Back to Journals » Research and Reports in Urology » Volume 12
Optimal Delivery of Follow-Up Care After Radical Cystectomy for Bladder Cancer
Authors Jensen BT , Lauridsen SV , Jensen JB
Received 1 July 2020
Accepted for publication 24 September 2020
Published 14 October 2020 Volume 2020:12 Pages 471—486
DOI https://doi.org/10.2147/RRU.S270240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Bente Thoft Jensen,1 Susanne Vahr Lauridsen,2 Jørgen Bjerggaard Jensen1,3
1Department of Urology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Urology, Copenhagen University Hospital, Denmark and WHO-CC, Clinical Health Promotion Centre, The Parker Institute Bispebjerg & Frederiksberg University Hospital, Copenhagen, Denmark; 3Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Correspondence: Bente Thoft Jensen Tel +45 21400299
Email [email protected]
Purpose of Review: To identify components representing optimal delivery of follow-up care after radical cystectomy because of bladder cancer and report the current level of evidence.
Methods: We conducted a systematic literature search of the following databases: Cochrane, MEDLINE, Embase, CINAHL, Web of Science, Physiotherapy Evidence Database and ClinicalTrials.gov. The search results were managed in Covidence Reference Manager and abstracts were screened by title. Articles relevant to the subject of interest were included and the results are reported narratively.
Results: Several studies have evaluated the positive impact of enhanced recovery after surgery (ERAS) on length of stay, albeit not on the further impact on 90-day postoperative complication rate, functional recovery, or mortality. Minimally invasive surgery may result in a slighter shorter length of stay compared to open surgery. Physical training combined with nutritional intervention can improve functional recovery up to one year after surgery. Nutritional supplements can preserve muscle and bone mass, and potentially improve recovery. Patient education in stoma care and prevention of infection can significantly improve self-efficacy and avoid symptoms of infection postoperatively. Moreover, specific devices like applications (apps) can support these efforts. Continued smoking increases the risk of developing postoperative complications while no evidence was found on the impact of continued alcohol drinking. Currently, there is no evidence on psychological well-being, sexual health, or shared decision making interventions with an impact on rehabilitation after radical cystectomy.
Conclusion: Data are scarce but indicate that peri- and postoperative multi-professional interventions can reduce prevalence of sarcopenia, and improve functional recovery, physical capacity, nutritional status, and self-efficacy in stoma care (level 1 evidence). Continued smoking increases the risk of complications, but the effects of a smoking and alcohol intervention remain unclear (level 3 evidence). The results of this review provide guidance for future directions in research and further attempts to develop and test an evidence-based program for follow-up care after radical cystectomy.
Keywords: muscle invasive bladder cancer, radical cystectomy, enhanced recovery, rehabilitation, recovery, self-efficacy
Plain Language Summary
The purpose of this review was to identify and report current evidence on optimal follow-up care after removal of the bladder and establishment of a new bladder reservoir because of advanced bladder cancer.
A comprehensive systematic literature search was carried out in leading scientific databases.
Evidence suggests that advanced nutritional interventions can maintain nutritional status and support the immune system, potentially helping recovery. Combined with nutritional interventions, physical resistance exercises, and endurance training, these can improve functional recovery. Patient education on stoma care and prevention of urinary tract infections can significantly improve self-efficacy and avoid infections postoperatively. Moreover, devices like apps can support these efforts. Currently, there is no specific evidence on early intervention covering psychological well-being, sexual health, or shared decision making. Continued tobacco smoking doubles the risk of developing complications. Smoking and alcohol cessation interventions have been proven to be effective in reducing complications after surgery in general, but there are still no studies specifically testing the effect of smoking and alcohol cessation interventions in relation to surgery for bladder cancer.
Introduction
Radical cystectomy (RC) remains the first-line treatment for patients with muscle invasive bladder cancer (MIBC), T2-T4aN0M0, and high grade non-invasive bladder cancer (NMIBC).1 RC is the most advanced standard procedure in major uro-oncological surgery, and involves removal of the bladder together with the prostate and seminal vesicles in men, and anterior vaginal wall, uterus, and adnexae in women, combined with extended lymph node dissection and construction of a urinary diversion. Despite increased adoption of enhanced recovery after surgery (ERAS) protocols, distinct anesthetic and pain management techniques, minimally surgical approaches, and enhanced postoperative care plans including progressive mobilization and nutritional care, perioperative outcomes following RC and survival rates remain unchanged overall. However, it is difficult to evaluate and compare different institutions.2–7
The RC procedure is an integral component of the management of advanced bladder cancer. However, patients with MIBC and high-grade NMIBC often have other urgent medical conditions, as part of a heavy comorbid burden, that require consideration during pre-evaluation before scheduled surgery.8 Candidates for RC (with or without preoperative neoadjuvant chemotherapy) constitute a group with high perioperative risk in the short term and weak health in the longer term. Patients are characterized by being around 70 years of age, with a high comorbidity index score on the Charlson Comorbidity Index; approximately 27% are at severe nutritional risk and 30% are current smokers. Overall, RC patients are considered to be a frail population with a high risk of perioperative morbidity reporting total complications rates of 64–90% within the first 3 months postoperatively.8–10 In combination with advanced age, the effects of malignancies can be even more devastating, as elderly patients often already have reduced physiological reserves, and comorbidities can limit treatment options and promote complications, thereby leading to greater impairment during the process of recovery.9
Most ERAS studies have defined successful recovery based on length of stay (LOS), postoperative morbidity, and readmission. However, these measures do not necessarily define recovery from the patient’s point of view. Since the recovery process is multifactorial, with influence from pre-, intra-, and postoperative factors, an increasingly widespread, rational approach is functional optimization before surgery, also known as prehabilitation.11 By contrast, rehabilitation interventions and follow-up care after discharge have not yet been a focus of interest, although they have been recognized as an issue worth exploring when LOS is significantly shortened and rehabilitation aspects are outsourced to primary care in most European countries. In public healthcare, rehabilitation interventions are categorized as a tertiary prevention approach, which aims to prevent or manage cancer-related and treatment-induced side effects. Evidently, survivors of MIBC experience higher physical and psychosocial side effects of their cancer diagnosis and treatment compared to other cancer sites.12 These negative side effects have a crucial impact on their health-related quality of life (HRQoL).13,14 Currently, the urologic community cannot offer evidence-based guidelines or survivorship programs to support rehabilitation and advise on optimal follow-up care after RC.1 However, increased awareness of the need for evidence-based follow-up care due to shortened LOS calls for urgent action in this area. Thus, this review aims to clarify and identify the level of evidence for current multi-professional rehabilitation interventions to guide optimal follow-up care after RC.
Purpose
To identify components representing optimal delivery of follow-up care after RC because of bladder cancer and report the current level of evidence with a focus on rehabilitation and the transition into survivorship.
