Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Optimal Management of Frontal Fibrosing Alopecia: A Practical Guide
Authors Imhof R, Tolkachjov SN
Received 1 July 2020
Accepted for publication 2 November 2020
Published 1 December 2020 Volume 2020:13 Pages 897—910
DOI https://doi.org/10.2147/CCID.S235980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Reese Imhof,1 Stanislav N Tolkachjov2
1Mayo Clinic Alix School of Medicine, Rochester, MN, USA; 2Epiphany Dermatology, Dallas, TX, USA
Correspondence: Stanislav N Tolkachjov Email [email protected]
Abstract: Frontal fibrosing alopecia (FFA) is a primary lymphocytic cicatricial alopecia that is often considered a clinical variant of lichen planopilaris (LPP) due to their shared histopathologic features. FFA is characterized by the recession of the frontal, temporal, or frontotemporal hairline; the clinical pattern is distinct and usually includes eyebrow hair loss, as well as other associated symptoms. Pruritus, facial papules, eyelash loss, body hair involvement, and trichodynia may also occur in addition to the frontotemporal recession and eyebrow loss classically seen. Early diagnosis and prompt treatment are critical as FFA is a progressive disorder that can result in permanent hair loss. FFA is challenging as patients may not present or be recognized until the disease has progressed. Additionally, there is currently no consensus or standard treatment regimen for FFA. While many different therapies have been reported as beneficial, there are a limited number of published guidelines for the treatment of FFA. This article is a review of the literature on treatment modalities for FFA and the objective is to offer a practical guide for clinicians on the evidence-based management options currently available in the literature.
Keywords: alopecia, frontal fibrosing alopecia, hair loss, scarring alopecia, treatment, management, hair, inflammatory hair disorder, lichen planopilaris, primary lymphocytic cicatricial alopecia, scarring
Introduction
First described in 1994 by Kossard,1 frontal fibrosing alopecia (FFA) is a type of primary lymphocytic cicatricial alopecia that is often considered a clinical variant of lichen planopilaris (LPP) due to their shared histopathologic features.2–6 Current evidence suggests the incidence of FFA may be increasing.7–9 FFA is characterized by the recession of the frontal, temporal, or frontotemporal hairline; the clinical pattern is distinct and usually includes eyebrow hair loss, as well as other associated symptoms.1,2,4 Pruritus, facial papules, eyelash loss, body hair involvement, and trichodynia may also occur in addition to the frontotemporal recession and eyebrow loss classically seen.4,5,10,11 Depression of frontal veins,12 hypopigmentation of the frontal hairline,13 and lonely hairs14,15 are other often reported clinical features. Early diagnosis and prompt treatment are critical as FFA is a progressive disorder that can result in permanent hair loss. However, even with aggressive therapy, FFA may progress. Furthermore, patients may experience depressive symptoms related to their hair loss.10 Table 1, formatted from “Reply to: ‘Updated diagnostic criteria for frontal fibrosing alopecia’” in the Journal of the American Academy of Dermatology, outlines the current established diagnostic criteria of FFA.15
 |
Table 1 FFA Diagnostic Criteria |
While FFA is usually seen in postmenopausal women,1,2 it has also been recognized in premenopausal women3–5,10 and men.4,16 The etiology and pathogenesis of FFA are not completely understood and remain areas of active investigation.17 Autoimmune, environmental, hormonal, and genetic factors have all been implicated in FFA.4,5,9,17 In genome-wide association studies, Tziotzios et al observed a genome-wide significant association with FFA at four genomic loci.18 Recent studies suggest that FFA appears to be associated with specific comorbidities (thyroid disease, lichen planus, and rosacea), hormone changes (hysterectomy, hormone replacement therapy, pregnancy, and drugs such as raloxifene), and possibly environmental exposures (facial creams, sun exposure or sunscreen ingredients, and dietary factors).17,19 However, further research is needed to better understand what role these potential risk factors play in FFA development.
FFA is challenging as patients may not present or be recognized until the disease has progressed. Diagnosis and disease stabilization generally take months to years, and recurrence is quite common.10 Additionally, there is currently no consensus or standard treatment regimen for FFA.7 Case reports and cohort studies have reported improvement and remission through the use of various therapies including 5-alpha-reductase inhibitors,4,10 topical and intralesional corticosteroids,4,5,10 topical and oral immunomodulators,5,10 hydroxychloroquine,4,5,10 isotretinoin,20 antibiotics,10,21,22 and pioglitazone.10,22 Although treatment outcomes are variable and there remains a lack of data on treatment efficacy, combination therapy, particularly including systemic treatments, does appear helpful in slowing disease progression and maintaining disease stabilization.10 Table 2 provides a current summary of FFA treatment options reported in the literature, while Figure 1 provides a suggested treatment algorithm. While many different therapies have been reported as beneficial, there are a limited number of published guidelines for the treatment of FFA. The objective of this article is to provide a review of the various treatment modalities for FFA and offer a practical guide for clinicians on the evidence-based management options currently available in the literature.
 |
Table 2 Summary Table of FFA Treatment Options Reported in the Literature |
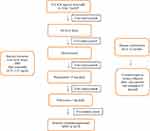 |
Figure 1 FFA treatment algorithm. |
Methods
For this review paper, we conducted a review of the literature to evaluate the treatment options available for FFA. An initial literature search was performed on Dec 30, 2019, using the National Library of Medicine PubMed search engine. Search terms included frontal fibrosing alopecia treatment and frontal fibrosing alopecia management and were limited to journal articles in the English language.
