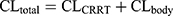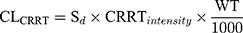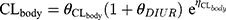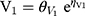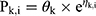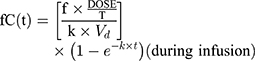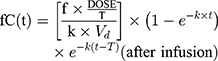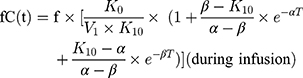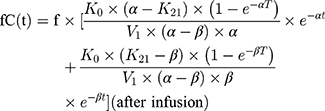Back to Journals » Infection and Drug Resistance » Volume 13
Optimal Empiric Treatment for Klebsiella pneumoniae Infections in Short-Stay ICU Patients During Continuous Renal Replacement Therapy: Results from a Population Pharmacokinetic/Pharmacodynamic Analysis
Authors Jin Y, Mao H, Liu B , Zhou F, Yang J, Xu L, Tong J , Huang C, Ding Y
Received 4 October 2020
Accepted for publication 30 October 2020
Published 19 November 2020 Volume 2020:13 Pages 4155—4166
DOI https://doi.org/10.2147/IDR.S284754
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Yuhong Jin,1 Haiyan Mao,1 Bingyang Liu,1 Fen Zhou,1 Junjie Yang,1 Lei Xu,1 Jingtao Tong,2 Chen Huang,3 Yi Ding1
1Department of Intensive Care, Lihuili Hospital, Ningbo Medical Center, Ningbo, People’s Republic of China; 2Department of Radiotherapy, Lihuili Hospital, Ningbo Medical Center, Ningbo, People’s Republic of China; 3Department of Respiratory Medicine, Lihuili Hospital, Ningbo Medical Center, Ningbo, People’s Republic of China
Correspondence: Chen Huang
Department of Respiratory Medicine, Lihuili Hospital, Ningbo Medical Center, Ningbo 315000, People’s Republic of China
Tel +86-574-87018701
Fax +86- 574-87392232
Email [email protected]
Yi Ding
Department of Intensive Care, Lihuili Hospital, Ningbo Medical Center, Ningbo 315000, People’s Republic of China
Tel +86-574-87018661
Email [email protected]
Objective: There is a paucity of published data to evaluate the efficacy and safety of imipenem (IPM) and piperacillin-tazobactam (PT) dosing regimens in the treatment of septic patients acquiring continuous renal replacement therapy (CRRT).
Methods and Materials: Critically-ill patients were grouped into short-stay and long-stay intensive care unit (ICU) patients. Pathogens were isolated from bloodstream infections in these patients. Minimum inhibitory concentration (MIC) value was determined by agar dilution method. Population PK models were introduced in this study, and differences in the likelihood of achieving efficacious and toxic exposures of IPM and PT for critically-ill patients were assessed.
Results: A total of 86 K. pneumoniae bloodstream infection associated isolates were collected, and the MIC50 and MIC90 for short-stay ICU patients were 0.5/4 mg/L and 32/128 mg/L, respectively. IMP 0.5g q8h reached 90% probability of target attainment (PTA) against isolates with MICs ≤ 2 mg/L and was recommended to empirically treat short-stay ICU patients during CRRT based on the target of 40% ƒT>MIC. However, based on a more aggressive target of 100% ƒT>MIC, all the simulated IMP regimens except for IMP 1g q6h failed to achieve > 80% cumulative fraction of response (CFR) in such patients. Unfortunately, the risk of drug-related toxicity for IMP 1g q6h was relatively high (50– 85%). For PT, even the regimen of 4/0.5g q6h failed to provide sufficient antimicrobial exposure in short-stay ICU patients acquiring CRRT.
Conclusion: No dose adjustment was required for the conventional IMP and PT regimens in the critically-ill population acquiring CRRT. Empirical treatment of IMP 0.5g q8h/q6h, not for PT, may provide sufficient antimicrobial exposure for short-stay ICU patients during CRRT. PT should be used in the knowledge of MIC results.