Methods
Search Strategy
The author group agreed the scope of the review in October 2019. A librarian from the Royal Danish Library conducted a systematic literature search between October 2019 and January 2020 in cooperation with BTJ and SV. The following databases were searched: Cochrane, MEDLINE, Embase, CINAHL, Web of Science, Physiotherapy Evidence Database, and ClinicalTrials.gov. The following search terms were used: urinary bladder neoplasms, physical activity, exercise, smoking cessation, alcohol cessation, nutritional support, stoma care, stoma education, sexual health, anxiety, depression, prehabilitation, health-related quality of life, self-efficacy, patient reported outcome, complication, unmet supported needs, psychosocial, physical function, physical capacity. The search was limited to patients aged 18 years or over. No language or date limits were applied.
Procedure
In an ERAS context, any multi-professional intervention occurring peri- and postoperatively defined “optimal follow-up care” and the level of evidence was identified. We included the following components in our definition of enhanced peri-operative recovery: shared decision making, bowel preparation, minimally invasive surgery, optimal pain management, sarcopenia, nutrition, physical exercises, patient education on stoma/neobladder care, smoking cessation, alcohol cessation, and sexual counseling. Moreover, follow-up care in this review is considered to be a natural continuation of efforts made in the prehabilitation period (Figure 1).10 Therefore, we also addressed the context of ERAS and the surgical challenge. The findings of the literature search were managed in Covidence. All titles and abstracts were screened individually by two authors, and in case of disagreement, conflicts were resolved by BTJ and SV. Articles were included according to the inclusion criteria outlined later. Moreover, we hand-searched articles and references when available material was deficient in several ways.
Outcomes of Interest
The outcomes of main interest were those reflecting impact on functional recovery, self-efficacy, HRQoL, and complications.
Criteria for Considering Studies for This Review
We primarily searched for randomized controlled trials (RCTs) and if no RCTs were identified, clinical studies (non-randomized) and cohort studies were included. Included studies had to report at least one outcome of interest. Qualitative studies were also included to add to the knowledge base for understanding barriers and unmet needs in follow-up care.
Results of the Search
The literature search retrieved 6092 articles including 764 duplicates. A total of 5328 titles and abstracts were screened for eligibility, out of which 83 articles were included in this review, with the hand-search yielding another 17 articles (Figure 2). The PRISMA flow chart shows the articles we have included in this narrative review.
The Context of ERAS and the Surgical Challenge
RC is considered to be a complex and major procedure involving several organs. Surgery causes a cascade of reactions including release of stress hormones and inflammatory mediators such as cytokines, which are responsible for systemic inflammatory response syndrome (SIRS).15 SIRS causes catabolism of glycogen, fat, and protein, and a consequence of protein catabolism is loss of muscle tissue, which is a short- or long-term burden for functional recovery. ERAS aims to reduce the stress response by combining into bundles various techniques used in the care of patients undergoing major surgery like RC. These bundles include preoperative counseling, preoperative nutritional care, avoidance of perioperative fasting and carbohydrate loading up to 2 h preoperatively, standardized anesthetic and analgesic regimens (epidural and non-opioid analgesia), epidural or regional anesthesia, minimally invasive techniques, thrombolytic prophylaxes, optimal pain control, early removal of drainage, early enteral (oral) nutrition, and progressive ambulation. The combination of these approaches has been shown to successfully reduce SIRS and organ dysfunction, and therefore greatly shorten the time required for hospital recovery after major surgery across Europe and the USA over the past two decades.5,11,16–18
ERAS strategies have been further developed into surgical-procedure-specific ERAS protocols (ERPs) with the establishment of the Enhanced Recovery After Surgery Society.19 Today, ERPs include multidisciplinary, evidence-based interventions in preoperative, intraoperative, and postoperative treatment and care that work synergistically to mitigate the undesirable effects of the surgical stress response.9 The procedure-specific ERAS pathway is a dynamic tool, which continuously adjusts to new evidence.
Changes of Focus in the ERAS Paradigm
ERAS has been a game changer in major urology surgery and has been standard procedure for almost two decades, although it lacks full implementation of all components for various reasons. Since ERPs involve multidisciplinary collaboration, a multitude of factors may contribute to delays in implementation due to an inherent risk of interdisciplinary disagreement. Furthermore, many specific protocols include recommendations translated from other procedures and so are not necessarily relevant or evidence-based for the specific procedure in question.7 However, the ERAS/ERP recommendations for RC are well described among the leading urological societies with high consensus on which elements should be integrated as a minimum in procedure-specific standards for patients undergoing RC because of MIBC or high-grade NMIBC.1,19
The basic question “why is the patient still in hospital ?” is the continuous driver in the concept of ERAS.7 The most common outcome measure used to assess the success of an ERAS protocol was, and still is, LOS in hospital. Many studies have shown reduced LOS and enhanced recovery after implementation of the ERAS concept while others report high adherence to ERP components but no reduction in LOS due to very early optimization of the pathways.5,11,16,17,20,21 Although LOS was the outcome of interest at the beginning of the ERAS epoch around year 2000, it is today considered to be a surrogate marker of recovery and usually not regarded as comparable between institutions, due to the variety of ERPs and the nature of different healthcare systems.22 Importantly, many surgical centers recognize that LOS does not necessarily reflect the true functional recovery of a patient and today the majority of patients in reality have to convalescence at home. Therefore, it is necessary to focus research efforts beyond LOS and readmission rate to understand the factors that influence return to normal daily living after early discharge, and increase awareness of multi-professional “post-discharge” efforts and the evidence base in order to reduce short- and long-term impairments after surgery. Consequently, there is a winds of change for the way we understand ERAS today. Parameters reflecting functional recovery, such as physical function, nutritional status, mental health, and self-efficacy, should be considered and monitored as outcome measures similar to LOS and complications, which are key indicators of recovery from baseline throughout the follow-up period.23,24 However, implementing such “add-ons” will require useful, relevant, and evidence-based recommendations for follow-up care.
Components Identified in the Perioperative ERAS Pathway and Level of Evidence
Prehabilitation
Prehabilitation consists of multiple interventions to capitalize on the waiting period before surgery including preoperative strategies that aim to optimize the patient`s physical condition to withstand the upcoming surgical challenge and to promote earlier postoperative recovery.25 Currently, there is no consensus on the optimal duration of prehabilitation before major urology surgery, and the time span from diagnosis to surgery varies between countries, mostly because of governmental demands. Prehabilitation has become an important and integrated part of the ERAS pathway, and optimal postoperative care builds on the endeavors of prehabilitation (Figure 1). The current level of evidence for prehabilitation in major bladder surgery has been published previously.10
 |
Figure 1 The Bladder Cancer Pathway. * If the patient is not a qualified candidate for neo.adj. chemotherapy the next step will be surgery. |
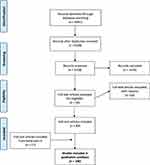 |
Figure 2 Prisma Flow-diagram - Optimal Follow-up Care after Radical Cystectomy. |
The components identified in the literature search for optimal follow-up care after surgery are each reported separately, and the associated level of evidence found for each component on outcomes after RC surgery is reported in Table 1.
 |
Table 1 Highest Level of Evidence Reported for Each Component and the Impact on the Defined Outcomes Using the Oxford Hierarchy of Evidence-Based Medicine |
Information/Shared Decision-Making
Shared decision making (SDM) has been defined as: an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options, to achieve informed preferences.26 The process of SDM can be used in pivotal decisions such as treatment strategy, to determine which urinary diversion option is best for a patient, and as part of goal setting for the rehabilitation period.27 Recently, a tool has been developed and tested which helps to highlight known patient values and preferences essential to establish the choice of urinary diversion.28 This tool is still to be tested in clinical settings.