Topical Agents
Topical Corticosteroids
Topical corticosteroids (tCS) are often part of first-line therapy for FFA given their minimal adverse side effect (ASE) profile.23,24 Of note, treatment with tCS as monotherapy is generally not recommended as studies have not found corticosteroids to be successful when used alone.2,5,11,25 In a recently published randomized controlled trial (RCT) evaluating the efficacy of treating FFA utilizing either monotherapy with topical clobetasol 0.05% and tacrolimus 0.1% compared to combination therapy with oral isotretinoin, the monotherapy was not as effective as combination therapy with oral isotretinoin.26 A systematic review on therapeutic options for FFA found that high or moderate potency tCS led to no response in 93% of cases.27 tCS when used in combination with other therapies has yielded mixed results. In a review of 148 patients with FFA treated at Mayo Clinic, topical clobetasol 0.05% solution was noted to be helpful in 69.1% of cases achieving slowing of disease progression (SDP) or disease stabilization (DS); however, this included combination therapy and other systemic treatments.10 In a large study by Vañó-Galván and colleagues that included 355 patients, tCS and topical minoxidil were used in conjunction with various systemic therapies, and the authors reported that the clinical response varied based on the systemic therapy utilized.4 In a cohort study of 72 patients by Heppt and colleagues, the authors found that combining high-potency tCS (topical clobetasol or betamethasone valerate) with a topical calcineurin inhibitor (tCI) (pimecrolimus 1% cream) initially improved hair loss in 64.6% of patients, while 22.9% experienced subjective worsening; however, all patients eventually experienced DS after 9 months of treatment, as well as decreased trichodynia in 33% and improved pruritus in 44%.28 Another retrospective review that included 106 patients with FFA found that 37.3% of patients achieved DS after a year of treatment with topical clobetasol 0.5% foam in combination with a 5α-reductase inhibitor (oral dutasteride, 0.5 mg, three times per week).29 Results from these larger reviews suggest that tCS are clinically beneficial in some FFA patients with active disease when combined with other treatments. However, it is important to consider the long-term ASE of extended tCS use, such as skin atrophy and telangiectasia.28,30 Of note, telangiectasia development may also interfere with the correct assessment of clinical and trichoscopic inflammatory signs.31 MacDonald and colleagues reported several patients with FFA developing follicular pustules and focal purpura secondary to tCS treatment.32 In order to decrease the risk of ASE, one proposal has been to alternate tCS with another topical therapy, such as tCI.33
Topical Calcineurin Inhibitors
tCI include pimecrolimus and tacrolimus, which exert immunomodulatory and anti-inflammatory effects through blockage of calcineurin phosphate and subsequent downstream decreases in cytokine production and T cell activation.33,34 tCI may help treat FFA, as one of the postulated pathophysiologic mechanisms of FFA is T-cell-mediated destruction of follicular stem cells.33 In their paper on FFA treatment options, Fertig and Tosti detail that tCI are part of their favored course of combination treatment, noting that they treat a large number of FFA patients and have had the best results using combination medical therapy with tCI, oral finasteride, hydroxychloroquine, and excimer laser.30 As described above, a recent RCT sought to investigate the efficacy of treating FFA with monotherapy using topical clobetasol 0.05% and tacrolimus 0.1% compared to combination therapy with oral isotretinoin. This study reported that monotherapy with topical clobetasol and topical tacrolimus was not as effective as the combination therapy that included oral isotretinoin.26 When Strazzulla and colleagues performed a retrospective cohort analysis involving 92 FFA cases, the authors compared topical tacrolimus 0.3% and topical clobetasol. The authors report that patients who were treated with tacrolimus 0.3% were more likely to achieve stabilization of hair loss within 3 months.22 As detailed previously, in the Heppt and colleagues’ cohort study, pimecrolimus 1% cream led to an improvement in combination with high-potency tCS.28 One systematic review on therapeutic options for FFA reported that tCI, when used as an adjuvant treatment, resulted in positive effects in over 50% of treated patients.27 In a study involving 29 patients, Zhang and colleagues reported that the most efficacious treatments at limiting hair loss in their population of patients included combination therapy with tCI, topical and intralesional corticosteroids, hydroxychloroquine, and excimer laser therapy.35 Reported side effects from tCI use include erythema and scaling.28 Based on the current available evidence, tCI have a place in FFA treatment and may be efficacious when combined with other treatments, perhaps even more so than tCS.
Topical Minoxidil
Topical minoxidil has a longstanding history of efficacy for pattern hair loss and is thought to increase hair growth and count by both inhibiting excess fibroblasts and increasing vascular endothelial growth factors.33,36,37 Using topical minoxidil as monotherapy is not recommended for patients with FFA, as it is unlikely to be beneficial.33 However, as an adjuvant, topical minoxidil should be considered to increase hair volume.38 Tosti and colleagues reported positive results when treating FFA patients with topical minoxidil 2% BID in conjunction with oral finasteride 2.5 mg per day, successfully halting disease progression in 50% of patients after 12–18 months of treatment.38 There are a number of smaller case series and case reports that describe topical minoxidil as part of treatment in patients who achieved DS.5 In a recent case report of a patient with longstanding FFA, hair and eyebrow regrowth was reported after treatment with a combination of oral finasteride and hydroxychloroquine, corticosteroid injections, and topical minoxidil.39 As with tCS and tCI, topical minoxidil may provide some benefit as part of a combination therapy regimen. It should particularly be considered in patients with mixed FFA and androgenetic alopecia, which is not an uncommon presentation.33
Intralesional Corticosteroids
