Back to Journals » Open Access Surgery » Volume 13
Optimal Management of Post-Laryngectomy Pharyngo-Cutaneous Fistula
Authors Molteni G, Sacchetto A, Sacchetto L , Marchioni D
Received 20 September 2019
Accepted for publication 23 February 2020
Published 3 March 2020 Volume 2020:13 Pages 11—25
DOI https://doi.org/10.2147/OAS.S198038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Luigi Bonavina
Gabriele Molteni, Andrea Sacchetto, Luca Sacchetto, Daniele Marchioni
Otolaryngology –Head and Neck Surgery, Department of Surgery, University of Verona, Verona, Italy
Correspondence: Andrea Sacchetto
Otolaryngology-Head and Neck Surgery, Department of Surgery, University of Verona, Verona, Italy
Tel +39-45-8122330
Fax +39-45-8122313
Email [email protected]
Abstract: The pharyngocutaneous fistula (PCF) is one of the most common post-operative complications in patients undergoing laryngectomy. Up till now, there is no universally accepted algorithm for managing of PCFs and several treatment modalities are used for wound healing. The English language literature was searched using PUBMED databases with the keywords “laryngectomy”, “pharyngocutaneous”, “fistula”, “treatment”, and “management” from January 1, 1999 to June 1, 2019; we selected 35 studies according to inclusion criteria and we conducted a systematic review of the articles. The analysis of the international literature shows a high variability of treatment approaches; there is no consensus about conservative treatment and waiting time, and neither about the indication for surgical treatment or the ideal surgical technique. A first attempt of a conservative measure is mandatory in all cases of PCF. In case of failure of conservative measures surgical treatment should be considered: direct closure and local flap are suitable for small defects, pedicled or free flaps showed good to excellent results in closure of large and complex cervical defects. Other non-invasive treatment such as hyperbaric oxygen therapy (HBOT) and negative pressure wound therapy (NPWT) showed promising results but in limited case series.
Keywords: laryngectomy, pharyngo-cutaneous fistula, reconstructive surgery, hyperbaric oxygen therapy, negative pressure wound therapy, laryngeal cancer
Introduction
The pharyngocutaneous fistula (PCF) is one of the most common post-operative complications in patients undergoing laryngectomy. The reports incidence of PCF is 14.3% for primary total laryngectomy (PTL) and 27.6% for salvage total laryngectomy (STL), according to the most recent meta-analysis published in the international literature.1 Several risk factors have been analyzed in order to understand the etiology of this major complication: previous radiotherapy (RT) or chemoradiotherapy (CHT), type of surgery, T and N stage, a short interval between the end of RT and laryngectomy, hemoglobin levels lower than 125 g/L preoperative as well as postoperative, comorbidities such as diabetes, liver diseases or hypothyroidism, and surgical aspects such as neck dissection, previous tracheotomy, surgical wound infection, resection of the pharynx and its closure after laryngectomy, or the use of non-irradiated tissue to reinforce the pharyngeal suture.2–5 The treatment of PCF is crucial in patients who underwent laryngectomy; the persistence of this abnormal communication is associated with delay in adjuvant treatment, prolonged hospital stay, requirement for reoperation, and mortality from, for example, carotid blowout or aspiration pneumonia. The increased incidence of post-laryngectomy PCF in the modern era of organ preservation therapy has driven considerable efforts to develop techniques to avoid and treat this complication.1 It is generally agreed that most PCFs respond well to conservative management, especially in non-irradiated patients. Surgical closure of PCFs is instead indicated when conservative measures fail, and the fistula persists.6 In addition, several new techniques have been recently developed to treat persistent PCF, such as vacuum-assisted closure system7 and hyperbaric oxygen therapy.8 The aim of this review is to evaluate the different treatment modalities published in the international literature, in order to define the optimal management of post-laryngectomy PCFs.
Methods
The PRISMA guidelines9 for systematic reviews were followed. The English language literature was searched using PUBMED databases with the keywords “laryngectomy”, “pharyngocutaneous”, “fistula”, “treatment”, “management” from January 1, 1999, to June 1, 2019. References for relevant articles were also searched. The rationale for these inclusion dates is to provide a contemporary view of post-laryngectomy PCFs treatment modalities and to select studies focused on management of this post-operative complication. Only studies meeting strict inclusion criteria were included. These are: 1) Site. Only studies reporting data from patients with primary squamous cell carcinoma of the larynx or hypopharynx were included. 2) Procedure. Only studies reporting data from patients undergoing total laryngectomy or total laryngectomy and partial pharyngectomy were included. 3) Complication. Only studies reporting data from patients developing post-operative PCFs. 4) Treatment. Only studies clearly reporting treatment modalities for fistula healing. The exclusion criteria were: 1) studied published earlier than 1 January 1999; 2) studies published as systematic review or meta-analysis; 3) studies not specifically providing data about treatment modalities (conservative, surgical or other) for PCFs; 4) studied published in languages different than English; 5) studies describing treatment of other post-operative fistulas, i.e. orocutaneous or laryngocutaneous fistulas. The corresponding author (AS) reviewed titles and abstracts, read full-text papers, extracted data and assessed quality. The primary data analysed were the different types of treatment (conservative, surgical or other) for PCFs. An analysis of the reported PCFs features was conducted to underline which characteristics are considered to choose each treatment. Finally, among PCFs treated surgically, we conducted a comparison of the results obtained with different reconstructive technique (local, pedicled and microvascular flaps). All the articles included in this systematic review met ethical criteria. The authors have no conflicts of interest in connection with the articles.