Although preoperative patient education and information are part of the standard ERAS protocol, and patients who use a decision aid are more often satisfied with their choice compared to those who receive standard counseling, no studies have evaluated the effect of using a SDM tool to support patients in health decisions when undergoing RC. One pilot study has evaluated an education and training intervention, and the authors suggest that the intervention provides MIBC patients with useful treatment information and gives them confidence in treatment decision making.29
Bowel Preparation
Mechanical bowel preparation (BP) aims to cleaning the bowel of fecal content, thereby reducing the rate of infectious complications following surgery. Traditionally, bowel cleansing was done using enemas in combination with oral laxatives over a period of diet restriction. Two RCTs evaluated the effect of three-day traditional BP compared to preoperative mechanical BP and found no evidence to show any advantage of three-day BP over limited BP.30,31 Gastrointestinal complications are very common following RC, and dysfunction like mild nausea, anorexia, and weight loss is expected. Prolonged return of bowel function may affect 12–40% of patients undergoing RC and often results in increased LOS. The effect of gum chewing in the immediate postoperative period on return of bowel function has been evaluated in three RCTs. They all found a stimulatory effect on bowel function recovery after cystectomy followed by ileum urinary diversion. Chewing gum was safe and simple, and could be routinely used for postoperative treatment after RC and ileum urinary diversion.32–34
Minimally Invasive Surgery
Minimally invasive surgery is characterized as a procedure that is less invasive than the classical gold standard, which in cystectomy is the open procedure. A conventional laparoscopic approach was introduced in cystectomy in a few centers but never gained popularity because of the complexity and lengthy learning curve, as well as unacceptably long time in the operating room (OR). Instead, robotic-assisted laparoscopic cystectomy (RALC) overcame most of the obstacles to become the new gold standard in several high-volume centers. However, RALC can present some confusing results as the urinary diversion can be done either extracorporeally by an open procedure, where the advantages of minimally invasive surgery are potentially lost, or as a complete intracorporeal procedure, which is the true minimally invasive procedure. Minimally invasive surgery may reduce the severity of SIRS compared to open surgery, and seems to be advantageous in elderly people with regard to complications.35 Five RCTs fulfilled the inclusion criteria of this review, and a recent Cochrane review has summarized the findings.36 They found that robotic cystectomy and open cystectomy may have similar outcomes with regard to rates of major complications and quality of life (low-certainty evidence). It is very uncertain whether the robotic approach reduces rates of minor complications (very low-certainty evidence), while the approach may reduce LOS in hospital slightly (low-certainty evidence).36 However, as all these RCT were randomizing between open cystectomy or RALC with extracorporeal urinary diversion, the true difference between open and minimally invasive procedures remains to be elucidated.
The Nutritional Component
Sarcopenia – a predictor of mortality after RC
Sarcopenia is defined as severe loss of skeletal muscle mass and is considered to be an important preoperative prognostic factor for cancer-specific survival in patients undergoing RC.37 Moreover, sarcopenia is associated with age, state of cancer, and nutritional status in MIBC patients and should be addressed accordingly in order to optimize the perioperative period and convalescence.38–40 RC patients are considered to be frail, defined as having decreased reserves and resistance to stressors, which increases the risk of adverse outcomes, such as the onset of disability and morbidity after major surgery. An important and fundamental component of frailty is sarcopenia, which is accelerated in patients with cancer compared to individuals of a similar age because SIRS, malnutrition, or cancer treatment itself contributes to progressive loss of skeletal muscle mass, strength, and physical performance.41 Causes of sarcopenia are multifactorial but may include a sedentary lifestyle and inadequate protein intake, and the condition is significantly associated with increased disease-specific all-cause mortality among bladder cancer patients.37,40 Older patients, like RC patients where the peak is around the age of 67 years, are less able to utilize amino acids for protein synthesis at muscle level, and almost 30% are at nutritional risk ahead of surgery.9,42 In addition, it is estimated that one-third of patients undergoing RC are sarcopenic and would benefit from a combined intervention of physical exercises and nutrition with protein supplements to attenuate the loss of lean leg mass and strength, and promote the recovery phase.25,37 Today, it is generally accepted that exercises provide the main anabolic stimulus while nutrition potentiates the muscle protein response. Consequently, these two components are synergistically related, and a combined intervention should be offered in both the perioperative period and post-discharge to counteract sarcopenia, maximize recovery, and reduce long-term impairments.25
Nutritional interventions
A total of five RCT studies, two follow-up studies, and one Cochrane review included the nutritional component (Table 1). Several studies have reported that nutrition is the key component in functional recovery, albeit improvements are greater when nutrition is combined with prolonged physical exercises, as these two factors are interrelated. The intention with nutritional assessment and supportive nutritional care is essential to maintain or improve a patient’s nutritional status, aiming to avoid sarcopenia, facilitate wound healing, support ambulation and physical training, and reduce the risk of postoperative complications and mortality.15
A postoperative delay in the return of bowel function is relatively common, which presents a challenge for providing sufficient oral intake of nutrition. Patients relatively often experience symptoms of ileus and abdominal pain on day 3 or 4 postoperatively, and reduced oral intake may necessitate parenteral treatment in order to avoid starvation and increased sarcopenia.43,44 Studies investigating the best modality for delivering nutritional support postoperatively to RC patients are few, and if oral intake was limited, parenteral nutrition was considered to be the standard mode of care until recently.