Intralesional corticosteroids (ILCs) are often considered part of the mainstay of treatment for FFA, as they suppress the inflammatory process, which in turn decreases T-cell-mediated destruction of follicular stem cells.33,40,41 Current evidence supports ILCs as part of first-line treatment for FFA. Vañó-Galván and colleagues reported an overall positive clinical response rate of 83% in patients with FFA who received ILCs’ injections every 3 to 6 months, with an average of eight injections in total. The authors noted that this was the most effective therapy after 5α-reductase inhibitors in their study, and concluded that treatment with ILCs and 5α-reductase inhibitors should be combined in patients who exhibit follicular hyperkeratosis and perifollicular erythema on the exam.4 A systematic review by Ho and Shapiro found that ILCs’ injections were one of the most beneficial therapies utilized, resulting in a positive treatment response in 88% of patients.23 In a retrospective cohort study involving 62 patients with FFA, Banka and colleagues reported that treatment with intralesional triamcinolone acetonide (0.5 to 3 mL per session, every 6 to 8 weeks) injected into the frontal scalp, was beneficial in DS after 4 to 5 sets of injections. The authors noted that symptomatic improvement and hairline stabilization were achieved in 97% of treated patients with ILCs.11 As previously detailed, Zhang and colleagues showed that a combination approach involving ILCs and tCS, tCI, hydroxychloroquine, and excimer laser therapy, were the most effective treatments in their study.35 In a retrospective review, Moreno-Ramirez and Camacho Martinez noted that all 15 patients who received 20 mg/mL of intralesional triamcinolone acetonide injections every 3 months to the frontal hairline and eyebrows achieved DS. Of note, nearly half of these patients were also concurrently treated with oral finasteride and topical minoxidil.42 In a cohort study on the use of ILCs as part of treatment for eyebrow hair loss in patients with FFA, Donovan and colleagues demonstrated that intralesional triamcinolone acetonide is effective at inducing eyebrow regrowth when used as part of combination therapy.40 In this study, intralesional triamcinolone acetonide injections were given at a dose of 10 mg/mL with 0.125 mL per eyebrow, in addition to adjuvant systemic therapy that included hydroxychloroquine, doxycycline, or mycophenolate mofetil (MMF).40 The authors also reported that none of the patients who received ILCs experienced atrophy.40 ILCs injections may also be beneficial in patients who have had longstanding disease when utilized as a part of a combination therapy regimen.39
Oral Anti-Inflammatory Agents
Hydroxychloroquine
Hydroxychloroquine is an antimalarial drug that is thought to benefit patients with FFA through its anti-lymphocytic effect, as it decreases the upregulation of T-cells.33 As FFA can often be treatment resistant with localized therapy alone, many patients need systemic treatments in order to achieve DS. Hydroxychloroquine is considered a first-line systemic agent due to its anti-inflammatory properties and relatively low ASE profile.23 There have been several studies investigating the therapeutic outcomes of patients with FFA treated with hydroxychloroquine. Ho and Shapiro reported, in a systematic review, that overall improvement in the Lichen Planopilaris Activity Index (LPPAI) score was seen in 74% of patients treated with hydroxychloroquine 200 to 400 mg per day alone, while stabilization was found in 71% of patients who received hydroxychloroquine as part of combination therapy.4,5,21,23,43,44 In their systematic review on therapeutic options for FFA, Rácz and colleagues reported that 33 of 114 patients were treated with either hydroxychloroquine or chloroquine and after 6 months of therapy, resulted in a good clinical response in 30% and a partial response (PR) in 39%.27 Vañó-Galván and colleagues reported results from treating 54 patients with oral hydroxychloroquine, between 200 and 400 mg daily. In their study, DS was achieved in 59%, and hair regrowth was reported in 15% of patients.4 Samrao and colleagues described treating 15 FFA patients with hydroxychloroquine and reported that 73% experienced a reduction in signs and symptoms after 6 months.21 Nearly half of patients with FFA treated with oral hydroxychloroquine at Mayo Clinic achieved SDP or DS.10 Fertig and Tosti also include oral hydroxychloroquine as a medication that has resulted in their most beneficial clinical response, in addition to tCI, oral finasteride, and excimer laser.30 Similarly, Zhang and colleagues also noted oral hydroxychloroquine as one of the most efficacious treatments in their study.35 In a study of male patients with FFA, Tolkachjov and colleagues described four male patients who received oral hydroxychloroquine as part of combination therapy with tCS and ILCs and a tCI, with three of the four patients achieving DS.16 In another retrospective study of male patients with FFA, 2 of the patients received oral hydroxychloroquine 200 mg per day and both achieved DS after over a year of treatment.45 Of note, Strazzulla and colleagues performed a retrospective review of 92 FFA patients and compared outcomes from patients who were treated with oral hydroxychloroquine with patients treated with anti-inflammatory antibiotics (doxycycline/tetracycline/minocycline), finding that while there were no significant differences in outcomes, patients treated with doxycycline, tetracycline, or minocycline were more likely to experience ASE.22 There have been no RCT on the use of hydroxychloroquine for FFA. While there are many studies reporting DS after treatment with hydroxychloroquine, it is important to note that many of these involved combination therapy with various other medications. When treating patients with hydroxychloroquine, note that it generally takes up to a year for peak effectiveness to be achieved.33 Additionally, while it is a rare ASE, retinopathy has been associated with the chronic use of hydroxychloroquine or chloroquine. Based on this, the American Academy of Ophthalmology recommends that all patients on hydroxychloroquine or chloroquine receive a baseline ophthalmologic examination as well as annual screenings after 5 years of use, with any patient who experiences vision loss advised to stop the medication and be evaluated by an ophthalmologist.23,46
Oral Tetracyclines
The tetracycline antibiotics, including minocycline and doxycycline, have been utilized as a treatment option for FFA owing to their known anti-inflammatory properties.33 Banka and colleagues reported 13 patients with FFA who all achieved DS after treatment with oral tetracycline 500 mg twice daily or doxycycline 100 mg twice daily, in combination with ILCs and tCS.11 In a Mayo Clinic retrospective review, of the patients with FFA treated with antibiotics, three patients treated with oral doxycycline achieved DS as defined by no further progression of clinical features of the disease, while seven experienced unaltered disease progression (UDP).10 Only one patient in this review was treated with oral minocycline and that patient achieved disease stabilization.10 Out of three patients with FFA who were treated at Duke University Hair Disorders Research and Treatment Center with a combination of oral doxycycline and dutasteride, two were reported to have achieved DS.44 As described previously, when compared to patients treated with oral hydroxychloroquine, Strazzulla and colleagues reported that patients treated with doxycycline, tetracycline, or minocycline had no significant differences in outcomes but were more likely to experience ASE including nausea, esophagitis, lightheadedness, photosensitivity, skin eruption, and candida infection.22 Similarly, Samrao and colleagues reported on four patients with FFA treated with doxycycline for 6 months, of which only one patient responded and one was noted to have a PR.21 Of these four patients, three experienced ASE including gastrointestinal upset and photosensitivity reactions.21 Overall from the current literature, responses to oral tetracyclines have been somewhat unpredictable.23 While some patients do appear to experience benefit, ASE of tetracycline-class antibiotics may limit their use.33
5α-Reductase Inhibitors