Results
The search identified 1625 titles. After removing duplicates and non-English manuscripts, 1073 articles were excluded. 124 full-text articles were identified for assessment of eligibility. Of these, 35 final papers were finally included. Figure 1 summarises the process of titles selection. The studies consisted of retrospective cohort studies (n = 20), case reports (n = 14) and short communication (n =1). No randomised or prospective studies were identified. All studies reported a male preponderance in patient characteristics. The mean age of patients ranged from 46 to 77.5. All the selected studies included patients affected by squamous cell carcinoma of the larynx undergoing total laryngectomy. PTL was the only primary surgical treatment performed in 4 articles, STL in 14 articles; in 17 studies the cohort consisted of patients that underwent both PTL or STL. The selected studies included patients treated with adjuvant therapies, i.e. radiotherapy and/or chemoradiation, in a percentage between 20% and 100%. Conservative treatment of PCF was reported as first treatment attempt in 23/35 studies. Surgical treatments were described in 29 papers, negative pressure wound therapy (NPWT) in 3 studies, hyperbaric oxygen therapy (HBOT) in 2 papers. Application of decellularized and lyophilized human amnion/chorion membrane (DLHACM) graft and of recombinant platelet-derived growth factor-BB (Becaplermin) was described in 1 article. Table 1 summarizes the main data of the selected papers.
 |  |  |  |
 |
Figure 1 Studies identification and selection process. |
Conservative Treatment
Conservative measures are described as the first treatment option in 23 studies. Only 11 of these reported the effectiveness of conservative treatment that ranges between 56% and 90%.
Negative-Pressure Wound Therapy (NPWT)
The use of NPWT is reported in 3 articles; Teixeira et al10 described it as a topical negative pressure dressing, in 1 case Loeck et al11 showed the results obtained with the endoscopic negative-pressure therapy (ENPT), and in another study Zhang et al12 illustrated the effectiveness of a continuous negative pressure-flush through a dual tube. The length of the treatment ranges between 14 and 60 days (mean 31.8 days). The rate of fistulas closure is reported between 87.5% and 100%.
Hyperbaric Oxygen Therapy (HBOT)
HBOT is described as treatment for PCFs in 2 articles. Abu Eta et al8 reported a closure rate of 87.5% in 8 patients treated, while Busoni et al13 a rate of 100% in 15 patients who underwent HBOT. Only Abu Eta et al8 reported the length of the treatment, that ranged between 14 and 45 days.
Surgical Treatment
Surgical treatments were described in 29 papers; the surgical procedures consisted of direct suture of PFCs, closure with local, pedicled or free flaps. Direct closure of PCF is described in 6 articles, the use of local flap in 1 article, pedicled flaps in 21 studies, and free flaps in 6 studies. Other surgical procedures are illustrated in 3 studies: Fink et al14 showed the results obtained with endoscopic surgical technique, Karakullucku et al15 presented a case of refractory PCF treated with positioning of a voice prothesis, and Wiseman et al16 described the results obtained using fibrin glue to reinforce direct closure of PCFs. A high variety of flaps is reported: pectoralis major flap (PMF) was used in 11 case series, deltopectoral (DP) flap in 4 cases; the supraclavicular artery pedicled flap, the temporoparietal fascia flap and the myocutaneous sternocleidomastoid flap were utilized in 2 studies. Less common is the use of the submandibular island flap (n =1), the platysma myocutaneous flap (n = 1), the internal mammary artery perforator flap (n = 1), the lower trapezius island myocutaneous flap (n = 1) and the pedicled latissimus dorsi musculocutaneous flap (n = 1). The reparation with free flaps was achieved using radial forearm free flap (RFFF) (n = 2), jejunal free flap (n = 2), anterolateral thigh (ALT) flap (n = 1), latissimus dorsi free flap (n = 1) and rectus abdominis free flap (n =1).
Discussion
The development of a PCF after laryngeal surgery represents an inconvenient and troublesome complication in the post-operative period. The prevention of this complication is crucial since it usually entails the prolongation of the hospital stay, it sometimes requires revision surgery and rarely puts at risk the life of patients. Numerous risk factors have been identified; among them, previous radiotherapy, pre-operative tracheotomy, neck dissection, tumoral staging and systemic factors (low level of haemoglobin and albumin) are strongly related to the development of fistulas.3,4 On the other hand, up till now, there is no universally accepted algorithm for managing of PCFs. Fistula healing rates between 65 and 94% are commonly reported using conservative measures; in previously irradiated necks, spontaneous closure is less commonly achieved with rates as low as 33% being reported.2 The analysis of the international literature shows a high variability of treatment approaches; there is no consensus about conservative treatment and waiting time, and neither about the indication for surgical treatment or the ideal surgical technique. In addition, the application of new therapies, such as NPWT ad HBOT is preliminary and the number of patients treated is still poor.