While the relationship between nutritional status and perioperative outcomes is accepted, there is limited evidence to support specific nutrition intervention protocols, as well as a lack of consistent guidelines and recommendations for nutrition supplementation in most postoperative ERPs.43,44 A few studies have investigated the effects and benefits of parenteral nutrition in improving recovery from RC.45,46 One minor RCT study concluded that, when compared to oral administration, parenteral administration led to increased infectious complications and higher costs during a patient’s hospital stay without any difference in LOS.46 Another single-center RCT study with a median follow-up of 50 months concluded parenteral treatment has a long-term negative influence on bowel function.47 In light of increasing awareness that postoperative infectious complications may be related to the nutrient component and SIRS, a pilot RCT study explored the immune response and postoperative complications in men consuming either specialized immune nutrition or an standard oral supplement before and after RC. The results showed that the group receiving specialized immune nutrition had better outcomes for immune response to surgery, late complications, and infection rates.48 Moreover, perioperative immune nutrition modulates the inflammatory response, which could indicate that an immune-enhanced formula may positively attenuate SIRS in patients undergoing RC, and may reduce infectious complications, thereby improving outcomes.49 Another prospective RCT study recently investigated the relationship between oral nutrition supplementation and sarcopenia. The study demonstrated that giving patients nutrition intervention with enriched supplements twice a day in the perioperative period may be more likely to preserve skeletal muscle mass and reduce the prevalence of sarcopenia, potentially improving outcomes and reducing readmissions.44
The Physical Component
Resistance-type exercise training has been established as an effective interventional strategy to counteract the age-related loss of muscle strength and performance in frail elderly people. A single daily session of resistance-type exercises increases both muscle protein synthesis and breakdown rates. Although exercise improves muscle protein balance, net balance will remain negative in the absence of food intake.50,51 To achieve a positive protein balance, protein ingestion before or after exercise is required to further enhance muscle protein synthesis rates and inhibit protein breakdown.51 Consequently, it has been proposed that dietary protein supplementation is required to preserve skeletal muscle mass during postoperative ambulation and prolonged exercise rehabilitation interventions, in order to more effectively counteract sarcopenia and frailty during follow-up care after major surgery.52
Clearly, there is ample evidence for the beneficial effects of long-term resistance-type exercise training on muscle mass and performance in healthy elderly people. In contrast, rehabilitation studies investigating the impact of such prolonged exercise interventions on elderly RC patients are scarce, while functional outcomes, except those involving stoma aspects, have not been a focus of interest in research until recently. A pilot RCT by Porserud et al investigated a 12-week postoperative exercise program for patients undergoing RC because of MIBC and found increased physical function and a positive impact on HRQoL in those who completed the program compared to controls.53 Another RCT study investigated the efficacy of a multi-model pre- and postoperative rehabilitation program on physical and nutritional recovery when entering the survivorship phase. Objective measures included leg extension power (proxy for physical capacity), handgrip strength (proxy for nutritional status), and oral nutritional intake. Compared to the standard, physical capacity in the intervention group was significantly superior at four months and maintained until 12 months postoperatively. In addition, handgrip strength (nutritional status) was significantly higher at discharge and five weeks postoperatively compared to the standard, and was maintained at four and 12 months.54 Like Porserud et al, Jensen et al found significantly higher HRQoL in the physical domain.55 A recent clinical study confirmed that both pre- and rehabilitation have a role in RC pathways. In a comprehensive cancer center, 32 patients undergoing a multimodal pre- and rehabilitation program, including physical and nutritional interventions, showed significant improvement in functional outcomes measured by the six-minute walk test (6MWT) and handgrip strength eight weeks after RC. Bio-impedance measures supported the improvements in bone and muscle mass.9 The same results were found in a Canadian RCT study evaluating whether multimodal prehabilitation programs were feasible for RC patients. Data for 40 patients confirmed improved functional capacity measured by the 6MWT at four and eight weeks postoperatively.56
A qualitative research study using semi-structured face-to-face interviews aimed to explore the determinants of physical activity in patients with bladder cancer before and after RC. A total of 30 interviews were conducted. Patients’ physical activity history was one of the factors that influenced their physical activity behavior after RC. Most people wanted to be “normal” again, and those who used to practice a particular sport or engage in various activities before treatment were motivated to pick up that sport or be active again. Perceived benefits of physical activity, such as feeling good, more energy, better sleep, muscle gain, clearer head, physical satisfaction, less boredom, relaxation, better control of pelvic floor muscles, less stiffness, and good recovery from surgery, can act as motivators for continuing physical activity. Conversely, if people had negative experiences, such as becoming unwell when using fitness equipment or related to carrying too much weight, they avoided physical activities in the future out of fear.57
To date, no specific exercise guidelines exist for bladder cancer patients although the international bladder cancer community hypothesizes that better physical condition among bladder cancer patients could possibly lead to reduced impairments after neoadjuvant chemotherapy and/or surgery, and positively reduce the impact of SIRS, thereby improving postoperative outcomes after RC. A recent review by Rammant et al of the scope of exercise and psychosocial rehabilitation interventions to improve health-related outcomes in patients with bladder cancer undergoing RC summarizes:
that there are few studies investigating the use of exercise or psychosocial interventions for people undergoing RC. There is some albeit weak evidence that pre- and postoperative exercise may improve HRQoL outcomes in some domains, especially physical fitness.58
The Urinary Diversion (Stoma/Neobladder)
Living with a urostomy- or intestinal stoma has been shown to considerably affect patients’ daily lives and can result in a wide range of impairments that impact on various aspects of daily living, such as those directly related to the stoma, fatigue, urinary tract infections, sexual health, and altered body image.59–62
Due to the short LOS after RC, the window for in-hospital educational activities is narrow. Thus, there is a need for a shared instrument to determine an individual’s ability to independently perform stoma care, and provide mutual agreement between a patient and healthcare providers involved in stoma education to ensure optimal follow-up care. The Urostomy Education Scale (UES) is the first unique reliable and valid tool to evaluate urostomy self-care skills among patients undergoing RC because of MIBC.63–65 Based on systematic literature reviews, the UES presents internationally recognized minimum standards for urostomy care categorized into seven skills considered necessary to perform a full change of a urostomy appliance. The tool provides a quantified expression of the patient’s actual level of stoma self-care skills at any point on the pathway, with the aim of improving communication between healthcare providers and the patient in case of complications or specific needs for guidance while in hospital and as part of follow-up care. To date, the UES has been translated and validated in various languages and has proven to be highly reliable.65 The UES has also been integrated into international guidelines and incorporated into electronic health records, eg, at Aarhus University Hospital, Denmark.