Keywords: Klebsiella pneumoniae infection, pharmacokinetic/pharmacodynamic, continuous renal replacement therapy; CRRT, intensive care unit; ICU
Introduction
The treatment of critically-ill patients with sepsis in intensive care units (ICU) is challenging because of disease complexity, often resulting in acute kidney injury and multiple organ dysfunction syndrome (MODS) as well as reduced antibiotic susceptibilities of nosocomial pathogens.1 In this setting, continuous renal replacement therapy (CRRT) is a well-established and effective method that can cause enhanced elimination of drugs to avoid overdosing-related toxicity.2 However, significant and unpredictable pharmacokinetic (PK) variability (i.e. the volume of distribution (Vd) and the clearance (CL)) in such patients has been found and these parameters may vary depending on the physical condition and the type and intensity of CRRT on elimination of drugs.3,4 Therefore, optimizing the dosing regimen in such circumstances is a crucial but difficult issue.
It has been stated that an increased risk of carbapenem-resistant Klebsiella pneumoniae (CRKP) infections and mortality was observed for patients with prolonged hospitalization in ICU.5–7 In other words, patients in ICU are relatively easily infected with CRKP. Notably, the risk of the duration of ICU stay for patients infected with these organisms is still unknown. A previous study revealed that low colonization prevalence of carbapenem-resistant Enterobacteriaceae among patients in short-stay hospital ICU patients, compared to long-term (3.3% vs. 30.4%, prevalence ratio, 9.2; 95% CI, 6.3–13.5).8 Early and appropriate antibiotic therapy in critically-ill patients is associated with the promising clinical outcome.9 Considering the fear of overdosing-related toxicity and the lack of therapeutic drug monitoring (TDM), the target antimicrobial concentration was suboptimal in some critically-ill patients during CRRT.10 Actually, there is a paucity of published data to evaluate the efficacy and safety of antibiotic dosing regimens in the treatment of septic patients during CRRT.
Given that the above situation, the Monte Carlo simulation (MCS) was introduced in our study to integrate population-PK parameters and population-minimum inhibitory concentration (MIC) pathogen data together so as to avoid underdosing and overdosing-related treatment failure.11 Imipenem (IPM) is one of the leading antibiotic with a broad-spectrum antibacterial activity against most Gram-positive, Gram-negative and anaerobic isolates, including multidrug-resistant isolates.12 And for piperacillin-tazobactam (PT), it has been demonstrated clinical efficacy in the treatment of community- or hospital-acquired infections.13,14 These two drugs are frequently used intravenous antimicrobials for the treatment of severe infections in ICU settings. The aim of our study was to: 1) determine the MIC distributions of IMP and PT against K. pneumoniae isolated from ICU; and 2) evaluate the efficacy and safety of these two antibiotics in critically-ill patients with sepsis during CRRT.
Methods and Materials
Bacterial Isolates and Susceptibility Testing
Clinical K. pneumoniae, isolated from a 29-bed and 22-bed medical ICU, were collected between January 2015 and December 2019. Strains were isolated and identified in accordance with clinical microbiological methods using the API20 system (bioMérieux, Durham, NC, USA). Furthermore, strains were re-identified by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF, Bruker) mass spectrometry (MS). The MICs for IMP and PT were tested using the standard agar dilution method, as recommended by the Clinical and Laboratory Standards Institute (CLSI).15 The results were interpreted based on the following: IMP ≤1 mg/L, 2 mg/L = intermediate, ≥4 mg/L = resistant; PT ≤16/4 mg/L = susceptible, 32/4–64/4 mg/L = intermediate, ≥128/4 mg/L = resistant. E. coli American Type Culture Collection (ATCC) 25,922 was used as a quality control.