Conservative Treatment
Conservative treatment is considered in most of the cases the first option for post-laryngectomy PCFs. According to several authors,4,17-19 it consists of medical treatment with antibiotics and anti–inflammatory drugs; suspension of oral feeding with position of a nasogastric tube or parenteral nutrition; and daily local wound care including drainage of fluids from the fistulous tract, local cleaning, removal of all necrotic tissue followed by curettage of the fistulous borders and pressure dressing above the neck flaps. Saki et al18 also suggested to sterilize the fistula from within by administering 10 mL of 0.25% acetic acid by mouth, while Guha et al20 underlined the importance of maintaining a good nutrition (monitored by albumin levels) and of keeping the Hb level above 10 g/dL. The length of conservative treatment represents a crucial point; several Authors consider there is no reason to wait any longer if PCF’s closure is not obtained in one month with medical management. Busoni et al13 proposed a precise flow chart of treatment: PCFs without major wound breakdown or vessel exposure are treated with conservative therapy for 3 weeks; surgical procedures or HBOT are utilized in case of failure of these measures. Other authors suggested similar therapeutic approaches, with waiting time that lasts 4 weeks,2,17,21 6 weeks,18,20 8 weeks,16,22 but also longer waiting period are described by Huang et al23 (5–18 weeks), Makitie et al24 (4–122 days), McLean et al25 (10–120 days). Iteld et al26 compared the results obtained between patients that underwent early (3–28 days) and late (5–12 months) repair of PCFs with ALT flap. They stated that in the “early reparation” group, surgical dissection in the neck was much easier and the resulting defects in the pharynx and cervical oesophagus were smaller. They advocated early surgical intervention when large PCFs develop; fistulas greater than 5 mm in diameter are unlikely to heal in the setting of prior irradiation and extensive surgery, and eventually will require surgical closure.26 Hirano et al27 described a case of a large PCF (diameter 5 cm x 5 cm) associated with neck abscess that was surgically repaired after 48 hrs from appearance of the fistula. Similarly, Sumarroca et al2 described surgical reparations of PCFs associated with cervical bleeding in an emergency context. Independently from the length of conservative management, it is reported a significant difference in effectiveness between irradiated and non-irradiated patients. The closure rate in non-irradiated patients is reported between 80 and 95%, while in irradiated patients is between 44 and 82%.21 Even though the percentage of closure is limited in this category of patient, conservative measures are mandatory to reduce the risk of neck infections and to obtain in some cases a reduction in the size of the fistula, which might allow the surgical closure of PCFs with less invasive procedures such as direct closure or local flaps.
Two substances have been recently described to treat PCFs: decellularized and lyophilized human amnion/chorion membrane (DLHACM) graft and recombinant platelet-derived growth factor-BB (Becaplermin). Kakabadze et al28 described the use of DLHACM in 8 patients that developed PCF after STL; all eight PCFs closed and the average time of healing of the wound was 18 days. The healing of PCF after using the DLHACM graft was possible due to the unique characteristics of the human amnion/chorion membrane, which possesses immunomodulative, anti-microbial, and anti–inflammatory properties, hastens fibrogenesis and angiogenesis, and increases extracellular matrix deposition. It is a non-invasive technique that prevents the donor morbidity and does not require other surgical interventions. The main drawback is the complex fabrication of the DLHACM, that requires decellularization of placentas, DNA quantification with spectrophotometer, histologic and immunochemical evaluation.
Jakubowicz et al29 report on the contribution of recombinant platelet-derived growth factor-BB (PDGF-BB, Becaplermin, Regranex; OMJ Pharmaceuticals, San German, PR) in treating recalcitrant post-laryngectomy fistulas. PDGF-BB is the only recombinant growth factor licensed for use in the United States and indicated only for diabetic foot ulcers; however, in case reports, Becaplermin has shown promise in resolving chronic orbital ulceration after exenteration for melanoma and improving healing in a chronic neck wound after radiation therapy. Giving these promising results in treatment of chronic wounds, Becaplermin gel was used to heal post-laryngectomy PCF in 2 patients after PTL or STL. After daily application of the growth factor, each wound exhibited an exuberant granulation response, with a 50% decrease in the size of wound at 1 week. The medication alone did not precipitate immediate closure of the fistula during the treatment period, even though the patients experienced eventual closure, with none having local recurrence of their cancer at 2 years’ follow-up. In addition, contraindications to its use include known hypersensitivity to any component of this product and known neoplasm at the site of application. These reports represent interesting non-invasive adjunctive measures that may have a role in refractory PCFs management in the future, none has been widely tested and still need further research for determining their efficacy and safety.
Surgical Treatment
Surgical closure of PCFs is indicated when conservative measures fail, and the fistula persists, exposing the patient to continuous salivary leak with risk of infection, aspiration as well as vessels exposure and even rupture.6 The wide variety of reconstructive procedures reported in the literature indicates the lack of a single, reliable operative approach. The principal of PCF closure is the same in all cases: a fistula must be closed by two epithelial surfaces, one to provide an internal lining and the second for external coverage.6 Many surgical options are available, and the choice of the surgical technique should be dictated by the diameter of the fistula but also by other factors, such as previous radiation history,21 amount of residual pharyngeal mucosa and condition of soft tissue.25 Guha et al20 distinguished 3 types of fistula according to the classification of Hawkes and Stell:30 type 1 fistula is less than 0.5 cm in diameter, type 2 is greater than 0.5 cm but less than 2 cm, type 3 is larger than 2 cm. The type of fistula dictated the surgical approach: for type 1 fistula, the two layers (skin and mucosa) are repaired locally; for type 2 fistulas, one layer is locally repaired and one distantly (DP or PMMC); finally type 3 fistulas are managed with distant flaps (DP and PMMC). Figure 2 summarizes the suggested approach to PCFs depending on defect size. The effectiveness of direct closure seems to be limited by the size of the fistula. In addition, McLean et al25 showed that the closure rate of small PCFs with direct suture is strongly influenced by the local conditions of skin and mucosa, and by previous RT. In fact, 6/7 irradiated patients developed recurrent fistula and required revision surgery with pedicled flap. Direct closure is thus not adequate for larger wounds, especially in radiation fields. Management options for this problem become very limited when this complication occurs after STL and failed organ preservation with chemoradiation because of a lack of recipient neck vessels for free tissue transfer and unfavourable local tissue for local flaps.31 In salvage patients, a higher closer rate might be obtained with the aid of fibrin glue, as reported by Wiseman et al.16 For the first time they used this material to reinforce the double layer direct suture of PCF that developed after STL; the described technique for incorporating fibrin glue into a PCF repair was successful and may be achieved with relative technical ease and minimal patient morbidity.