In order to prevent or reduce post-surgical impairment, it is crucial to enable patients to confidently return to their daily activities, working life, and former level of quality of life (QoL), or at least obtain an acceptable QoL66 A recent systematic review identified this as a process from acceptance, through adaptation to autonomy.67 There is strong evidence that stoma self-care ability is the most important variable in predicting positive adjustment to life with a stoma. It has been suggested that confidence in changing the stoma appliance and stoma self-care skills significantly increase patients’ perceptions of QoL.68–71 In a selected patients, the choice of an orthotopic neobladder may serve as the best preservation of body image without compromising cancer control. However, patients must be fully educated and committed to the labour-intensive rehabilitation process, including being able to perform self-catheterization if necessary. A meta-analysis of >2000 patients showed a 4–25% rate of clean intermittent self catheterization (CISC) for incomplete emptying indicating that assessment of manual dexterity to perform CISC before surgery is needed. Functional outcome of maintaining daytime continence has been shown to be 13% of patients. The rate of nocturnal incontinence is usually higher due to lack of the guarding reflex from loss of the native bladder and is in the range of 15–40%. Current studies show that HRQoL differences mainly stem from preservation of body image, particularly with orthotopic neobladders.72 No prospective studies were identified evaluating the effect of an educational intervention to educate patients in the rehabilitation period to distinguishing symptom normality and thus optimize care. Currently, patient education mainly take place during patients’ recovery in hospital after surgery although preoperative stoma education and stoma site marking are increasingly common.73,74 Interestingly, a major cohort study recently reported that patients’ experiences after stoma surgery vary in terms of the lifestyle advice they receive. Only 19% of respondents reported that they had received information, advice, or support about physical activity, diet, alcohol, and smoking, but most would welcome more guidance as part of follow-up care. Developing interventions that support health professionals to provide evidence-based lifestyle advice, while also addressing patients’ concerns about their stoma, could help patients to change their health behaviors and improve satisfaction with care.75,76
A prospective RCT study, including 104 RC patients, found significantly improved stoma care skills up to one year after surgery among those who had been given systematic pre- and postoperative stoma education.77 Moreover, in a randomized study, preventive educational initiatives aiming to avoid urinary tract infections (UTIs) and including the use of cranberry capsules in the postoperative period have been shown to reduce UTIs.62 Another approach to reach out to patients and/or health-care staff to support follow-up care, is a home care mobile app, which provides stoma care support weekly, or when needed, using a smartphone. Patients in the intervention group had significantly higher self-efficacy and fewer psychosocial challenges six months after discharge.78 Overall, the evidence suggests that systematic, proactive, and comprehensive patient education interventions that include multitude lifestyle factors, preventive UTI strategies, and proactive stoma care may reduce impairments and improve follow-up care.62,75,76,79,80
Smoking
Smoking is the greatest single risk factor for the development of bladder cancer,81 responsible for up to 50% of bladder cancer diagnoses.82 For bladder cancer patients undergoing RC, continued smoking almost doubles the risk of surgical complications, particularly wound dehiscence and myocardial infarction, which, while common, are burdensome for the individual patient and costly for society.83,84 There is evidence that smoking cessation 4–8 weeks before surgery halves the risk of postoperative complications while one brief intervention preoperatively does not reduce postoperative complications.85 While the relationship between smoking and postoperative complications is well established, exactly how long the patient needs to be abstinent to reduce the risk is still unknown. Only one study has explored patients’ involvement in a smoking cessation intervention in relation to RC,86 but the results of the intervention have not yet been published.87 A smoking cessation intervention is seen as a relevant offer for patients undergoing major bladder cancer surgery, and side effects of surgery like nausea, oral thrush, or changes in taste due to medication were factors supporting cessation. Despite the fact that cessation during hospitalization felt easy for all participants, returning to everyday life challenged their continued smoking abstinence, and this points to the importance of addressing smoking cessation at each follow-up meeting in the urological outpatient clinic.86
Alcohol Drinking
Risky drinking increases the endocrine stress response to surgery, leading to deterioration of existing conditions and thus increasing the risk of postoperative morbidity.88 Similarly, risky drinking reduces the immune capacity, leading to an increased risk of infection and impaired wound healing.89 The threshold for an increased risk of complications may be as low as >2 drinks per day,90 and because these patients are often relatively healthy, staff may not be alerted to their risky alcohol intake at admission to surgery.91 In general, complete alcohol cessation 4–8 weeks before surgery seems to reduce the number of complications after planned surgery while no effect was found on number of deaths and LOS.92 An article describing the protocol for a study evaluating the effect of an intensive alcohol and smoking cessation intervention for patients undergoing RC was identified,87 but the results have not yet been published. During the rehabilitation period, patients are recommended to follow the guidelines of their local health authorities.
Sexual Counseling
Sexual dysfunction is a common issue among patients undergoing RC. Despite efforts having been made to provide nerve sparing surgery, there is still a lack of routine provider-led sexual health counseling for patients undergoing RC. A cross-sectional survey of members of the Society of Urologic Oncology showed that the majority did not routinely counsel patients about sexual dysfunction or options for referral to sexual health services.93 Men were more likely to receive sexual counseling than women. In general, sexual function among women undergoing RC is poorly studied, with limitations related to the use of validated questionnaires.94
One RCT study was identified that evaluated the effect of intra-cavernous injection (ICI) therapy for erectile dysfunction (ED) after non-nerve-sparing (NNS) radical pelvic surgery on patient compliance. The study concluded that ICI-oriented sexual counseling increased the efficacy of treatment.95 No studies were found on sexual function among women.
Discussion
The components included in this review may contribute to the process of establishing a bundle of basic and robust interventions that can form the basis of a program of evidence-based follow-up care after RC. However, despite increasing recognition of the urgent need for evidence-based recommendations to support optimal follow-up care, evidence in this area remains scarce: a situation recognized earlier by Rammant et al.57,58
The World Health Organization (WHO) provides a general definition of rehabilitation, but there is a more specific definition of rehabilitation in cancer care:
cancer rehabilitation is a concept that is defined by the patient and involves supporting a person with cancer to obtain maximum physical, social, psychological, and vocational functioning within the limit by the disease and its treatment.96
Therefore, it is essential that cancer rehabilitation and survivorship care comprise different approaches. Patients have a plethora of individual needs and concerns after RC, which are reported in several qualitative studies.13,14,57,76,86,97 A better understanding of these needs and behaviors following surgery, and patients’ individual experiences and attitudes towards receiving lifestyle advice, could help to identify specific gaps and inform follow-up interventions in future. SDM tools, which can support the patient and bladder team in making an informed decision on strategy for surgery and goal setting for rehabilitation, are a related area that also currently lacks evidence. While these tools may be essential for successful rehabilitation and the whole course of treatment and care, unfortunately, no studies to date have evaluated the effect of using such tools to support health decisions when undergoing RC.
One tool has been developed to support patients in deciding what kind of urinary diversion will fit best into their everyday life.28 More information about this tool can be found at: https://decisionaid.ohri.ca/docs/das/Surgery_for_Bladder_Cancer.pdf
Sarcopenia is a fundamental component in the concept of frailty, and importantly, approximately 30% of patients undergoing RC are considered to be both sarcopenic and at nutritional risk at the time of surgery.37,98 Recent studies have associated patients being sarcopenic with increased complications and overall mortality after RC. This may be the reason for increasing interest in this area, which can hopefully promote systematic multimodal interventions and guide healthcare staff to understand and act on the importance of early implementation of both pre- and rehabilitation interventions. More studies focusing on physical exercises and nutrition with protein supplements to counteract sarcopenia during follow-up care would be a welcome addition to the literature.25,37,38,99
An increasing number of procedure-specific studies have evaluated the efficacy of combined and separate interventions involving physical exercises and/or nutrition components. Both an RCT study and minor follow-up studies have provided evidence that a combination of these interventions can maintain or even improve functional recovery in practice, and sustain muscle and bone mass during the rehabilitation period and follow-up care.9,11,54,56 Porserud investigated a 12-week postoperative exercise program for patients undergoing RC because of MIBC and found increased physical function and positive impact on HRQoL in those who completed the program compared to controls.53 The study had some limitations with respect to recruitment and major dropout due to critical illness. However, only one RCT study has reported the positive impact of such combined interventions over a long-term follow-up period,54 showing that this is clearly a valuable area for further research.
As RC involves a resection of the intestine to establish a urinary diversion, which has a major impact on bowel function, nutritional care is a key component of rehabilitation, to counter the ileus symptoms and nausea suffered by patients immediately after surgery. Nutritional supplements can help to meet the challenges posed by patients’ inability to achieve sufficient oral intake. Chewing gum is an accepted and evidence-based intervention to stimulate small bowel moments and counteract ileus, but no interventions to prevent nausea were found.