The 5-α-reductase-inhibitors (5α-RI) finasteride and dutasteride act as antiandrogens, preventing the enzyme 5-α-reductase from converting testosterone into dihydrotestosterone.32,47 While often used to treat androgenetic alopecia, they have also shown to be effective in patients with FFA. Murad and Bergfeld reported the 5α-RI as being one of the most efficacious treatment options currently available for FFA.47 Ho and Shapiro found that 5α-RI were the most beneficial oral therapy for FFA out of all the studies they included in their systematic review.23 They reported that treatment with finasteride 1 to 5 mg per day or dutasteride 0.5 mg per day stabilized hair loss in 88% (158/180) of patients.4,20,28,38,42,44,48–51 In their systematic review, Rácz and colleagues reported that oral finasteride or dutasteride was most often provided as treatment; approximately 45% of patients in their review showed a good response, while 45% exhibited a PR.27 Furthermore, in their review, Fertig and Tosti have also included oral finasteride as one of their preferred combination medical therapies.30 Vañó-Galván and colleagues reported on 120 patients with FFA who were treated with 5α-reductase inhibitors.4 Of those who received finasteride 2.5 to 5 mg daily, 47% (48/102) showed symptomatic improvement and 52.9% (54/102) experienced DS. Of those who received dutasteride 0.5 mg weekly, 44.4% (8/18) exhibited improvement while 55.6% (10/18) achieved DS.4 Another large review of 106 patients performed by Moreno-Arrones and colleagues found that 37.3% of patients achieved DS after receiving combination therapy with oral dutasteride, 0.5 mg, three times per week, in combination with tCS.29 In a recent large study of 224 patients with FFA, Pindado-Ortega and colleagues reported that dutasteride was the most effective therapy with a dose-dependent response compared to other systemic therapies or no systemic treatment.52 In a retrospective review from Babahoseini and colleagues that included 26 patients with FFA, 5-alpha reductase inhibitors, in combination with systemic retinoids (isotretinoin), were described as the most effective therapeutic options for patients who were treated for FFA at their institution.50 In another retrospective study by Georgala and colleagues, of 13 patients who received dutasteride, 0.5 mg daily, 6 patients (46.1%) achieved stabilization and 2 (15.3%) experienced hair regrowth after 12 months, while all patients remained in remission after 18 months.51 In the Mayo Clinic study, of the 12 patients who were treated with oral finasteride, 10 achieved DS, defined as no further progression of clinical features of the disease, while two continued to have UDP.10 Oral finasteride was noted to be one of the most helpful treatments in patients who achieved SDP or DS, in addition to topical clobetasol 0.05% solution and oral hydroxychloroquine.10 Similarly in the Duke study, Ladizinski and colleagues found that the majority of patients on dutasteride-experienced DS.44 Moreno-Ramirez and Camacho Martinez described seven patients, all of whom achieved DS after an average of 2 years, after being treated with oral finasteride 2.5 mg per day, in combination with ILCs and topical minoxidil.42 Other case reports and series report hair regrowth in FFA patients treated with 5α-RI.39,48,49,53 It has been recommended that patients who seek to halt disease progression consider 5α-RI as part of their treatment regimen.33 In their published treatment algorithm, Ho and Shapiro recommended initiating patients with hairline recession greater than or equal to 1 cm on initial combination therapy with finasteride 5 mg per day if premenopausal or dutasteride 0.5 mg per day if postmenopausal, in addition to intralesional triamcinolone acetonide 2.5 mg/mL per month, and a topical protocol consisting of tacrolimus 0.3% in Cetaphil cleanser, clobetasol solution, and minoxidil 5% solution.23 Although they are felt to be relatively safe, there are some ASE to consider with 5α-RI. In patients who may become pregnant, 5α-RI are category X during pregnancy and have been associated with feminization of male fetuses, such that the recommendation was made to initiate birth control prior to starting treatment in order to help mitigate this potential risk.23,33,54 It is also recommended that finasteride be used over dutasteride in patients who may become pregnant, as finasteride has a comparatively shorter half-life.23,54 Additionally, other reported possible ASE of 5α-RI include psychiatric and sexual ASE, as well as a potential increase in breast cancer risk.33 While 5α-RI have shown benefit in patients with FFA, RCTs are still necessary.
Peroxisome Proliferator-Activated Receptor γ (PPAR-γ) Agonists
PPAR-γ agonists, such as pioglitazone, have been proposed as a possible treatment for scarring alopecias including FFA.23,44,55 PPAR-γ is a transcription factor that works in the regulation of peroxisome and lipid metabolism, with evidence suggesting that it serves a vital role in the function of pilosebaceous units and decreased expression is found in scarring alopecia.33,56,57 Although PPAR-γ agonists such as pioglitazone may potentially be efficacious for treating FFA, there are currently few studies specifically investigating this, as most studies have reported on patients with LPP rather than FFA. In one case of a patient who was reported to have received pioglitazone as monotherapy for FFA, disease stabilization was not achieved after 8 months of treatment.44 In a Mayo Clinic retrospective review, only one patient was treated with oral pioglitazone and did achieve disease stabilization defined by no further progression of clinical features of the disease.10 Additionally, in a retrospective cohort study, four patients were diagnosed with FFA and treated with pioglitazone, with 75% (3/4) of the patients demonstrating disease stabilization and mild regrowth.55 Although there remains a lack of evidence, PPAR-γ agonists have shown some promise in the few reported cases and additional research is certainly warranted to further evaluate their efficacy. It is important to consider potential ASE, however, as PPAR-γ agonists have been associated with heart failure, hypoglycemia, edema, upper respiratory tract infection, decreased bone mineral density and fractures.30,33
Systemic Immunosuppressant Agents
Methotrexate
There are only a few cases reporting on methotrexate use for patients with FFA and results have been mixed. In a Duke University study, three patients were reported to have received methotrexate therapy, and only one achieved stabilization of disease.44 In a Mayo Clinic study, only two patients were reported to have received methotrexate treatment, but both achieved DS as defined by no further progression of clinical features of the disease.10 It is advised that potent immunosuppressant agents such as methotrexate are reserved for patients with refractory disease.23 Additionally, future studies are required before recommendations can be made. MTX is associated with a variety of ASE including hepatotoxicity, nephrotoxicity, gastrointestinal disorders, pneumonitis, hematologic disorders, dermatitis and infections, as well as being category X during pregnancy.58
Mycophenolate Mofetil
There are few studies reporting on the use of MMF, a systemic immunosuppressive medication, for the treatment of FFA. In their systematic review, Ho and Shapiro found that MMF use resulted in improvement in 60% (3/5) of patients.2,21,23 In a Mayo Clinic retrospective review, out of eight patients who were treated with MMF for FFA, five achieved disease stabilization, while three experienced UDP.10 Additional studies with larger sample sizes are warranted before any conclusions regarding efficacy can be drawn.