Population PK Model
The population PK (PPK) models for IPM and PT in critically-ill patients during CRRT were derived from previous studies.16,17 The PPK model for IPM was a one-compartment model parameterized on CL and V1. The equations were displayed as follows:16
where Sd referred to saturation coefficients and the value was assumed to be 1, WT referred to weight, CRRTintensity referred to the sum of ultrafiltration rate and dialysis rate, divided by body weight. θ was population estimate, and the typical parameters for CLbody and V1 were 6.11 and 34.2, respectively. The high- and low-intensity were 40 mL/kg/h and 20 mL/kg/h, respectively. θDIUR was an indicator variable with a value of 0 for patients with anuria. The inter-individual variability (IIV) for CLbody and Vd were 36.6% and 47.2%, respectively. The fraction of unbound IPM was 0.8±0.16.18
Piperacillin is regarded as the main agent exerting bactericidal effects against isolates. Thus, the parameters of piperacillin were used for MCS. The PPK model for piperacillin in ICU patients with anuria undergoing CRRT was a two-compartment model.17 The CRRTintensity in this model was in the range of 35–45 mL/kg/h. As the patients in the PPK model were anuric and on dialysis, it was not possible to evaluate a covariate effect of creatinine clearance on elimination. IIV was implemented on the structural PK parameters as follows:
where Pk,i represents the estimated kth PK parameter for the ith individual calculated from the population PK parameter θk of the typical patient whilst ηk, i represents the deviation from the typical PK parameter assuming log-normal distribution. The typical PK parameters of CL, V1, V2, and Q were 3.3, 6.77, 10.0, and 15.4, respectively. CL and V1 were scaled to WT in line with allometry, where weight was normalized to 70 kg for a typical adult and scaled using a fixed exponent of 0.75 for CL and 1 for V1. The IIV in CL, V2 and Q were 10.9%, 26.0% and 54.8%, respectively. The fraction of unbound piperacillin was 0.78±0.13.19
Pharmacokinetics/Pharmacodynamics Target (PK/PD)
As the drug of beta-lactam, IMP and PT displays time-dependent bactericidal effect against K. pneumoniae, and the percentage of time during a dosing interval that free plasma concentrations exceed the MIC (%ƒT>MIC) is the best PD index most closely linked to clinical efficacy. In general, the PD targets were ≥40% ƒT>MIC for IPM and ≥50% ƒT>MIC for PT.20 Since the simulated subjects were critically-ill patients with sepsis, ≥100% ƒT>MIC for IPM and PT was also suggested for the promising outcomes.21,22 To evaluate the risk of potentially toxic in critically-ill patients, we used the trough concentrations of >10 mg/L for IPM and>150 mg/L for piperacillin to be a threshold for expected toxicity.23,24 All the simulated dosing regimens were conducted to measure the possibility of clinical cure and toxic effects.
Monte Carlo Simulation
The initial 48 hours of free serum concentration–time profiles were simulated for IPM and PT dosing regimens using the parameters derived from PPK models. A 1-compartmental model for IMP with constant intravenous input and first-order elimination was used:
where ƒ is the unbound fraction, C(t) is the drug concentration at a specific time, T is the infusion time, k is the elimination rate constant, Vd is volume of distribution, and t is the time from infusion initiation.
A 2-compartmental model for piperacillin with constant intravenous input and first-order output was used:
where  and
and 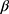 are hybrid first-order elimination rate constants of the distribution and elimination phase, V1 is the volume of distribution of the central compartment and k21 is the transfer rate constant from the peripheral compartment to the central compartment.
are hybrid first-order elimination rate constants of the distribution and elimination phase, V1 is the volume of distribution of the central compartment and k21 is the transfer rate constant from the peripheral compartment to the central compartment.