Interestingly, a few minor RCT studies have investigated the effects and benefits of parenteral nutrition versus oral supplements postoperatively. These studies found that parenteral nutrition leads to increased infectious complications and higher costs during a patient’s hospitalization without any positive effect on LOS.46 Moreover, it has also been reported that parenteral nutrition has a long-term negative effect on bowel function.45 Although these were minor studies, the results challenge the use of conservative treatment with aggressive parenteral interventions to prevent starvation. Another new approach to supporting nutritional status and reducing SIRS is the use of specialized immune nutrition versus oral supplements perioperatively, where the immune nutrition modulates the inflammatory response, reducing infection rates and late complications.48,49 Although the results are encouraging, the authors acknowledge that the small sample size limits the possibility of translating them directly into clinical practice, but a large multisite study focusing on an ERAS context is under consideration. However, a Cochrane review exploring the evidence and quality of the studies found that the quality of evidence is low, and flagged the urgent need for high-quality research to better inform nutritional support interventions for people undergoing RC because of bladder cancer.100
With regard to other important lifestyle factors such as smoking and risky drinking, which have a huge impact on physiological functions, there is generally strong evidence to support preoperative interventions to prevent postoperative complications if they are initiated 4–8 weeks before major surgery. There is still a lack of evidence to support short-term interventions, which is relevant to RC pathways, although studies have been performed, but are yet to be published. A qualitative study has focused on patients’ experiences of undergoing smoking and/or alcohol cessation interventions during the perioperative period. Patients felt these were easy to follow and experienced the interventions as an integrated part of preparing for surgery. However, maintaining abstinence after returning to everyday life after discharge was challenging, highlighting that smoking and alcohol interventions need specific attention if they are to be an effective part of follow-up care.86
No evidence was found about the effects of psychosocial interventions on outcomes, but it does not necessarily follow that further investigation of this area is not needed.13 Due to the general prognosis of MIBC, patients are often anxious before and after treatment, but unfortunately the standard follow-up care currently provided seldom involves any shared discussion of the psychosocial burden after treatment so the lack of evidence to promote such interventions remains. Likewise, the effects of major invasive surgery on patients’ sexual health lack clinical attention and may not be of interest to professionals during follow-up care unless individual patients are outspoken about the issue. Men are more likely to receive counseling, and one study recorded a positive effect in conjunction with ICI therapy. Unfortunately, the need for sexual counseling and how it might be provided are poorly studied areas for women undergoing RC.
Conclusion
Published data indicate that a bundle of multi-professional interventions can improve postoperative functional recovery, physical capacity, nutritional status, and self-efficacy in stoma care, and reduce the prevalence of sarcopenia. There is currently little evidence to suggest that these interventions can improve HRQoL. Continued tobacco smoking doubles the risk of complications. Whether it is possible to reduce this risk through smoking cessations interventions is still unclear. The situation is similar with regard to the consequences of continued alcohol drinking in relation to surgery. No evidence was found in relation to reducing complications or mortality. The current evidence identified is in general of low quality albeit able to guide directions for future research and attempts to develop and test evidence-based programs for optimal follow-up care after RC. The results highlight the need for high-quality studies investigating not only the physiological components that cause the majority of problems during hospitalization, but also the educational and unmet needs of patients throughout the survivorship phase. This will contribute to the integration of patient-related outcomes and the establishment of optimal follow-up care programs.
Disclosure
Prof. Dr. Jørgen Bjerggaard Jensen reports grants from Ferring, Photocure ASA, medac and Cephaid, and personal fees from Ferring, Olympus and Cephaid. He is proctor for Intuitive Surgery outside the submitted work. The authors have no other potential conflicts of interest to report for this work.
References
1. Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi:10.1016/j.eururo.2016.06.020
2. Schiffmann J, Gandaglia G, Larcher A, et al. Contemporary 90-day mortality rates after radical cystectomy in the elderly. Eur J Surg Oncol. 2014;40(12):1738–1745. doi:10.1016/j.ejso.2014.10.004
3. Dell’Oglio P, Tian Z, Leyh-Bannurah SR, et al. Short-form charlson comorbidity index for assessment of perioperative mortality after radical cystectomy. J Natl Compr Canc Netw. 2017;15(3):327–333.
4. Ploussard G, Shariat SF, Dragomir A, et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol. 2014;66(2):361–370.
5. Pang KH, Groves R, Venugopal S, Noon AP, Catto JWF. Prospective implementation of enhanced recovery after surgery protocols to radical cystectomy. Eur Urol. 2017.
6. Zehnder P, Studer UE, Skinner EC, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112(2):E51–8.
7. Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia. 2020;75(Suppl 1):e54–e61.
8. Elmussareh M, Simonsen PC, Young M, Kingo PS, Jakobsen JK, Jensen JB. Correlation between organ-specific co-morbidities and complications in bladder cancer patients undergoing radical cystectomy. Scand J Urol. 2018;52(5–6):395–400.
9. Bente Thoft Jensen GD, Jørgen Bjerggaard J, Mallory Bowker CR. Implementing a multimodal prehabilitation program prior to radical cystectomy in a comprehensive cancer center: a pilot study to assess feasibility and outcomes. Urol Nurs. 2019;39(6):303–313.
10. Jensen BT, Lauridsen SV, Jensen JB. Prehabilitation for major abdominal urologic oncology surgery. Curr Opin Urol. 2018;28(3):243–250. doi:10.1097/MOU.0000000000000487
11. Jensen BT, Petersen AK, Jensen JB, Laustsen S, Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol. 2015;49(2):133–141. doi:10.3109/21681805.2014.967810
12. Hansen MB, Ross L, Petersen MA, Groenvold M. Age, cancer site and gender associations with symptoms and problems in specialised palliative care: a large, nationwide, register-based study. BMJ Support Palliat Care. 2019. doi:10.1136/bmjspcare-2019-001880
13. Mohamed NE, Chaoprang Herrera P, Hudson S, et al. Muscle invasive bladder cancer: examining survivor burden and unmet needs. J Urol. 2014;191(1):48–53. doi:10.1016/j.juro.2013.07.062
14. Mohamed NE, Pisipati S, Lee CT, et al. Unmet informational and supportive care needs of patients following cystectomy for bladder cancer based on age, sex, and treatment choices. Urol Oncol. 2016;34(12):
15. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutri. 2017;36(3):623–650. doi:10.1016/j.clnu.2017.02.013
16. Saar M, Ohlmann CH, Siemer S, et al. Fast-track rehabilitation after robot-assisted laparoscopic cystectomy accelerates postoperative recovery. BJU Int. 2013;112(2):E99–E106. doi:10.1111/j.1464-410X.2012.11473.x
17. Pruthi RS, Nielsen M, Smith A, Nix J, Schultz H, Wallen EM. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg. 2010;210(1):93–99. doi:10.1016/j.jamcollsurg.2009.09.026
18. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi:10.1016/S0002-9610(02)00866-8
19. Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutri. 2013;32(6):879–887. doi:10.1016/j.clnu.2013.09.014
20. Smith J, Meng ZW, Lockyer R, et al. Evolution of the Southampton Enhanced Recovery Programme for radical cystectomy and the aggregation of marginal gains. BJU Int. 2014;114(3):375–383.
