.2174/156720110791011774
30. Patel S, Ryals RC, Weller KK, Pennesi ME, Sahay G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release. 2019;303:91–100. doi:10.1016/j.jconrel.2019.04.015
31. Zeng C, Zhang C, Walker PG, Dong Y. Formulation and delivery technologies for mRNA vaccines. Curr Top Microbiol Immunol. 2020. doi:10.1007/82_2020_217
32. Simberg D, Weisman S, Talmon Y, Barenholz Y. DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Crit Rev Ther Drug Carrier Syst. 2004;21(4):257–317. doi:10.1615/CritRevTherDrugCarrierSyst.v21.i4.10
33. Kranz LM, Diken M, Haas H, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi:10.1038/nature18300
34. Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22. doi:10.1016/j.addr.2020.06.022
35. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi:10.1186/s12943-021-01335-5
36. Thevenot J, Troutier AL, David L, Delair T, Ladavière C. Steric stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromolecules. 2007;8(11):3651–3660. doi:10.1021/bm700753q
37. Ringer S. Regarding the action of hydrate of soda, hydrate of ammonia, and hydrate of potash on the ventricle of the frog’s heart. J Physiol. 1882;3(3–4):195–202.196. doi:10.1113/jphysiol.1882.sp000095
38. Lee JA. Sydney Ringer (1834–1910) and Alexis Hartmann (1898–1964). Anaesthesia. 1981;36(12):1115–1121. doi:10.1111/j.1365-2044.1981.tb08698.x
39. Wang L, Wang X, Li X. Isotonic sodium bicarbonate-triggered emodin release from borate stabilized emodin nanoparticles-loaded polymeric microgel films. Int J Pharm. 2014;469(1):80–87. doi:10.1016/j.ijpharm.2014.04.046
40. Misra A, Jinturkar K, Patel D, Lalani J, Chougule M. Recent advances in liposomal dry powder formulations: preparation and evaluation. Expert Opin Drug Deliv. 2009;6(1):71–89. doi:10.1517/17425240802652309
41. Jensen GM. The care and feeding of a commercial liposomal product: liposomal amphotericin B (AmBisome(®)). J Liposome Res. 2017;27(3):173–179. doi:10.1080/08982104.2017.1380664
42. Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311. doi:10.1016/j.jconrel.2009.10.024
43. Wroblewska L, Kitada T, Endo K, et al. Mammalian synthetic circuits with RNA binding proteins for RNA-only delivery. Nat Biotechnol. 2015;33(8):839–841. doi:10.1038/nbt.3301
44. Muralidharan B, Bakthavachalu B, Pathak A, Seshadri V. A minimal element in 5ʹUTR of insulin mRNA mediates its translational regulation by glucose. FEBS Lett. 2007;581(21):4103–4108. doi:10.1016/j.febslet.2007.07.050
45. Chen X, Wang X, Wang Y, et al. Improved tumor-targeting drug delivery and therapeutic efficacy by cationic liposome modified with truncated bFGF peptide. J Control Release. 2010;145(1):17–25. doi:10.1016/j.jconrel.2010.03.007
46. Che J, Okeke CI, Hu ZB, Xu J. DSPE-PEG: a distinctive component in drug delivery system. Curr Pharm Des. 2015;21(12):1598–1605. doi:10.2174/1381612821666150115144003
47. Guerrero-Cázares H, Tzeng SY, Young NP, Abutaleb AO, Quiñones-Hinojosa A, Green JJ. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014;8(5):5141–5153. doi:10.1021/nn501197v
48. Tavernier G, Andries O, Demeester J, Sanders NN, De Smedt SC, Rejman J. mRNA as gene therapeutic: how to control protein expression. J Control Release. 2011;150(3):238–247. doi:10.1016/j.jconrel.2010.10.020
49. Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi:10.1126/science.1093620
50. Zukancic D, Suys EJA, Pilkington EH, Algarni A, Al-Wassiti H, Truong NP. The importance of poly(ethylene glycol) and lipid structure in targeted gene delivery to lymph nodes by lipid nanoparticles. Pharmaceutics. 2020;12(11):1068. doi:10.3390/pharmaceutics12111068
51. Zhuang Y, Ma Y, Wang C, et al. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: role of lymphatic trafficking and biodistribution. J Control Release. 2012;159(1):135–142. doi:10.1016/j.jconrel.2011.12.017
52. Carralot JP, Probst J, Hoerr I, et al. Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. CMLS. 2004;61(18):2418–2424. doi:10.1007/s00018-004-4255-0
53. Alberer M, Gnad-Vogt U, Hong HS, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human Phase 1 clinical trial. Lancet. 2017;390(10101):1511–1520. doi:10.1016/S0140-6736(17)31665-3
54. Butreddy A, Dudhipala N, Janga KY, Gaddam RP. Lyophilization of small-molecule injectables: an industry perspective on formulation development, process optimization, scale-up challenges, and drug product quality attributes. AAPS PharmSciTech. 2020;21(7):252. doi:10.1208/s12249-020-01787-w
55. Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61(10):3592–3597. doi:10.1128/aem.61.10.3592-3597.1995
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.