A 1000 patients MCS was conducted to calculate the probability of target attainment (PTA) and cumulative fraction of response (CFR) of each dosage regimen against bacterial population using Crystal Ball software (version 11.1.2.4; Oracle) to evaluate clinical efficacy and safety. The following dosage regimens were simulated: IPM 0.5g q8h/q6h as intermittent infusion (II) and 1g q8h/q6h as II, PT 2/0.25g q6h as II and 4-h infusion (extended-infusion, EI), 4/0.5g q12h/q8h/q6h as II and EI. Optimal dosing regimens were selected if they provided ≥90% PTA and the risk of drug-related toxicity was low. The CFR was calculated as the proportion of %PTA of each MIC according to the MIC distributions. An optimal regimen was defined as reaching ≥90% CFR against a population of organisms whereas a CFR between 80% and 90% was associated with moderate probabilities of success.25,26
Statistical Analysis
A previous multidrug-resistant organism surveillance network involving ICUs of long-term (>10) and short-stay (<10 days) acute care hospitals was conducted to ascertain the prevalence of colonization by carbapenem-resistant Enterobacteriaceae.8 Thus, the critically-ill patients with sepsis were grouped to short-stay and long-term ICU patients in our study. Comparisons between groups used the chi-square tests as appropriate. A P value of 0.05 was considered statistically significant, and all analyses and figures were performed using R v.4.0.2.
Results
Microbiological and Clinical Data
A total of 86 K. pneumoniae bloodstream isolates were collected from ICU in this study. The carbapenem-resistant rate was 42% (36/86). The in vitro activities of IMP and PT against the tested isolates were summarized in Table 1. In our study, the median lengths of ICU stay before onset of carbapenem-susceptible K. pneumoniae and CRKP bloodstream infections (BSIs) were 3 and 27.5 days, respectively. We used the duration of ICU stay to assess the risk for acquisition of CRKP BSIs. The chi-square analysis revealed that short-stay ICU patients was tended to be infected with carbapenem-susceptible K. pneumoniae BSIs (P<0.0001, Figure 1). In the subgroup of short-stay, the MIC50 for IMP and PT against this collection of isolates were 0.5 and 4 mg/L, while the MIC90 were raised to 32 and 128 mg/L, respectively.
 |
Table 1 MICs of K. pneumoniae Against Tested Agents |
Monte Carlo Simulation
The relationships between MIC and PTA for different dosing regimens were presented in Figures 2 and 3. For critically-ill patients requiring CRRT, body weight showed an impact on the achievement of PTA for the simulated regimens. For example, the cut-off for achieving >90% PTA was 64 mg/L in patients weighting 50 kg for the simulated regimens of PT 4/0.5g q8h/q6h II/EI, while this was lowered to 32 mg/L in patients weighting 100 kg, based on the PK/PD target of 40% ƒT>MIC (Figure 2). For patients with severe life-threatening infections, 100% ƒT>MIC might be considered as a more appropriate target. Similar trends were also observed in the different weighted patients. It is worth noting that only the regimen of PT 4/0.5g q6h EI reached 90% PTA against isolates with a MIC of 64 mg/L in the 50 kg ICU patients. And for isolates with a MIC of 32 mg/L, PT 4/0.5g q6h II/EI achieved the promising PTA target regardless of body weight.
For all the simulated IMP regimens, the PTAs were almost 100% against isolates with MIC ≤2 mg/L regardless of body weight based on the target of 40% ƒT>MIC (Figure 3). However, the value was lowered to <0.125 mg/L for IMP 0.5g q8h II when the target of 100% ƒT>MIC was used. None of the simulated regimens reached ≥90% PTA against a MIC of 2 mg/L regardless of body weight or CRRT modality. Increasing the daily dose could improve the cut-off value. The achievable MIC value was ≤1 mg/L in critically-ill patients administered with IMP 1g q6h II during CRRT.