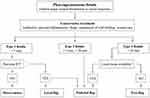 |
Figure 2 Flow chart of the suggested surgical treatments according to PCF size. |
The goals of surgical reconstruction should also focus on minimizing surgical morbidity; according to this consideration, the use of local flaps might reduce length and invasiveness of surgery. Nevertheless, despite many advantages, the closure of PCFs with these techniques is limited in case of large tissue defects and previous RT. These flaps have been used with some success for relatively minor fistulas without significant radiation damage or fibrosis. A localised skin flap was utilized by Kilic et al32 for PCFs with small skin defect; a single-lobed skin flap was successfully used in 4 patients with no recurrence of fistulas and no complications. This technique is especially useful for small PCFs in nonirradiated necks but can be selectively applied in non-heavily irradiated cases with good tissue vascularity.
Pedicled Flaps
The use of pedicled flap for PCFs reparation is widely described in the literature; among the selected articles, 21 studies illustrated their use for fistulas’ healing.
Pectoralis Major Flap (PMF)
The PMF is one of the most common flaps in surgical treatment of PCFs. It shows several advantages: ease of harvest, proximity to the defect, versatility in flap design and highly reliable vascularity. It is often very bulky, with adipose tissue between muscle and skin, and thus it is generally indicated in cases of large substance loss in the pharyngo-laryngeal area.21,33 The issue of bulkiness of the PMMC is, however, controversial and none of the selected studies reported this. The harvesting of the flap might cause a significant morbidity with aesthetic sequelae, especially in female patients; nevertheless, recent studies34 showed favourable aesthetic outcomes at the donor site in most of these patients. The PMF can be used both as myocutaneous (PMMC) and fasciomuscular flap; Sumarroca et al2 described their experience with PMF and suggested that the choice of using a myocutaneous flap or a fasciomucscular flap should depend basically on the size of the defect and the morphology of the patient. A muscle flap without skin paddle is used when the reconstruction of less than a third of the hypopharynx is required, or when the thickness of the skin paddle was considered excessive. In the case of large PCFs requiring reconstruction of more than a third of the hypopharynx, a myocutaneous flap is used. They also suggested the placement of salivary bypass tube in case of surgical closure of PCFs; this expedient reduced the percentage of fistula rate (37% in patients with salivary bypass tube versus 56% in patients without) and the length of hospitalization. The reparation with PMF, and more commonly with PMMC, is reported in case of failure of conservative treatment,2,13,18,24,35 failure of direct closure,21,25 or of other flaps.36 It is otherwise suggested in case of large fistulas20,25 or PCFs with major wound breakdown and vessel exposure.2,13,35 Guha et al20, for example, suggested the use of these pedicled flaps in case of type 2 or 3 fistula (i.e. larger than 0.5 or 2 cm). They described the use of both DP and PMMC in 3 cases of type 3 PCFs. For the closure of these fistulas both flaps are harvested at the same side; PMMC is used to repair the pharyngeal defect, whereas DP is utilized to close the skin defect. The percentage of recurrence of fistula in these case series ranged between 0 and 44%; Sumarroca et al2 analysed the main risk factors in PCFs’ recurrence and stated that the use of the PMF in an emergency context increased the risk of fistula recurrence almost nine-fold. When cervical bleeding is associated with pharynx and cervical dehiscence the pectoralis flap is sutured on infected tissue; these patients, furthermore, are often hemodynamically unstable. According to these results, Sumarroca et al2 suggested that in the emergency setting is preferable to perform a pharyngostoma and to defer the reconstruction of the PCF with a PMF until local and systemic conditions improve. Hamahata et al37 compared the surgical results obtained with the conventional double layer suture operation using PMMC flap and the triple-layer suture operation with “frilled” PMMC flap. In the first group of patients 3/14 patients required re-operation for fistula recurrence, whereas in the second group this complication never occurred.
Deltopectoral Flap (DP)
The DP, elevated from the deltopectoral region and based on an axial vascularization coming from cutaneous branches of intercostal arteries, has long been the flap of choice for closure of large PCFs. Many Authors33,38 comment that this flap usually requires two reconstructive procedures, does not provide big amounts of tissue bulk, and often leaves major aesthetic sequelae. This flap was described in 3 articles and utilized for closure of large PCFs,20,22 in case of failure of direct closure20 or combined to free flaps for treatment of large PCFs after chemoradiotherapy and STL associated with neck abscess and internal jugular vein thrombosis.27
Temporoparietal Fascia Flap (TPFF)
The TPFF may represent in selected cases a new tool for the reconstructive surgeon, when classic reconstructive solutions are likely to fail. It is a fasciocutaneous island flap pedicled on the temporoparietal branch of the superficial temporalis artery. Abt et al39 and Fabrizio et al38 showed the results obtained with TPFF; both authors performed reparation in patients that underwent STL after chemoradiotherapy and later developed large PCFs refractory to conservative and previous surgical treatments. One of the main advantages of this flap in case of patients who underwent STL is that the pedicle is not at risk of RT damage; in addition, TPFF is feasible also in case of failure of previous flaps, as reported by Abt et al.39 However, this flap option has obvious major limitations regarding pedicle length and size of harvested tissue.
Sternocleimastoid (SCM) Flap
The use of sternocleidomastoid muscle is uncommon in literature; the harvesting is limited in case of previous radical neck dissection and difficult if patients underwent pre- or post-operative RT. Kilic et al32 described the use of a myocutaneous SCM flap in 3 patients with good results. Raman et al40 described a surgical method in which the sternocleidomastoid muscle without the cutaneous cover is used for the closure of a PCF in a patient who underwent PTL.
Fasciocutaneous Supraclavicular Flap
Pabiszczak et al41 presented the outcome of a regional pedicled fasciocutaneous supraclavicular flap in 6 patients who underwent STL after chemoradiotherapy; the percentage of failure was 17% (1/6 flaps). The fasciocutaneous supraclavicular flap, owing to its small thickness, plasticity, good vascularity and proximity to the primary operative site could be an effective alternative to other free and pedicled flaps. It is characterized by a low rate of complications of the donor site, it is quick and easy to harvest and consequently especially recommended in patients with accompanying general medical conditions. The span of contraindications is overall limited and could overcome limitations caused by previous bilateral neck dissection and irradiation of the operated site.