21. Collins JW, Patel H, Adding C, et al. Enhanced recovery after robot-assisted radical cystectomy: EAU robotic urology section scientific working group consensus view. Eur Urol. 2016;70(4):649–660. doi:10.1016/j.eururo.2016.05.020
22. Kehlet H. Postoperative length of stay–evidence-based or elastic meter-measure? Ugeskr Laeger. 2004;166(37):3175.
23. Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncologica (Stockholm, Sweden). 2017;56(2):128–133. doi:10.1080/0284186X.2016.1266081
24. Pozzar RA, Berry DL. Gender differences in bladder cancer treatment decision making. Oncol Nurs Forum. 2017;44(2):204–209.
25. Gillis C, Buhler K, Bresee L, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. 2018;155(2):391–410.e4. doi:10.1053/j.gastro.2018.05.012
26. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi:10.1007/s11606-012-2077-6
27. Rose A, Rosewilliam S, Soundy A. Shared decision making within goal setting in rehabilitation settings: A systematic review. Patient Educ Couns. 2017;100(1):65–75. doi:10.1016/j.pec.2016.07.030
28. McAlpine K, Lavallée LT, Stacey D, et al. Development and acceptability testing of a patient decision aid for urinary diversion with radical cystectomy. J Urol. 2019;202(5):1001–1007. doi:10.1097/JU.0000000000000341
29. Mohamed N, Leung TM, Mehrazin R, Sfakianos J, Knauer C. PD15-03 an intervention to improve bladder cancer knowledge and treatment decision making in patients with muscle invasive bladder cancer: a pilot study. J Urol. 2018;199(4S):e310–e1. doi:10.1016/j.juro.2018.02.802
30. Xu R, Zhao X, Zhong Z, Zhang L. No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: a randomized controlled trial of 86 patients. Int Urol Nephrol. 2010;42(4):947–950. doi:10.1007/s11255-010-9732-9
31. Aslan G, Baltaci S, Akdogan B, et al. A prospective randomized multicenter study of Turkish Society of Urooncology comparing two different mechanical bowel preparation methods for radical cystectomy. Urol Oncol. 2013;31(5):664–670. doi:10.1016/j.urolonc.2011.03.009
32. Wang Y, Meng YS, Fan Y, et al. [Effect of gum chewing on bowel function recovery in patients after radical cystectomy with urinary diversion]. Beijing Da Xue Xue Bao Yi Xue Ban. 2016;48(5):822–824.
33. Kouba EJ, Wallen EM, Pruthi RS. Gum chewing stimulates bowel motility in patients undergoing radical cystectomy with urinary diversion. Urology. 2007;70(6):1053–1056.
34. Choi H, Kang SH, Yoon DK, et al. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: a prospective randomized comparative study. Urology. 2011;77(4):884–890.
35. Lauridsen SV, Tonnesen H, Jensen BT, Neuner B, Thind P, Thomsen T. Complications and health-related quality of life after robot-assisted versus open radical cystectomy: a systematic review and meta-analysis of four RCTs. Syst Rev. 2017;6(1):150.
36. Rai BP, Bondad J, Vasdev N, et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst Rev. 2019;4.
37. Mayr R, Fritsche HM, Zeman F, et al. Sarcopenia predicts 90-day mortality and postoperative complications after radical cystectomy for bladder cancer. World J Urol. 2018;36(8):1201–1207.
38. Psutka SP, Carrasco A, Schmit GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120(18):2910–2918.
39. Smith AB, Deal AM, Yu H, et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol. 2014;191(6):1714–1720.
40. Hirasawa Y, Nakashima J, Yunaiyama D, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol. 2016;23(Suppl 5):1048–1054.
41. Gewandter JS, Dale W, Magnuson A, et al. Associations between a patient-reported outcome (PRO) measure of sarcopenia and falls, functional status, and physical performance in older patients with cancer. J Geriatr Oncol. 2015;6(6):433–441.
42. Jensen BT, Laustsen S, Petersen AK, et al. Preoperative risk factors related to bladder cancer rehabilitation: a registry study. Eur J Clin Nutr. 2013.
43. Declercq P, De Win G, Van der Aa F, et al. Reduced length of stay in radical cystectomy patients with oral versus parenteral post-operative nutrition protocol. Int J Clin Pharm. 2015;37(2):379–386.
44. Ritch CR, Cookson MS, Clark PE, et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: results of a prospective randomized controlled trial. J Urol. 2019;201(3):470–477.
45. Declercq P, Van der Aa F, De Pourcq L, Spriet I. Impact of an oral nutrition protocol in patients treated with elective radical cystectomy: a long term follow-up. Int J Clin Pharm. 2019;41(2):408–413.
46. Roth B, Birkhauser FD, Zehnder P, et al. Parenteral nutrition does not improve postoperative recovery from radical cystectomy: results of a prospective randomised trial. Eur Urol. 2013;63(3):475–482.
47. Vidal A, Arnold N, Vartolomei MD, et al. Oncological and functional outcomes of postoperative total parenteral nutrition after radical cystectomy in bladder cancer patients: A single-center randomized trial. Int J Urol. 2016;23(12):992–999.
48. Hamilton-Reeves JM, Bechtel MD, Hand LK, et al. Effects of immunonutrition for cystectomy on immune response and infection rates: a pilot randomized controlled clinical trial. Eur Urol. 2016;69(3):389–392.
49. Hamilton-Reeves JM, Stanley A, Bechtel MD, et al. Perioperative immunonutrition modulates inflammatory response after radical cystectomy: results of a pilot randomized controlled clinical trial. J Urol. 2018;200(2):292–301.
50. Koopman R, Verdijk L, Manders RJ, et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84(3):623–632.
51. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–331.
52. Tieland M, Dirks ML, van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–719.
53. Porserud A, Sherif A, Tollback A. The effects of a physical exercise programme after radical cystectomy for urinary bladder cancer. A pilot randomized controlled trial. Clin Rehabil. 2014;28(5):451–459.
54. Jensen BT, Borre M, Borre M, Soendergaard I, Jensen JB. One year follow up of the efficacy of physical prehabilitation in radical cystectomy pathways - secondary results from a randomized controlled trial. Eur Urol Suppl. 2018;17(2):e1556.
55. Jensen BT, Jensen JB, Laustsen S, Petersen AK, Sondergaard I, Borre M. Multidisciplinary rehabilitation can impact on health-related quality of life outcome in radical cystectomy: secondary reported outcome of a randomized controlled trial. J Multidiscip Healthc. 2014;7:301–311.
56. Minnella EM, Awasthi R, Bousquet-Dion G, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. LID. S2405-4569(19):30153. doi:10.1016/j.euf.2019.05.016
57. Rammant E, Fonteyne V, Decaestecker K, et al. Understanding physical activity behavior in patients with bladder cancer before and after radical cystectomy: a qualitative interview study. Clin Rehabil. 2019;33(4):750–761.
58. Rammant E, Decaestecker K, Bultijnck R, et al. A systematic review of exercise and psychosocial rehabilitation interventions to improve health-related outcomes in patients with bladder cancer undergoing radical cystectomy. Clin Rehabil. 2018;32(5):594–606.
59. Shih C, Porter MP. Health-related quality of life after cystectomy and urinary diversion for bladder cancer. Adv Urol. 2011;2011:715892.
60. Novotny V, Hakenberg OW, Froehner M, et al. Systematic assessment of complications and outcome of radical cystectomy undertaken with curative intent in patients with comorbidity and over 75 years of age. Urol Int. 2013;90(2):195–201.