The possibility of developing drug-related toxicity of each simulated regimen was presented in Figures 4 and 5. The MICs of 1 and 64 mg/L was used to calculate the PTA of different dosing regimens of IMP and PT reaching 100% ƒT>MIC, respectively. Considering efficacy from the PTA target and the probability of developing toxicity, the regimen of PT 4/0.5g q6h II was suggested for 75/100 kg critically-ill patients infected with organism with high MICs (32–64 mg/L) during CRRT (Figure 4). And if the body weight of ICU patients was 50 kg, the optimal regimen was PT 4g q8h II. For the IMP regimens, a higher risk of drug-induced toxicity was observed when compared to PT. When CRRT with lower effluent rate of 20 mL/kg/h was prescribed, the probability of toxicity was >50% for IMP 1g q6h II regardless of body weight (Figure 5). Elevated effluent rate (40 mL/kg/h) resulted to the probability of toxicity in the range of 20%–-50%. In addition, the regimen of IMP 0.5g q6h II had greater therapeutic effect and lower toxicity as well as better economic than IMP 1g q8h II.
The results of Cmin were also calculated and showed that the Cmin in IMP regimens was highly heterogeneous. The median values of Cmin in all the simulated IMP regimens were 1.5–12.5 mg/L, whereas the minimum values were all <0.1 mg/L (Table 2). Figures S1 and S2 showed the simulated median and 95% prediction interval of 0–48 h free IMP and piperacillin concentrations in 75 kg criticallyill patients requiring CRRT.
 |
Table 2 The Simulated Cmin of PT and IPM Dosing Regimens in Critically-Ill Patients Requiring CRRT |
As the CFR values were similar for patients in different body weight, 75kg critically-ill patients was presented in Table 3. For the tested K. pneumoniae isolated form short-stay ICU patients, all of the simulated IMP regimens reached ≥80% and 50–82% CFRs based on targets of 40% and 100% ƒT>MIC, respectively. And for the simulated PT regimens, the decreasing CFRs (70–75%) were found against the above strains regardless of II or EI. However, against all the K. pneumoniae isolated from BSIs in ICU, the worse outcomes were achieved with the CFR values of 40–65% for IMP and 45–60% for PT.
 |
Table 3 Cumulative Fraction of Response (CFR) to IPM and PT Against the Tested K. pneumoniae Isolated from ICU Patients |
Discussion
IMP and PT are the widely prescribed drugs and account for a large proportion of antibiotic consumption in ICU settings. Notably, the antimicrobial concentration in BSI are known to vary greatly in critically-ill patients during CRRT. Such patients may be administrated with suboptimal dosing regimens in fear of drug-related toxicity. Thus, sufficient antimicrobial exposure remains a pivotal challenge for ICU clinicians. In this study, the Bayesian-based dosing regimens, based on the PPK models, were conducted to provide individualised dosing regimens from a patient’s own physiopathological characteristics. We evaluated the possibility of clinical cure and the risk of drug-related toxicity simultaneously to optimize the dosing regimens for ICU patients requiring CRRT. Our findings highlighted that empiric treatment of PT 4/0.5g q6h II or EI failed to provide sufficient antimicrobial exposure to treat K. pneumoniae BSIs for short-stay ICU patients regardless of the PK/PD targets. Conversely, IMP 0.5g q8h II was recommended to treat in such patients based on the target of 40% ƒT>MIC, and increasing the daily dosage could be used in severe infections caused by multidrug-resistant isolates (up to ≤8 mg/L). However, based on a more aggressive target of 100% ƒT>MIC, all the simulated IMP regimens except for IMP 1g q6h II failed to achieve >80% CFR in treating short-stay ICU patients with sepsis in the CRRT intensity of 20 mL/kg/h. Unfortunately, the risk of drug-related toxicity for IMP 1g q6h II was relatively high (50–85%). For the remaining simulated IMP regimens, the potentially toxic possibility was low (0–30%) in different CRRT modalities. Thus, no dose adjustment was required for the daily dose of IMP ≤3 g in ICU patients during CRRT.