Submental Artery Island Flap
The submental artery island flap is a versatile and durable reconstructive flap for closure of facial defects. It can be easily raised on one side and rotated to the whole homolateral face and neck. In selected cases, the flap may be used for closure for PCFs, also in heavily irradiated tissue. The major drawback of this technique is a previous bilateral neck dissection where both facial arteries may have been closed. However, in patients who have undergone previous ipsilateral neck dissections, the submental artery island flap can be raised on the contralateral side pedicle successfully and inset into the defect. Demir et al33 described the closure of PCFs in 9 patients that underwent PTL and adjuvant RT; no intra- or post-operative complications were reported, and the closure rate was 100% at the last follow-up.
Trapezius Island Myocutaneous Flap (TIMF)
The folded extended vertical lower TIMF based on the transverse cervical pedicle was designed to repair large PCFs (diameter larger than 1.8 cm) developing after STL in eight patients.23 According to the authors, the flap offers several advantages: the flap creation is technically simple, and the operating time is relatively short; the flap donor site can be primarily closed; the thin pedicle allows easy transfer of the island flap (which can be tunnelled into a defect in the head and neck); the flap can be used in patients who have previously undergone radiation of the head and neck; donor site morbidity is low; and the donor site is well-hidden. However, care must be taken when placing the flap in patients who have undergone radical neck dissection associated with damage to the transverse cervical pedicle.
Internal Mammary Artery Perforator Flap (IMAP)
Pirgousis et al31 reported the use of the IMAP flap in 2 patients that developed post-laryngectomy PCFs; one patient underwent 13 reconstructive surgeries with local and pedicled flaps; the other patient suffered from a PCF with carotid artery exposure after STL. The pedicled nature of this island flap is particularly suited to close defects in previously irradiated and/or dissected vessel-depleted neck. The authors suggested that the flap could be suitable for defect reconstruction in patients with vessel-depleted necks, where a thin flap is desired. The harvest of the IMAP flap is straightforward without the necessity of microvascular anastomosis and with minimal donor site morbidity.
Delayed Platysma Myocutaneous Turnover Flap
The use of delayed platysma myocutaneous turnover flap was described by Neubauer et al.42 Turnover flaps have been firstly used in the lower extremities to close wounds. They were recently utilized for repair of two PCFs after STL. Delay technique is a well-established phenomenon to improve the blood supply and increase survival to a flap; it involves raising a skin flap with a single pedicle, partially devascularizing tissue for a period of time before transposing the tissue. This procedure, according to the authors, could increase vascularization of the flap and surrounding tissue thus reducing the risk of flap failure.
Free Flaps
When local tissues are unavailable and unable to provide even one epithelial surface for fistula closure, the reconstruction of pharyngeal and cervical tissue is still more challenging and far more complex requiring mobilization of distant tissues for both internal lining and external coverage. The use of free tissues has several advantages: the donor site is far from previously irradiated or infected tissues; the flaps are well vascularized and can be tailored to repair even complex PCFs. The main limitation that might be encountered in using free flaps for closing such complex neck wounds is the availability of suitable neck vessels for microvascular anastomosis; in addition, the fragility and the comorbidities of these patients might contraindicate these surgical procedures. When these situations can be overcome, free flaps are becoming more and more common in preventing e treating PCFs.
The use of RFFF was described in two articles for treatment of large and/or refractory PCFs. This free flap provides many advantages, such as reliability, easy elevation, one-stage procedure, minimal donor morbidity, and allowance for a two-team approach. The largest case series was presented by Bohannon et al;36 22 free flaps were performed in 20 patients. Approximately 86% of patients had radial forearm flap (RFFF) reconstruction, but other flaps included anterolateral thigh (ALT) and rectus free flaps were utilized. In this patient series donor neck vessels were used 54.5%, most commonly the facial vessels, and internal mammary vessel was used in 45.5% of patients. It is interesting to underline that the fistula closure rate with the initial free flap procedure was 50%. Ten patients (50%) had a leak on barium swallow or refistulized requiring additional procedures. After a second free flap, simultaneous pectoralis pedicled flap, or additional local flaps, an overall closure rate of 85% was achieved. These data suggest that there is some difficulty in closing PCFs with a free skin flap in the first repair surgery only; consequently, the authors suggested the use of both RFFF and pedicled pectoralis muscle to reconstitute both the pharyngeal lining and external skin. A smaller case series with promising results has been published by Min Yoo et al,43 that used RFFF “cork designed” to repair two large PCFs without recurrence neither complications.
Other Free Flaps
Few other free flaps are described in the literature (jejunal flap, ALT, latissimus dorsi free flap)24 for PCFs’ treatment, most commonly in case of large pharyngeal and skin defects and often in patients who underwent STL. Free jejunal flap is described by Hirano et al27 and McLean et al;25 in both cases, the free flaps were harvested together with a pedicled (DP or PMF) flap to obtain a complete closure of pharynx and cervical skin. This free flap is thin, pliable, well vascularized, and anatomically reliable; it was chosen to repair the inside of the PCF because of its retractility and low rate of fistula formation. Nevertheless, the use of both jejunal flap and DP pedicled flap has few disadvantages: the DP flap needs another skin graft for wound healing, which results in too many donor sites in critical patients, and the pedicle of the DP needs a secondary revision procedure. In addition, there is a risk of leakage with harvesting an intestine flap in laparotomy, and it will prolong the time of oral intake.27 McLean et al25 utilized jejunal free flap for circumferential pharyngeal reconstruction when there was insufficient remaining pharyngeal wall for closure with PMF. Both cases were successful but a PMF was required for neck closure, prolonging significantly the surgery.