Oral Prednisone
As with the other systemic immunosuppressant agents discussed, there are few studies evaluating treatment with oral prednisone for FFA. Kossard and colleagues reported that in patients with rapidly progressive hair loss from FFA, oral prednisone 25 to 50 mg per day slowed rapid hair loss in half (2/4) of cases, however patients with slowly progressive hair loss showed no response.2 In their FFA treatment algorithm, Ho and Shapiro suggested that patients with rapidly progressive FFA can be treated with oral prednisone 40 mg per day for 1 week, with a taper by 5 mg per week for 8 weeks, followed by the initiation of maintenance therapy.23
Other Systemic Therapies
Oral Retinoids
Systemic retinoids such as oral isotretinoin are vitamin A derivatives that have historically been utilized to treat moderate to severe acne. Systemic retinoids have also previously been used to treat LPP and it is thought that they may play a role in hair follicular keratinocyte antigen expression, reducing inflammatory infiltrates and suppressing T-cell-mediated destruction.33,59 Based on this, treatment with systemic retinoids has been proposed in patients with FFA. In a retrospective cohort study conducted by Rakowska and colleagues, 40 patients with FFA were treated with systemic retinoids; with 29 patients receiving 20 mg of isotretinoin daily and 11 patients receiving 20 mg of acitretin daily, while a control group of 14 patients received finasteride 5 mg daily.20 Of the patients who were treated with isotretinoin, 76% (22/29) achieved DS by 1 year and 72% (21/29) had no disease progression following the discontinuation of treatment.20 Of the patients who were treated with acitretin, 73% (8/11) achieved both DS by 1 year and no further disease progression following the discontinuation of treatment.20 Of the patients treated with finasteride, only 43% (6/14) achieved both DS by 1 year and no further disease progression following the discontinuation of treatment.20 No patients in this study reported ASE.20 Of note, patients who were treated with systemic retinoids as monotherapy showed higher rates of DS than the patients who were treated with finasteride.20 A recently published RCT investigated the efficacy of treating FFA with oral isotretinoin. Specifically, Mahmoudi and colleagues reported utilizing either monotherapy with topical clobetasol 0.05% and tacrolimus 0.1%, compared to combination therapy with oral isotretinoin.26 The authors reported that monotherapy with topical clobetasol and tacrolimus was not as effective as the combination therapy that included oral isotretinoin.26 In a review of 26 patients with FFA, Babahoseini and colleagues reported that systemic retinoids (isotretinoin) in combination with 5-alpha reductase inhibitors were the most effective therapeutic options for FFA patients treated at their institution.50 A case series by Pham and colleagues reported on three patients with FFA and associated facial papules who were treated with oral isotretinoin after multiple other therapies had failed. All of these patients achieved symptomatic improvement including improvement in their facial papules.59 Additionally, two of the three patients experienced eyebrow hair regrowth.59 In a case report, Pirmez and colleagues reported the results of utilizing isotretinoin in three patients with FFA who also had the characteristic facial papules.60 Patients were reported to have received isotretinoin for 3 months, at 20 mg per day, titrated up to 40 mg per day.60 Of note, these patients were also receiving combination therapy with finasteride 5 mg per day and topical therapy. Although the authors noted that hairline involvement remained unchanged, the patients did achieve complete remission of their FFA associated facial papules.60 Pedrosa and colleagues also reported on the utilization of systemic retinoids, as part of combination therapy with finasteride and topical pimecrolimus, for FFA patients with facial papules.61 In this series, 10 patients received low-dose oral isotretinoin (10 mg every other day) and all patients experienced a notable reduction in facial papules.61 Although there are a limited number of studies to date, results have been promising and suggest that retinoids may be beneficial in treating FFA. Systemic retinoids do have an ASE profile that must be considered, including adverse reproductive, neurological, and cutaneous effects, as well as being category X during pregnancy due to known teratogenicity.32,62
Naltrexone
Naltrexone is a non-selective antagonist of opioid receptors that, within a specific dosage window, is thought to have immunomodulatory effects and has been suggested for use in autoimmune disorders.23,63 Naltrexone has been proposed as a possible treatment option for scarring alopecias including FFA, but there is a need for further research.23 To date, only one case report describes the use of low-dose naltrexone, 3 mg per day, in combination with pioglitazone 15 mg per day, to successfully treat a patient with LPP.64
Oral Minoxidil
Recent studies have investigated the efficacy of low-dose oral minoxidil (0.25–1.25 mg/d) and low-dose sublingual minoxidil in the treatment of LPP and other alopecias including androgenetic alopecia, chronic telogen effluvium, and monilethrix, with promising results shown including increased hair thickness in LPP.65–68 Although no studies have been published to date on the efficacy of oral minoxidil for FFA, it is nevertheless a potential consideration for adjunctive therapy, particularly if the FFA is unmasking androgenetic alopecia. Future studies are needed to investigate the efficacy of oral minoxidil specifically for patients with FFA.
Platelet-Rich Plasma
Platelet-rich plasma (PRP) is an autologous blood product that has received increased attention in the dermatologic fields of hair loss, cosmetic dermatology, and wound healing.69 One theory behind why PRP may be beneficial in patients with hair loss is due to the concentrated number of growth factors in PRP, some of which may stimulate hair regrowth.69 Although there remains a dearth of well-designed studies, there is some growing evidence to suggest that PRP may be beneficial when utilized as part of combination therapy for androgenetic alopecia treatment.69 PRP has also been explored for the treatment of LPP, with studies reporting positive results in improving hair thickening when used as adjunctive treatment.70,71 Özcan and colleagues recently reported on using PRP for the treatment of resistant FFA.72 Currently, there is not enough evidence to make recommendations on utilizing PRP for FFA treatment; however, we recommend using either three treatments, each separated by month duration and then annual or every 6-month follow-up treatment, or starting ongoing monthly therapies and reevaluating efficacy every 6 months. Consideration of cost and lack of efficacy data should be discussed with the patient. As with any hair treatments, meticulous serial photographs are helpful in assessing efficacy.
Low-Level Lasers
Excimer Laser
Excimer laser, via delivery of 308 nm of ultraviolet-B light, has been utilized as a means of treatment for a number of dermatologic disorders including LPP, alopecia areata, psoriasis, and vitiligo.33,73,74 Excimer laser is hypothesized to benefit inflammatory skin diseases via modulation of T-cells and cytokines.33,73 In their survey of 29 patients with FFA, Zhang and colleagues noted that excimer laser therapy was one of the most efficacious at limiting hair loss, as well as intralesional and tCS, tCI, and oral hydroxychloroquine.35 Similarly, Fertig and Tosti also included excimer laser treatment as one of the methods resulting in the best clinical response in their large FFA practice, in conjunction with oral hydroxychloroquine, finasteride, and tCI.30 Unfortunately, there are no other studies reporting on excimer therapy use in patients with FFA and additional evidence is needed.