From several previous surveillance studies, K. pneumoniae is one of the most prevalent bloodstream isolates in ICUs.27,28 Thus, it is critical to know local trends in resistance and population-MIC distributions as well as the best regimens to maximise the efficacy and minimise the probability of toxic effects. In our study, the resistance of K. pneumoniae to IMP was 41.9%, which was lower than that of previous studies reported.27,28 This may be that severe infections have been occurred in some patients before they were admitted to ICU. As excepted, IMP and PT failed to achieve promising CFRs against all the tested K. pneumoniae because of highly resistant pathogens. In this regard, the length of IUC stay was regarded as the index to discriminate whether organisms were carbapenem-resistant. It has been demonstrated that admission to ICU, exposure to carbapenems, previous antibiotic use, longer length of hospitalization and mechanical ventilation were associated with CRKP infection.29 Our findings revealed that short-stay ICU patients tended to infect with carbapenem-susceptible K. pneumoniae (P<0.0001). However, there were still 6 critically-ill patients infected with CRKP in the duration of short-stay. Most of them had several chronic diseases and ever hospitalized in the wards and treated with antibiotics. Thus, this maybe promote to the selective pressure on carbapenem-resistant germs.
Our simulation results demonstrated that IMP 0.5g q8h II could be empirically treated in short-stay ICU patients infected with K. pneumoniae BSI regardless of CRRT intensity or body weight. Similar results were also observed in a previous PK/PD study involving critically-ill patients acquiring CRRT, which supported the regimen of IMP 1 g/day to treat most common gram-negative pathogens with a MIC of ≤2 mg/L, and 2 g/day or more to treat and prevent resistance in pathogens with higher MICs (MIC=4–8 mg/L).30 However, when the target of 100% ƒT>MIC was adopted, the cut-off for achieving >90% PTA was lowered to ≤1 mg/L, and the values of CFRs were disappointing. The reason partly may be the presence of CRKP. Besides, the great inter‐individual variability of PK in the critically-ill patients and the contribution of CRRT to total clearance were also responsible for the unpredictable and fluctuating drug concentrations, since the the lower limit of simulated Cmin was relatively low (<0.05 mg/L, Table 2). Given the increased Vd and the above situation in critically-ill patients with sepsis, maybe a front-loaded dose of carbapenems during the first 24–48 hours was required, regardless of organ function. Of note, IMP 1g q6h II could lead to the risk of IMP-induced toxicity with a 48.8%, 35.5% and 21.3% likelihood of toxicity for 50 kg, 75 kg and 100 kg patients in the CRRT intensity of $0 mL/kg/h, respectively. The values were higher in CRRT with lower effluent rate of 20 mL/kg/h (Figure 5). TDM is recommended if applicable, and clinical monitoring of IMP adverse reactions should be concerned.
For PT, recommended dosing regimens from previous studies in a short or extended infusion were evaluated in our study. Based on the target of 100% ƒT>MIC, PT 4/0.5g q6h II could be used for infections caused by microorganisms with a MIC of ≤16 mg/L in patients receiving CRRT. Actually, for many of the clinical strains isolated from critically-ill patients in ICU the MICs were ≥32 mg/L (Table 1). Following the above regimen would be underdosed in such circumstance. Increasing the dose to 4/0.5g q8h or more should be considered if treating a relatively drug-resistant pathogen (MIC 32–64 mg/L) for patients receiving CRRT since its low risk of drug-related toxicity (Figures 2 and 4). The results of a PPK study reported by Asín-Prieto et al was in line with our findings and commented that the administration of PT 24 g/day by continuous infusion could achieve >90% PTA against MICs up to 32 mg/L.19 Actually, PT should not be used in in severe pre-ICU infections unless MIC results were released. It is noteworthy that critically-ill patients weighting 50 kg should not be administered the regimen of 24 g/day as the potential of toxicity was recognized to be high (Figure 4). Besides, the combination of PT and vancomycin should be avoided, as the odds of acute kidney injury with vancomycin plus PT were increased versus vancomycin monotherapy (OR, 3.40; 95% CI, 2.57–4.50), versus vancomycin plus cefepime or carbapenem (OR, 2.68; 95% CI, 1.83–3.91).31