An interesting surgical option was proposed by Iteld et al:26 the use of the anterolateral thigh (ALT) free flap with transverse cervical vessels as recipient vessels. This procedure was performed in 9 patients that previously underwent STL or PTL; in all patients, the complex defects were safely repaired with a single ALT free flap. The use of transverse cervical vessels as recipient vessels avoids surgical dissection around the carotid artery and internal jugular vein, thus reducing the risk of intraoperative complication. The authors suggested to manage complicated PCFs in patients who have had previous radiotherapy and extensive neck surgery directly excising the fistula and scar tissue without exposing the major vessels, and to simultaneously reconstructing the pharyngoesophageal and neck or tracheostoma defects with a multi–island anterolateral thigh free flap.
Endoscopic Surgical Procedure
Fink et al14 recently reported a novel endoscopic approach to the repair of a PCF after STL. The fistula tract was explored with the flexible scope and a 19-gauge needle was passed externally into the neopharynx at the lateral border of the fistula. Then, a 2–0 polyglactin 910 suture was placed through the needle and visualized entering the neopharynx; using palpation to guide placement, the needle was then replaced at the desired exit site for the suture. The suture was then passed retrograde from the endolarynx through the needle to the external neck. This procedure was repeated such that sutures were placed circumferentially around the fistula to quilt it to the anterior platysma skin flap. The mucosalization of the defect and the elimination of the fistulous tract are achieved by reapproximating the platysma to the pharyngeal closure and using the vascularized local tissue to seal the mucosal defect, which then quickly remucosalizes. All patients had closure of PCFs in a radiated field with the above-described endoscopic procedure.14 Only certain fistulae are well suited to this technique; it requires an intact platysma skin flap over the fistula and the ability to access the fistula transorally. In addition, closure is accomplished more simply with smaller fistulae. This procedure, less invasive compared to other surgical technique, showed promising results also in irradiated patients and further studies are mandatory to extend the application of the endoscopic procedure.
Other Treatments
Due to the complexity of reconstruction and potential severe surgical morbidities and mortality, some otherwise eligible patients with benign fistulas are discouraged from undergoing complicated reconstructions. In the last decades, two non-invasive treatments have been used for healing of PCFs: the negative-pressure wound therapy (NPWT) and the hyperbaric oxygen therapy (HBOT). The main advantage of these treatments is the low invasiveness, that allows their application also in fragile and complex patients.
Negative-Pressure Wound Therapy (NPWT)
Negative-pressure wound therapy (NPWT) consists of application of a pressure difference between a wound sealed by an airtight dressing and the external environment, which transforms this open wound into a controlled closed wound allowing management of secretions.44 It is thought to work by decreasing interstitial edema and removing wound exudate, increasing perfusion and neovascularization at the wound site, compartmentalizing the wound, and possibly reducing the bacterial load at the wound site.7 The main expected benefit is the reduction of size and complexity of the wound. The NPWT device is contraindicated in the following situations: uncontrolled wound infection, presence of necrotic tissue requiring debridement, absence of interface between the gastrointestinal tract and the negative-pressure system, non-revascularized arterial disease, residual tumor after resection, presence of more than 50% of fibrin or pressure ulcer. Other significant limitations are the difficulty to maintain an airtight in case of fistulas situated too close to the tracheostoma, and the high costs of the device, even though it has been estimated that NPWT might significantly reduce the hospital stay and thus the costs for each patient treated.
NPWT was also successful in treatment of 2 PCFs that developed after pharyngeal reconstruction with RFFF or PMF.10 In this case, an adhesive gel was placed around the aperture of the fistula in order to protect the skin, improve the adhesiveness, and achieve an adequate sealing. It is interesting to note that both patients were discharged home 6 days after initiating NPWT, given the favourable clinical evolution, and could continue treatment in an outpatient basis, thus reducing the length of hospital stay. Loeck11 and Zhang12 proposed modifications of the classical NPWT; the former used endoscopic negative-pressure therapy (ENPT) in 1 patient while the latter treated 1 patient with continuous negative pressure-flush through a dual tube. The ENPT consisted of placement of a drain device at the wound site, and application of a controlled continuous negative pressure after connecting drainage tube to an electronic vacuum pump. The distal end of the drainage tube is covered with an open-pore polyurethane foam drainage (OPD) or a thin double-layered open-pore film membrane (OFD; Suprasorb CNP Drainagefolie; Lohmann & Rauscher, Rengsdorf, Germany). After 14 days of treatment, a complete closure of the PCF was reported. Zhang et al12 used continuous negative pressure-flush through a dual tube in a complex PCF, after failure of surgical repair with PMF. In this case the length of treatment was 58 days, due to persistent leakage and infection of the wound. The application of NPWT is nowadays limited and only few data are reported in the literature. It is a promising therapy that may play a role in treatment of complex PCFs in patients that cannot underwent further surgical procedures due to severe comorbidities; the difficulties in obtaining an adequate sealing of the wound and the high costs of the device represent significant drawbacks of the technique.
Hyperbaric Oxygen Therapy (HBOT)
HBOT raises oxygen levels in hypoxic tissues, stimulates angiogenesis and fibroplasias enhancing the wound healing rate in hypoxic, irradiated tissue. Use of HBOT in patients with various comorbidities who have failed conservative and surgical management for complex pathologies has gained acceptance. However, use of HBOT for PCFs’ closure is scarcely addressed in the literature.8,13 Busoni et al13 recommended HBOT especially for salvage patients who cannot receive further adjuvant therapy. The authors elaborated a fistula treatment flow chart; the use of HBOT as complementary therapy in conservative management is strongly suggested in patients that underwent STL. The Authors used HBOT in 15 patients and stated that the treatment is highly effective, since in all cases it eventually promoted complete healing.13 Abu Eta et al8 showed instead a closure rate of 87.5% (1/8 failure) with HBOT; patients with PCFs that failed conservative measures were selected for this treatment whose length was reported between 4 and 45 days (average 28 days). The data published in the literature are limited but promising; the successful application of HBOT in salvage patients may suggest the routine use of this therapy as complementary conservative measure, since the standard conservative management in these conditions has proven to be often uneffective.