61. Stelton S, Zulkowski K, Ayello EA.Practice implications for peristomal skin assessment and care from the 2014 world council of enterostomal therapists international ostomy guideline. Adv Skin Wound Care. 2015;28(6):275–284.
62. Temiz Z, Cavdar I. The effects of training and the use of cranberry capsule in preventing urinary tract infections after urostomy. Complement Ther Clin Pract. 2018;31:111–117.
63. Kristensen SA, Laustsen S, Kiesbye B, Jensen BT. The Urostomy Education Scale: a reliable and valid tool to evaluate urostomy self-care skills among cystectomy patients. J Wound Ostomy Continence Nurs. 2013;40(6):611–617.
64. Kristensen SA, Jensen BT. Testing inter-rater reliability of the urostomy education scale. Eur J Oncol Nurs. 2016;20:17–23.
65. Thoft Jensen B, de Blok W, Kiesbye B, Kristensen SA. Validation of the Urostomy Education Scale: the European experience. Urol Nurs. 2013;33(5):219–229.
66. Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63(5):295–317.
67. Capilla-Diaz C. Bonill-de Las Nieves C, Hernandez-Zambrano SM, et al. Living with an intestinal stoma: a qualitative systematic review. Qual Health Res. 2019;29(9):1255–1265.
68. Metcalf C. Clinical Stoma care: empowering patients through teaching practical skills. Brit J Nurs. 1999;8(9):593–600.
69. Brown H, Randle J. Living with a stoma: a review of the literature. J Clin Nurs. 2005;14(1):74–81.
70. Wu HKM, Chau JPC, Twinn S. Self-efficacy and quality of life among stoma patients in Hong Kong. Cancer Nurs. 2007;30(3):186–193.
71. O’Connor G. Teaching stoma-management skills: the importance of self-care. Brit J Nurs. 2005;14(6):320–324.
72. Lee RK, Abol-Enein H, Artibani W, et al. Urinary diversion after radical cystectomy for bladder cancer: options, patient selection, and outcomes. BJU Int. 2014;113(1):11–23.
73. Colwell JC, Gray M. Does preoperative teaching and stoma site marking affect surgical outcomes in patients undergoing ostomy surgery? J Wound Ostomy Continence Nurs. 2007;34(5):492–496.
74. Haugen V, Bliss DZ, Savik K. Perioperative factors that affect long-term adjustment to an incontinent ostomy. J Wound Ostomy Continence Nurs. 2006;33(5):525–535.
75. Beeken RJ, Haviland JS, Taylor C, et al. Smoking, alcohol consumption, diet and physical activity following stoma formation surgery, stoma-related concerns, and desire for lifestyle advice: a United Kingdom survey. BMC Public Health. 2019;19(1):574.
76. Taylor C, Munro J, Goodman W, et al. Experiences of wearing support garments by people living with a urostomy. Br J Nurs. 2019;28(22):S26–s33.
77. Jensen BT, Kiesbye B, Soendergaard I, Jensen JB, Kristensen SA. Efficacy of preoperative uro-stoma education on self-efficacy after Radical Cystectomy; secondary outcome of a prospective randomized controlled trial. Eur J Oncol Nurs. 2017;28:41–46.
78. Wang QQ, Wang J, Zhao J, et al. Effects of a home care mobile app on the outcomes of discharged patients with a stoma: a randomised controlled trial. J Clin Nurs. 2018;27(19–20):3592–3602.
79. Faury S, Koleck M, Foucaud J, M’Bailara K, Quintard B. Patient education interventions for colorectal cancer patients with stoma: A systematic review. Patient Educ Couns. 2017;100(10):1807–1819.
80. Faury S, Quintard B. StomieCare: individual psycho-educational intervention with cognitive-behavioral techniques for people with rectal cancer and temporary stoma. J Ther Comportementale Cogn. 2019.
81. Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol. 2016;31(9):811–851.
82. Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–745.
83. Sathianathen NJ, Weight CJ, Jarosek SL, Konety BR. Increased surgical complications in smokers undergoing radical cystectomy. Bladder Cancer. 2018;4(4):403–409.
84. Hemal S, Krane LS, Richards KA, Liss M, Kader AK, Davis RL
85. Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014;3:Cd002294.
86. Lauridsen SV, Thomsen T, Kaldan G, Lydom LN, Tonnesen H. Smoking and alcohol cessation intervention in relation to radical cystectomy: a qualitative study of cancer patients’ experiences. BMC Cancer. 2017;17(1):
87. Lauridsen SV, Thomsen T, Thind P, Tonnesen H. STOP smoking and alcohol drinking before OPeration for bladder cancer (the STOP-OP study), perioperative smoking and alcohol cessation intervention in relation to radical cystectomy: study protocol for a randomised controlled trial. Trials. 2017;18(1):329.
88. Eliasen M, Gronkjaer M, Skov-Ettrup LS, et al. Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2013;258(6):930–942.
89. Tønnesen H. Alcohol abuse and postoperative morbidity. Dan Med Bull. 2003;50(2):139–160.
90. Bradley KA, Rubinsky AD, Sun H, et al. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. J Gen Intern Med. 2011;26(2):162–169.
91. Bradley KA, Rubinsky AD, Sun H, et al. Prevalence of alcohol misuse among men and women undergoing major noncardiac surgery in the Veterans Affairs health care system. Surgery. 2012;152(1):69–81.
92. Egholm JWM, Pedersen B, Møller AM, Adami J, Juhl CB, Tønnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database Syst Rev. 2018;11.
93. Gupta N, Kucirka LM, Semerjian A, et al. Comparing provider-led sexual health counseling of male and female patients undergoing radical cystectomy. J Sex Med. 2020;17(5):949–956.
94. Smith AB, Crowell K, Woods ME, et al. Functional outcomes following radical cystectomy in women with bladder cancer: a systematic review. Eur Urol Focus. 2017;3(1):136–143.
95. Titta M, Tavolini IM, Dal Moro F, Cisternino A, Bassi P. Sexual counseling improved erectile rehabilitation after non-nerve-sparing radical retropubic prostatectomy or cystectomy–results of a randomized prospective study. J Sex Med. 2006;3(2):267–273.
96. Gudbergsson SB, Dahl AA, Loge JH, Thorsen L, Oldervoll LM, Grov EK. What is covered by “cancer rehabilitation” in PubMed? A review of randomized controlled trials 1990–2011. J Rehabil Med. 2015;47(2):97–106.
97. Paterson C, Jensen BT, Jensen JB, Nabi G. Unmet informational and supportive care needs of patients with muscle invasive bladder cancer: a systematic review of the evidence. Eur J Oncol Nurs. 2018;35:92–101.
98. Jensen BT, Dalbagni G, Borre M, Love-Retinger N. Preoperative nutritional status and the impact on radical cystectomy recovery: an international comparative study. Urol Nurs. 2016;36(3):133–40, 52.
99. Mayr R, Gierth M, Zeman F, et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle. 2018;9(3):505–513.
100. Burden S, Billson HA, Lal S, Owen KA, Muneer A. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst Rev. 2019;5:Cd010127.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