The tazobactam concentration was not simulated in the present study. It is unknown whether tazobactam accumulates in patients with no residual renal function during CRRT. Asín-Prieto et al conducted a simulation of tazobactam 0.5 g q4h in patients with CrCL 50 mL/min undergoing CRRT.19 A relative accumulation was observed in their research, but it did not lead to toxic concentration. And similar tazobactam concentration has also been shown in a PK study of critically-ill patients, including anuric renal failure, treated with PT 4/0.5g q8h during continuous veno-venous hemofiltration.32
There are several limitations in this study. First, the population PK model of IMP was developed from 17 critically-ill patients with anuria, oliguria or preserved diuresis.16 And for PT, 10 patients with anuria receiving CRRT were included in their study. As the significant PK variability has been found in critically-ill patients during CRRT, the PK models of IMP and PT, used in our study, may not be fully evaluated. Second, the therapeutic or potentially toxic targets were derived from previous studies. Thus, our simulation results may be varied depending on the different targets. Some authors have commented that a more aggressive target of up to 100% ƒT>4MIC may be required to achieve adequate antimicrobial exposure in critically-ill patients. Following this criteria, limited beta-lactams dosing regimens could be used in severe life-threatening infections. And maybe, none of the regimens could empirically treat infections caused by multidrug-resistant pathogens. It is debatable which target is more suitable. Third, combination antibiotic therapies are often empirically prescribed in ICU settings. Unfortunately, MCS is helpless in such situations. Finally, our MIC distributions against K. pneumoniae were derived from BSIs in ICU, which may may not be representative of the MIC distributions in other regions.
Conclusion
The PPK model was introduced in our study to evaluate a truly optimized regimen. No dose adjustment was required for the conventional IMP and PT regimens in the critically-ill population acquiring CRRT. TDM clinical monitoring of IMP adverse reactions should be concerned for IMP 1g q6h II. Empirical treatment of IMP 0.5g q8h/q6h, not for PT, may provide sufficient antimicrobial exposure for short-stay ICU patients during CRRT. PT should be used in the knowledge of MIC results and de-escalation therapy.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients according to the ethical protocol approved by the Ethics Committee of Lihuili Hospital, Ningbo Medical Center (no. KY2019PJ028).
Funding
This work was supported by the Natural Science Foundation of Ningbo (2019A610232 and 2019A610226); and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021KY305).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43(6):816–828. doi:10.1007/s00134-017-4755-7
2. Uchino S, Bellomo R, Morimatsu H, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33(9):1563–1570. doi:10.1007/s00134-007-0754-4
3. Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–851. doi:10.1097/CCM.0b013e3181961bff
4. Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med. 2009;37(7):2268–2282. doi:10.1097/CCM.0b013e3181aab3d0
5. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
6. Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20(12):1357–1362. doi:10.1111/1469-0691.12747
7. Zheng B, Xu H, Lv T, et al. Stool samples of acute diarrhea inpatients as a reservoir of ST11 hypervirulent KPC-2-producing Klebsiella pneumoniae. mSystems. 2020;5(3). doi:10.1128/mSystems.00498-20.
8. Lin MY, Lyles-Banks RD, Lolans K, et al. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57(9):1246–1252. doi:10.1093/cid/cit500
9. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi:10.1097/01.CCM.0000217961.75225.E9
10. Jamal JA, Economou CJ, Lipman J, Roberts JA. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care. 2012;18(5):460–471. doi:10.1097/MCC.0b013e32835685ad
11. Nielsen EI, Friberg LE. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol Rev. 2013;65:1053–1090. doi:10.1124/pr.111.005769
12. Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother. 2006;58(5):916–929. doi:10.1093/jac/dkl354
13. Bao H, Lv Y, Wang D, Xue J, Yan Z. Clinical outcomes of extended versus intermittent administration of piperacillin/tazobactam for the treatment of hospital-acquired pneumonia: a randomized controlled trial. Eur J Clin Microbiol Infect Dis. 2017;36(3):459–466. doi:10.1007/s10096-016-2819-1
14. Ng TM, Khong WX, Harris PN, et al. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLoS One. 2016;11(4):e0153696. doi:10.1371/journal.pone.0153696
15. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 30th edition. Available from: http://www.clsi.org/. Accessed January 21, 2020.