Conclusions
Review of the literature about PCF after laryngectomy showed a wide variety of treatment modalities and treatment pathways. The choice of the best therapy for PCFs healing should consider the features of the fistula (size, location, local conditions of skin and mucosa) but also the general conditions of the patient and the previous administered treatments. A first attempt of a conservative measure is mandatory in all cases of PCF. In case of failure of conservative measures surgical treatment should be considered: direct closure and local flap are suitable for small defects, pedicled or free flaps showed good to excellent results in closure of large and complex cervical defects. Major flaps are suggested in case of PCFs that develop after STL, but also in case of PCFs associated with cervical infections and vessels exposure. PMF and RFFF are the most used flaps in the literature. Other non-invasive treatments such as HBOT and NPWT showed promising results but in limited case series.
Disclosure
All of the authors have read and approved the manuscript, and none of them have any financial relationships to disclose. The authors report no conflicts of interest in this work.
References
1. Sayles M, Grant DG. Preventing pharyngo-cutaneous fistula in total laryngectomy: a systematic review and meta-analysis. Laryngoscope. 2014;124(5):1150–1163. doi:10.1002/lary.24448
2. Sumarroca A, Rodríguez-Bauzà E, Lop-gros J, et al. Repair of post-laryngectomy pharyngocutaneous fistulas using a pectoralis major flap. Braz J Otorhinolaryngol. 2019;85(3):351–356. doi:10.1016/j.bjorl.2018.03.002
3. Mattioli F, Bettini M, Molteni G, et al. Analysis of risk factors for pharyngocutaneous fistula after total laryngectomy with particular focus on nutritional status. Acta Otorhinolaryngol Ital. 2015;35(4):243–248.
4. Virtaniemi JA, Kumpulainen EJ, Hirvikoski PP, et al. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck. 2001;23(1):29–33. doi:10.1002/(ISSN)1097-0347
5. Šifrer R, Aničin A, Pohar MP, et al. Pharyngocutaneous fistula: the incidence and the risk factors. Eur Arch Otorhinolaryngol. 2016;273(10):3393–3399. doi:10.1007/s00405-016-3963-z
6. Magdy EA. Surgical closure of postlaryngectomy pharyngocutaneous fistula: a defect based approach. Eur Arch Otorhinolaryngol. 2008;265(1):97–104. doi:10.1007/s00405-007-0414-x
7. Andrews BT, Smith RB, Hoffman HT, et al. Orocutaneous and pharyngocutaneous fistula closure using a vacuum-assisted closure system. Ann Otol Rhinol Laryngol. 2008;117(4):298–302. doi:10.1177/000348940811700410
8. Abu Eta R, Eviatar E, Gavriel H. Hyperbaric oxygen therapy as an alternative to surgery for non-healing pharyngocutaneous fistula. Eur Arch Otorhinolaryngol. 2016;273(11):3857–3861. doi:10.1007/s00405-016-4002-9
9. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
10. Teixeira S, Costa J, Bartosch I, et al. Management of pharyngocutaneous fistula with negative-pressure wound therapy. J Craniofac Surg. 2017;28(4):e364–e367. doi:10.1097/SCS.0000000000003682
11. Loeck J, von Lücken HJ, Kehrl W, et al. Endoscopic negative pressure therapy (ENPT) of a post-laryngectomy pharyngocutaneous fistula: first report of a new treatment method. HNO. 2019;67(Suppl 2):77–79. doi:10.1007/s00106-019-0653-3
12. Zhang X, Liu F, Lan X, et al. Continuous negative pressure-flush through a dual tube for the treatment of a complicated pharyngeal fistula: a case report. Oncol Lett. 2016;11(3):1815–1818. doi:10.3892/ol.2016.4152
13. Busoni M, Deganello A, Gallo O. Pharyngocutaneous fistula following total laryngectomy: analysis of risk factors, prognosis and treatment modalities. Acta Otorhinolaryngol Ital. 2015;35(6):400–405. doi:10.14639/0392-100X-626
14. Fink DS, Peña S, Hanby D, et al. Repair of pharyngocutaneous fistula after total laryngectomy: a novel endoscopic approach. Head Neck. 2015;37(7):E81–E84. doi:10.1002/hed.v37.7
15. Karakullukcu MB, Lohuis PJ, van den Brekel MW, et al. Sealing of small postlaryngectomy pharyngocutaneous fistulas with voice prosthesis. Laryngoscope. 2010;120(10):1971–1973. doi:10.1002/lary.v120:10
16. Wiseman S, Hicks W
17. Galli J, De Corso E, Volante M, et al. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Otolaryngol Head Neck Surg. 2005;133(5):689–694. doi:10.1016/j.otohns.2005.07.025
18. Saki N, Nikakhlagh S, Kazemi M. Pharyngocutaneous fistula after laryngectomy: incidence, predisposing factors, and outcome. Arch Iran Med. 2008;11(3):314–317. doi:08113/AIM.0013
19. Pantvaidya GH, Raina S, Mondal A, et al. Total laryngectomy: surgical morbidity and outcomes - a case series. Indian J Cancer. 2017;54(4):621–625. doi:10.4103/ijc.IJC_463_17
20. Guha G, Saha S, Kundu I. Surgical repair of postlaryngectomy pharyngocutaneous fistulas. Indian J Otolaryngol Head Neck Surg. 2007;59(2):103–107. doi:10.1007/s12070-007-0032-3
21. Redaelli de Zinis LO, Ferrari L, Tomenzoli D, et al. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Head Neck. 1999;21(2):131–138. doi:10.1002/(SICI)1097-0347(199903)21:2<131::AID-HED6>3.0.CO;2-F
22. Dedivitis RA, Ribeiro KC, Castro MA, et al. Pharyngocutaneous fistula following total laryngectomy. Acta Otorhinolaryngol Ital. 2007;27(1):2–5.