Light-Emitting Diodes
Superluminescent diodes are a fast-growing form of photobiomodulation therapy, with a wide range of dermatologic indications, that have recently been suggested as potentially beneficial in scarring alopecias.75 Although evidence is currently limited in terms of FFA treatment, one pilot study reported a reduction in symptoms and improved outcomes.75 The study by Gerkowicz and colleagues included 16 female patients, eight with FFA and eight with LPP, all of whom received superluminescent diode irradiations as part of combination therapy once a week for 10 weeks.75 The authors found that therapy was safe and well-tolerated, with a significant reduction in both the LPPAI score and Frontal Fibrosing Alopecia Severity Score (FFASS), as well as significantly increased number of thick hairs, although the number of medium-sized hairs and thin hairs did not significantly change.75 This study suggests that superluminescent diode treatment may be beneficial as adjuvant therapy in FFA patients, although additional studies with larger sample sizes and a control group are necessary.75
Hair Transplantation
In patients with FFA, medical therapy may be able to stabilize and prevent further hair loss, but prior scar tissue can impede hair regrowth and leave patients with permanent areas of alopecia.23 As such, patients may want to pursue hair transplantation as a reconstructive procedure. Unfortunately in patients with FFA, trauma from the transplantation process may aggravate hair loss in some cases.23,76,77 In the largest study to date, Vañó-Galván and colleagues reviewed 51 patients with FFA who underwent hair transplant utilizing the strip technique in 86% and the follicular unit extraction technique in 14%.78 After 1 year, the mean graft survival rate was 87%; after 5 years, the mean graft survival rate was 41%.78 The authors concluded that although reported patient satisfaction was high, the result of hair transplantation in patients with FFA is temporary.78 Overall, there are few studies investigating hair transplantation for patients with FFA. The current literature consists primarily of case reports with mixed results. In their systematic review, while Ho and Shapiro described that hair transplantation usually results in positive effects in the period immediately after the procedure; only 33% of patients had lasting results at 2 years post-transplant.23,76,79,80 Additionally, biopsy results from a patient 4 years post-transplant failure demonstrated histologic findings of FFA.23,79 Cranwell and Sinclair reported on a patient with FFA that underwent transplantation after achieving DS. While this patient initially achieved a satisfactory outcome, ultimately the patient developed implant folliculitis that required removal.53 In terms of eyebrow hair transplantation for patients with FFA, one study investigated the outcomes in ten patients who underwent eyebrow hair transplantation, with the transplanted hairs harvested from non-affected follicles of the occipital scalp.81 The authors reported that although most patients achieved positive results in the short term, the majority lost the transplanted hairs after 3 to 4 years, with only one patient retaining the transplant after 4 years follow-up.81 In patients with FFA who wish to pursue hair transplantation, it has been suggested that DS be a pre-requisite and that patients be counseled regarding expectations, given that disease may recur and transplantation may fail over time.33
Sunscreen Controversy
In recent literature, environmental factors are increasingly implicated as playing a role in the pathogenesis of FFA in susceptible individuals, with some studies suggesting an association between FFA and leave-on facial skincare products and sunscreens.82–84 This is further supported by a study that found titanium nanoparticles in the hair shafts of a patient with FFA, which are an active ingredient in ultraviolet-blocking sunscreen.85 Additionally, Cranwell and Sinclair reported a case of hair regrowth following sunscreen cessation.86 However, the methodology and statistical reporting behind some of these studies has been challenged.87 In a survey-based case series of 56 patients with FFA treated at Mayo Clinic, while a high rate of daily sunscreen product use was reported, when disease progression was compared between patients who used daily facial sunscreen products with those who did not, there was not a significant association found between daily facial sunscreen product use and UDP.88 Additionally, it has been noted that FFA can involve areas of the scalp where sunscreen is not applied, such as the occipital scalp.89 It is important to note that this issue remains controversial as there is currently no conclusive evidence to elucidate the role, if any, that sunscreen products play in FFA pathogenesis.23,88–90 Additional studies are needed to further address this issue.
Abbreviations
5α-RI, 5-α-reductase-inhibitors; ASE, adverse side effects; BID, twice daily; DS, disease stabilization; FFA, frontal fibrosing alopecia; FFASS, Frontal Fibrosing Alopecia Severity Score; ILCs, intralesional corticosteroids; LPP, lichen planopilaris; LPPAI, Lichen Planopilaris Activity Index; MMF, mycophenolate mofetil; PRP, platelet-rich plasma; PPAR-γ, Peroxisome proliferator-activated receptor γ; PR, partial response; RCT, randomized-controlled trial; SDP, slowing of disease progression; tCS, topical corticosteroids; tCI, topical calcineurin inhibitors; UDP, unaltered disease progression.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130(6):770–774. doi:10.1001/archderm.1994.01690060100013
2. Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36(1):59–66.
3. Faulkner CF, Wilson NJ, Jones SK. Frontal fibrosing alopecia associated with cutaneous lichen planus in a premenopausal woman. Australas J Dermatol. 2002;43(1):65–67. doi:10.1046/j.1440-0960.2002.00558.x
4. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70(4):670–678. doi:10.1016/j.jaad.2013.12.003
5. Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160(1):75–79. doi:10.1111/j.1365-2133.2008.08861.x
6. Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont TH, Price VH. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J Am Acad Dermatol. 2005;52(4):637–643. doi:10.1016/j.jaad.2004.07.069
7. To D, Beecker J. Frontal fibrosing alopecia: update and review of challenges and successes. J Cutan Med Surg. 2018;22(2):182–189.
8. Rudnicka L, Rakowska A. The increasing incidence of frontal fibrosing alopecia: in search of triggering factors. J Eur Acad Dermatol Venereol. 2017;31(10):1579–1580. doi:10.1111/jdv.14582
9. Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5(5):309–315. doi:10.1159/000496708
10. Imhof RL, Chaudhry HM, Larkin SC, Torgerson RR, Tolkachjov SN. Frontal fibrosing alopecia in women: the mayo clinic experience with 148 patients, 1992–2016. Mayo Clin Proc. 2018;93(11):1581–1588. doi:10.1016/j.mayocp.2018.05.036
11. Banka N, Mubki T, Bunagan MJK, McElwee K, Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53(11):1324–1330. doi:10.1111/ijd.12479
12. Vañó-Galván S, Rodrigues-Barata AR, Urech M, et al. Depression of the frontal veins: a new clinical sign of frontal fibrosing alopecia. J Am Acad Dermatol. 2015;72(6):1087–1088. doi:10.1016/j.jaad.2015.02.1129
13. Lin J, Valdebran M, Bergfeld W, Conic RZ, Piliang M, Atanaskova Mesinkovska N. Hypopigmentation in frontal fibrosing alopecia. J Am Acad Dermatol. 2017;76(6):1184–1186. doi:10.1016/j.jaad.2017.01.001
14. Tosti A, Miteva M, Torres F. Lonely hair: a clue to the diagnosis of frontal fibrosing alopecia. Arch Dermatol. 2011;147(10):1240. doi:10.1001/archdermatol.2011.261
15. Tolkachjov SN, Chaudhry HM, Imhof RL, Camilleri MJ, Torgerson RR. Reply to: “updated diagnostic criteria for frontal fibrosing alopecia”. J Am Acad Dermatol. 2018;78(1):e23–e24. doi:10.1016/j.jaad.2017.09.027
16. Tolkachjov SN, Chaudhry HM, Camilleri MJ, Torgerson RR. Frontal fibrosing alopecia among men: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2017;77(4):683–690. doi:10.1016/j.jaad.2017.05.045
17. Tavakolpour S, Mahmoudi H, Abedini R, Kamyab Hesari K, Kiani A, Daneshpazhooh M. Frontal fibrosing alopecia: an update on the hypothesis of pathogenesis and treatment. Int J Womens Dermatol. 2019;5(2):116–123. doi:10.1016/j.ijwd.2018.11.003
18. Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10(1):1150. doi:10.1038/s41467-019-09117-w
19. Moreno-Arrones OM, Saceda‐Corralo D, Rodrigues‐Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44(4):404–410. doi:10.1111/ced.13785
20. Rakowska A, Gradzińska A, Olszewska M, Rudnicka L. Efficacy of isotretinoin and acitretin in treatment of frontal fibrosing alopecia: retrospective analysis of 54 cases. J Drugs Dermatol. 2017;16(10):988–992.
21. Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163(6):1296–1300. doi:10.1111/j.1365-2133.2010.09965.x
22. Strazzulla LC, Avila L, Li X, Lo Sicco K, Shapiro J. Prognosis, treatment, and disease outcomes in frontal fibrosing alopecia: a retrospective review of 92 cases. J Am Acad Dermatol. 2018;78(1):203–205. doi:10.1016/j.jaad.2017.07.035
23. Ho A, Shapiro J. Medical therapy for frontal fibrosing alopecia: a review and clinical approach. J Am Acad Dermatol. 2019;81(2):568–580. doi:10.1016/j.jaad.2019.03.079
24. Dina Y, Aguh C. An algorithmic approach to the treatment of frontal fibrosing alopecia-a systematic review. J Am Acad Dermatol. 2018. doi:10.1016/j.jaad.2018.10.043
25. Rallis E, Gregoriou S, Christofidou E, Rigopoulos D. Frontal fibrosing alopecia: to treat or not to treat? J Cutan Med Surg. 2010;14(4):161–166. doi:10.2310/7750.2010.09041
26. Mahmoudi H, Rostami A, Tavakolpour S, et al. Oral isotretinoin combined with topical clobetasol 0.05% and tacrolimus 0.1% for the treatment of frontal fibrosing alopecia: a randomized controlled trial [published online ahead of print, 2020 Apr 20]. J Dermatolog Treat. 2020;1–7.
27. Rácz E, Gho C, Moorman PW, Noordhoek Hegt V, Neumann HA. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27(12):1461–1470. doi:10.1111/jdv.12139
28. Heppt MV, Letulé V, Laniauskaite I, et al. Frontal fibrosing alopecia: a retrospective analysis of 72 patients from a German Academic Center. Facial Plast Surg. 2018;34(1):88–94.
29. Moreno-Arrones OM, Saceda-Corralo D, Fonda-Pascual P, et al. Frontal fibrosing alopecia: clinical and prognostic classification. J Eur Acad Dermatol Venereol. 2017;31(10):1739–1745. doi:10.1111/jdv.14287
30. Fertig R, Tosti A. Frontal fibrosing alopecia treatment options. Intractable Rare Dis Res. 2016;5(4):314–315. doi:10.5582/irdr.2016.01065
31. Saceda-Corralo D, Moreno-Arrones ÓM, Fonda-Pascual P, et al. Steroid-induced changes noted on trichoscopy of patients with frontal fibrosing alopecia. J Am Acad Dermatol. 2018;79(5):956–957. doi:10.1016/j.jaad.2018.05.001
32. MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67(5):955–961. doi:10.1016/j.jaad.2011.12.038
33. Gamret AC, Potluri VS, Krishnamurthy K, Fertig RM. Frontal fibrosing alopecia: efficacy of treatment modalities. Int J Women's Health. 2019;11:273–285. doi:10.2147/IJWH.S177308
34. Wong E, Kurian A. Off-label uses of topical calcineurin inhibitors. Skin Therapy Lett. 2016;21(1):8–10.
35. Zhang M, Zhang L, Rosman IS, Mann CM. Frontal fibrosing alopecia demographics: a survey of 29 patients. Cutis. 2019;103(2):E16–E22.
36. Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11(4):e9860. doi:10.5812/ijem.9860
37. Blumeyer A, Tosti A, Messenger A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–S57. doi:10.1111/j.1610-0379.2011.07802.x
38. Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52(1):55–60. doi:10.1016/j.jaad.2004.05.014
39. Batra P, Sukhdeo K, Shapiro J. Hair loss in lichen planopilaris and frontal fibrosing alopecia: not always irreversible. Skin Appendage Disord. 2020;6(2):125–129. doi:10.1159/000505439
40. Donovan JC, Samrao A, Ruben BS, Price VH. Eyebrow regrowth in patients with frontal fibrosing alopecia treated with intralesional triamcinolone acetonide. Br J Dermatol. 2010;163(5):1142–1144. doi:10.1111/j.1365-2133.2010.09994.x
41. Martínez-Pérez M, Churruca-Grijelmo M. Frontal fibrosing alopecia: an update on epidemiology and treatment. Actas Dermosifiliogr. 2015;106(9):757–758.
42. Moreno-Ramírez D, Camacho Martínez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19(6):700–705. doi:10.1111/j.1468-3083.2005.01291.x
43. Chiang C, Sah D, Cho BK, Ochoa BE, Price VH. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62(3):387–392. doi:10.1016/j.jaad.2009.08.054
44. Ladizinski B, Bazakas A, Selim MA, Olsen EA. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68(5):749–755. doi:10.1016/j.jaad.2012.09.043
45. Alegre-Sánchez A, Saceda-Corralo D, Bernárdez C, Molina-Ruiz AM, Arias-Santiago S, Vañó-Galván S. Frontal fibrosing alopecia in male patients: a report of 12 cases. J Eur Acad Dermatol Venereol. 2017;31(2):e112–e114. doi:10.1111/jdv.13855
46. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. American Academy of Ophthalmology Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–1394. doi:10.1016/j.ophtha.2016.01.058
47. Murad A, Bergfeld W. 5-alpha-reductase inhibitor treatment for frontal fibrosing alopecia: an evidence-based treatment update. J Eur Acad Dermatol Venereol. 2018;32(8):1385–1390. doi:10.1111/jdv.14930
48. Donovan JC. Finasteride-mediated hair regrowth and reversal of atrophy in a patient with frontal fibrosing alopecia. JAAD Case Rep. 2015;1(6):353–355. doi:10.1016/j.jdcr.2015.08.003
49. Katoulis A, Georgala S, Bozi E, Papadavid E, Kalogeromitros D, Stavrianeas N. Frontal fibrosing alopecia: treatment with oral dutasteride and topical pimecrolimus. J Eur Acad Dermatol Venereol. 2009;23(5):580–582. doi:10.1111/j.1468-3083.2008.02963.x
50. Babahosseini H, Tavakolpour S, Mahmoudi H, et al. Lichen planopilaris: retrospective study on the characteristics and treatment of 291 patients. J Dermatolog Treat. 2019;30(6):598–604. doi:10.1080/09546634.2018.1542480
51. Georgala S, Katoulis AC, Befon A, Danopoulou I, Georgala C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61(1):157–158. doi:10.1016/j.jaad.2008.12.026
52. Pindado-Ortega C, Saceda-Corralo D, Moreno-Arrones OM, et al. Effectiveness of dutasteride in a large series of patients with frontal fibrosing alopecia in real clinical practice. J Am Acad Dermatol. 2020. doi:10.1016/j.jaad.2020.09.093
53. Cranwell WC, Sinclair R. Familial frontal fibrosing alopecia treated with dutasteride, minoxidil and artificial hair transplantation. Australas J Dermatol. 2017;58(3):e94–e96.
54. Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59(4):
55. Mesinkovska NA, Tellez A, Dawes D, Piliang M, Bergfeld W. The use of oral pioglitazone in the treatment of lichen planopilaris. J Am Acad Dermatol. 2015;72(2):355–356. doi:10.1016/j.jaad.2014.10.036
56. Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129(5):1243–1257. doi:10.1038/jid.2008.369
57. Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. 2009;145(12):1363–1366. doi:10.1001/archdermatol.2009.283
58. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027
59. Pham CT, Hosking AM, Cox S, Mesinkovska NA. Therapeutic response of facial papules and inflammation in frontal fibrosing alopecia to low-dose oral isotretinoin. JAAD Case Rep. 2020;6(5):453–456.
60. Pirmez R, Duque-Estrada B, Barreto T, Quintella DC, Cuzzi T. Successful treatment of facial papules in frontal fibrosing alopecia with oral isotretinoin. Skin Appendage Disord. 2017;3(2):111–113. doi:10.1159/000464334
61. Pedrosa AF, Duarte AF, Haneke E, Correia O. Yellow facial papules associated with frontal fibrosing alopecia: a distinct histologic pattern and response to isotretinoin. J Am Acad Dermatol. 2017;77(4):764–766. doi:10.1016/j.jaad.2017.04.1118
62. McLane J. Analysis of common side effects of isotretinoin. J Am Acad Dermatol. 2001;45(5):S188–S194. doi:10.1067/mjd.2001.113719
63. Li Z, You Y, Griffin N, Feng J, Shan F. Low-dose naltrexone (LDN): a promising treatment in immune-related diseases and cancer therapy. Int Immunopharmacol. 2018;61:178–184. doi:10.1016/j.intimp.2018.05.020
64. Strazzulla LC, Avila L, Lo Sicco K, Shapiro J. Novel treatment using low-dose naltrexone for lichen planopilaris. J Drugs Dermatol. 2017;16(11):1140–1142.
65. Vañó-Galván S, Trindade de Carvalho L, Saceda-Corralo D, et al. Oral minoxidil improves background hair thickness in lichen planopilaris [published online ahead of print, 2020 Apr 11]. J Am Acad Dermatol. 2020.
66. Ramos PM, Sinclair RD, Kasprzak M, Miot HA. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: a randomized clinical trial. J Am Acad Dermatol. 2020;82(1):252–253. doi:10.1016/j.jaad.2019.08.060
67. Perera E, Sinclair R. Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. F1000Res. 2017;6:1650. doi:10.12688/f1000research.11775.1
68. Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2(3):212–215.
69. Santos LDN, Shapiro J. What’s new in hair loss. Dermatol Clin. 2019;37(2):137–141. doi:10.1016/j.det.2018.11.002
70. Jha AK. Platelet-rich plasma for the treatment of lichen planopilaris. J Am Acad Dermatol. 2018;79(5):e95–e96. doi:10.1016/j.jaad.2018.05.029
71. Jha AK. Platelet-rich plasma as an adjunctive treatment in lichen planopilaris. J Am Acad Dermatol. 2019;80(5):e109–e110. doi:10.1016/j.jaad.2018.09.013
72. Özcan D, Tunçer Vural A, Özen Ö. Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: a case report. Dermatol Ther. 2019;32(5):e13072. doi:10.1111/dth.13072
73. Navarini AA, Kolios AG, Prinz-Vavricka BM, Haug S, Trüeb RM. Low-dose excimer 308-nm laser for treatment of lichen planopilaris. Arch Dermatol. 2011;147(11):1325–1326. doi:10.1001/archdermatol.2011.335
74. Mehraban S, Feily A. 308nm excimer laser in dermatology. J Lasers Med Sci. 2014;5(1):
75. Gerkowicz A, Bartosińska J, Wolska-Gawron K, Michalska-Jakubus M, Kwaśny M, Krasowska D. Application of superluminescent diodes (sLED) in the treatment of scarring alopecia - A pilot study. Photodiagnosis Photodyn Ther. 2019;28:195–200. doi:10.1016/j.pdpdt.2019.09.006
76. Liu YCS, Jee SH, Chan JYL. Hair transplantation for the treatment of lichen planopilaris and frontal fibrosing alopecia: a report of two cases. Australas J Dermatol. 2018;59(2):e118–e122. doi:10.1111/ajd.12682
77. Kossard S, Shiell RC. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int J Dermatol. 2005;44(4):321–323. doi:10.1111/j.1365-4632.2004.02251.x
78. Vañó-Galván S, Villodres E, Pigem R, et al. Hair transplant in frontal fibrosing alopecia: a multicenter review of 51 patients. J Am Acad Dermatol. 2019;81(3):865–866. doi:10.1016/j.jaad.2019.05.031
79. Jimenez F, Poblet E. Is hair transplantation indicated in frontal fibrosing alopecia? The results of test grafting in three patients. Dermatol Surg. 2013;39(7):1115–1118. doi:10.1111/dsu.12232
80. Nusbaum BP, Nusbaum AG. Frontal fibrosing alopecia in a man: results of follicular unit test grafting. Dermatol Surg. 2010;36(6):959–962. doi:10.1111/j.1524-4725.2010.01580.x
81. Audickaite A, Alam M, Jimenez F. Eyebrow hair transplantation in frontal fibrosing alopecia: pitfalls of short- and long-term results. Dermatol Surg. 2019. doi:10.1097/DSS.0000000000002207
82. Mirmirani P, Tosti A, Goldberg L, Whiting D, Sotoodian B. Frontal fibrosing alopecia: an emerging epidemic. Skin Appendage Disord. 2019;5(2):90–93. doi:10.1159/000489793
83. Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175(4):762–767. doi:10.1111/bjd.14535
84. Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177(1):260–261. doi:10.1111/bjd.15311
85. Brunet-Possenti F, Deschamps L, Colboc H, et al. Detection of titanium nanoparticles in the hair shafts of a patient with frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32(12):e442–e443. doi:10.1111/jdv.14967
86. Cranwell WC, Sinclair R. Frontal fibrosing alopecia: regrowth following cessation of sunscreen on the forehead. Australas J Dermatol. 2019;60(1):60–61. doi:10.1111/ajd.12833
87. Seegobin SD, Tziotzios C, Stefanato CM, Bhargava K, Fenton DA, McGrath JA. Frontal fibrosing alopecia: there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175(6):1407–1408. doi:10.1111/bjd.15054
88. Imhof RL, Larkin SC, Cantwell HM, Torgerson RR, Tolkachjov SN. The association of frontal fibrosing alopecia with skin and hair care products: a survey-based case series of 56 patients seen at Mayo Clinic [published online ahead of print, 2020 May 10]. J Am Acad Dermatol. 2020.
89. Felmingham C, Yip L, Tam M, Nixon RL. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182(2):481–482. doi:10.1111/bjd.18380
90. Robinson G, McMichael A, Wang SQ, Lim HW. Sunscreen and frontal fibrosing alopecia: a review. J Am Acad Dermatol. 2020;82(3):723–728. doi:10.1016/j.jaad.2019.09.085
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