16. Li S, Xie F. Population pharmacokinetics and simulations of imipenem in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2019;53(1):98–105. doi:10.1016/j.ijantimicag.2018.10.006
17. Bue M, Sou T, Okkels ASL, et al. Population pharmacokinetics of piperacillin in plasma and subcutaneous tissue in patients on continuous renal replacement therapy. Int J Infect Dis. 2020;92:133–140. doi:10.1016/j.ijid.2020.01.010
18. Lewis SJ, Kays MB, Mueller BA. Use of monte carlo simulations to determine optimal carbapenem dosing in critically ill patients receiving prolonged intermittent renal replacement therapy. J Clin Pharmacol. 2016;56(10):1277–1287. doi:10.1002/jcph.727
19. Asin-Prieto E, Rodriguez-Gascon A, Troconiz IF, et al. Population pharmacokinetics of piperacillin and tazobactam in critically ill patients undergoing continuous renal replacement therapy: application to pharmacokinetic/pharmacodynamic analysis. J Antimicrob Chemother. 2014;69(1):180–189. doi:10.1093/jac/dkt304
20. Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis. 1998;27(1):10–22. doi:10.1086/514622
21. Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35(2):156–163. doi:10.1016/j.ijantimicag.2009.10.008
22. Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. doi:10.1093/cid/ciu027
23. Beumier M, Casu GS, Hites M, et al. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81:497–506.
24. Lamoth F, Erard V, Asner S, Buclin T, Calandra T, Marchetti O. High imipenem blood concentrations associated with toxic encephalopathy in a patient with mild renal dysfunction. Int J Antimicrob Agents. 2009;34(4):386–388. doi:10.1016/j.ijantimicag.2009.06.001
25. Drusano GL, Preston SL, Hardalo C, et al. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother. 2001;45(1):13–22. doi:10.1128/AAC.45.1.13-22.2001
26. Bradley JS, Dudley MN, Drusano GL. Predicting efficacy of antiinfectives with pharmacodynamics and Monte Carlo simulation. Pediatr Infect Dis J. 2003;22(11):982–992. doi:10.1097/01.inf.0000094940.81959.14
27. Abdulall AK, Tawfick MM, El Manakhly AR, El Kholy A. Carbapenem-resistant gram-negative bacteria associated with catheter-related bloodstream infections in three intensive care units in Egypt. Eur J Clin Microbiol Infect Dis. 2018;37(9):1647–1652. doi:10.1007/s10096-018-3294-7
28. Polemis M, Tryfinopoulou K, Giakkoupi P, Vatopoulos A. Eight-year trends in the relative isolation frequency and antimicrobial susceptibility among bloodstream isolates from Greek hospitals: data from the Greek electronic system for the surveillance of antimicrobial resistance - WHONET-Greece, 2010 to 2017. Euro Surveill. 2020;25(34). doi:10.2807/1560-7917.ES.2020.25.34.1900516
29. Liu P, Li X, Luo M, et al. Risk factors for carbapenem-resistant klebsiella pneumoniae infection: a meta-analysis. Microb Drug Resist. 2018;24(2):190–198. doi:10.1089/mdr.2017.0061
30. Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother. 2005;49(6):2421–2428. doi:10.1128/AAC.49.6.2421-2428.2005
31. Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46(1):12–20. doi:10.1097/CCM.0000000000002769
32. van der Werf TS, Mulder PO, Zijlstra JG, Uges DR, Stegeman CA. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med. 1997;23(8):873–877. doi:10.1007/s001340050424
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.