23. Huang ZQ, Zhou B, Chen WL, et al. Use of a folded extended vertical lower trapezius island myocutaneous flap to repair large pharyngocutaneous fistulae developing after salvage total laryngectomy. Int J Oral Maxillofac Surg. 2018;47(10):1268–1273. doi:10.1016/j.ijom.2018.05.019
24. Mäkitie AA, Niemensivu R, Hero M, et al. Pharyngocutaneous fistula following total laryngectomy: a single institution’s 10-year experience. Eur Arch Otorhinolaryngol. 2006;263(12):1127–1130. doi:10.1007/s00405-006-0152-5
25. McLean JN, Nicholas C, Duggal P, et al. Surgical management of pharyngocutaneous fistula after total laryngectomy. Ann Plast Surg. 2012;68(5):442–445. doi:10.1097/SAP.0b013e318225832a
26. Iteld L, Yu P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J Reconstr Microsurg. 2007;23(6):339–345. doi:10.1055/s-2007-992343
27. Hirano T, Iwasaki T, Fujita K, et al. Repair of a large pharyngocutaneous fistula with a free jejunal patch flap after salvage laryngectomy: a case report. Microsurgery. 2017;37(1):61–65. doi:10.1002/micr.v37.1
28. Kakabadze Z, Mardaleishvili K, Loladze G, et al. Clinical application of decellularized and lyophilized human amnion/chorion membrane grafts for closing post-laryngectomy pharyngocutaneous fistulas. J Surg Oncol. 2016;113(5):538–543. doi:10.1002/jso.24163
29. Jakubowicz DM, Smith RV. Use of becaplermin in the closure of pharyngocutaneous fistulas. Head Neck. 2005;27(5):433–438. doi:10.1002/hed.20182
30. Hawkes AC, Stell PM. Results of closure of pharyngocutaneous fistulae. Clin Otolaryngol Allied Sci. 1980;5(4):249–253. doi:10.1111/j.1365-2273.1980.tb01654.x
31. Pirgousis P, Fernandes R. Use of the internal mammary artery perforator flap for repair of pharyngocutaneous fistulas in the vessel-depleted neck. J Oral Maxillofac Surg. 2011;69(4):1225–1228. doi:10.1016/j.joms.2010.04.021
32. Kiliç C, Tuncel U, Cömert E. Pharyngocutaneous fistulae after total laryngectomy: analysis of the risk factors and treatment approaches. B-ENT. 2015;11(2):95–100.
33. Demir Z, Velidedeoğlu H, Celebioğlu S. Repair of pharyngocutaneous fistulas with the submental artery island flap. Plast Reconstr Surg. 2005;115(1):38–44.
34. Aničin A, Šifrer R, Strojan P. Pectoralis major myocutaneous flap in primary and salvage head and neck cancer surgery. J Oral Maxillofac Surg. 2015;73(10):2057–2064. doi:10.1016/j.joms.2015.05.016
35. Walton B, Vellucci J, Patel PB, Jennings K, McCammon S, Underbrink MP. Post-laryngectomy stricture and pharyngocutaneous fistula: review of techniques in primary pharyngeal reconstruction in laryngectomy. Clin Otolaryngol. 2018;43(1):109–116. doi:10.1111/coa.2018.43.issue-1
36. Bohannon IA, Carroll WR, Magnuson JS, Rosenthal EL. Closure of post laryngectomy pharyngocutaneous fistulae. Head Neck Oncol. 2011;3:29. doi:10.1186/1758-3284-3-29
37. Hamahata A, Beppu T, Saitou T, et al. The usefulness of triple layers suturing technique with frilled pectoralis major musculocutaneous flap for pharyngocutaneous fistula. J Plast Reconstr Aesthet Surg. 2014;67(1):e32–e33. doi:10.1016/j.bjps.2013.08.016
38. Fabrizio T, Donati V, Nava M. Repair of the pharyngocutaneous fistula with a fasciocutaneous island flap pedicled on the superficial temporalis artery. Plast Reconstr Surg. 2000;106(7):1573–1576. doi:10.1097/00006534-200012000-00020
39. Abt NB, Srikanth P, Puram SV, et al. Repair of complex pharyngocutaneous fistula using a staged temporoparietal fascial flap. Am J Otolaryngol. 2017;38(2):254–256. doi:10.1016/j.amjoto.2016.11.016
40. Raman R, Arumainathan UD. Closure of a pharyngocutaneous fistula using a sternomastoid muscle flap. Can J Plast Surg. 2005;13(1):49. doi:10.1177/229255030501300107
41. Pabiszczak M, Banaszewski J, Pastusiak T, et al. Supraclavicular artery pedicled flap in reconstruction of pharyngocutaneous fistulas after total laryngectomy. Otolaryngol Pol. 2015;69(2):9–13. doi:10.5604/00306657.1147032
42. Neubauer P, Cañadas K, Sasaki CT. Delayed platysma myocutaneous turnover flap for repair of pharyngocutaneous fistula. Am J Otolaryngol. 2015;36(1):93–96. doi:10.1016/j.amjoto.2014.08.015
43. Min Yoo W, Suk Pae N, Pio Hong J, et al. Treatment of pharyngocutaneous fistulae with a cork-design radial forearm free flap. J Reconstr Microsurg. 2006;22(7):483–487. doi:10.1055/s-2006-951311
44. Loaec E, Vaillant PY, Bonne L, et al. Negative-pressure wound therapy for the treatment of pharyngocutaneous fistula. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(6):351–355. doi:10.1016/j.anorl.2013.12.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
