Back to Journals » Clinical Ophthalmology » Volume 16
Ophthalmic Manifestations of Newly Diagnosed Acute Leukemia Patients in a Tunisian Cohort
Authors Sayadi J , Gouider D , Allouche Y , Choura R, Cherni I, Sayadi M, Benneji H, Zghal I, Malek I, Nacef L
Received 4 April 2022
Accepted for publication 26 August 2022
Published 14 October 2022 Volume 2022:16 Pages 3425—3435
DOI https://doi.org/10.2147/OPTH.S365648
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jihene Sayadi,1 Dhouha Gouider,1 Yasmine Allouche,1 Racem Choura,1 Ines Cherni,1 Malek Sayadi,2 Hend Benneji,2 Imene Zghal,1 Ines Malek,1 Leila Nacef1
1Department A, Hedi Raies Institute of Ophthalmology, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia; 2Department of Hematology, Aziza Othmana Hospital, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
Correspondence: Dhouha Gouider, Department A, Hedi Raies Institute of Ophthalmology, Faculty of Medicine of Tunis, University of Tunis El Manar, Bab Saadoun, Tunis, Tunisia, Tel +21640660114, Email [email protected]
Purpose: To describe ocular manifestations of acute leukemia in a Tunisian cohort and to assess the associations between ophthalmic findings and epidemiological, clinical, and biological features of the disease.
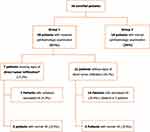
Methods: A prospective study included patients newly diagnosed with acute leukemia referred to our clinics between January 2019 and July 2020. All patients underwent a complete ophthalmic evaluation and spectral-domain optical coherence tomography (SD-OCT) at presentation, then every two months during one year. We defined two groups: Group 1 included patients with leukemic ophthalmopathy and group 2 included patients with normal ophthalmic examination.
Results: Forty-six patients were enrolled. The mean age of patients was 32.1± 15.3 years. The sex ratio M/F was 1.55 (28 male patients and 18 females). Twenty-nine patients (63%) had acute myeloid leukemia (AML), and 17 (37%) had acute lymphoblastic leukemia (ALL). The average follow-up was 9.1 months (range: 3– 12 months). We observed ophthalmic manifestations in 28 patients (61%). Among them, 17 (61%) had vision-threatening complications. The posterior segment was the most common site of ocular involvement (82% of group1). Primary leukemic infiltration (Disc edema, ptosis, exophthalmos) was present in 13 eyes (14.1%). Twenty-seven eyes (29.3%) had secondary involvement lesions (Subconjunctival hemorrhage, periorbital ecchymosis, retinal/sub-hyaloid hemorrhage, dilated/tortuous veins). Twenty-one eyes (22.8%) showed other ocular manifestations which etiopathogenesis is not yet fully understood (White-centred hemorrhages, cotton-wool spots, serous retinal detachment, hemorrhagic pigment epithelial detachment). Leukemic retinopathy was significantly more frequent in adults (23/39 and 1/7 in adult and pediatric groups, respectively; p=0.003). Patients suffering from AML were more likely to have secondary ocular involvement (20/29 and 7/17 in AML and ALL patients, respectively; p=0.047). Retinal hemorrhages were statistically associated with anemia and thrombocytopenia (p=0.041 and p=0.034; respectively).
Conclusion: Leukemic ophthalmopathy seems to be frequent and may lead to severe visual impairment. An ophthalmic assessment complemented with SD-OCT has paramount importance in all newly diagnosed acute leukemic patients.
Keywords: acute leukemia, ocular manifestations, leukemic retinopathy, retinal hemorrhage
Introduction
Acute leukemia is a neoplastic hematological condition resulting from the uncontrolled proliferation of immature white blood cells.1 The ophthalmic involvement may be asymptomatic. It can also be the revealing feature of the disease or an isolated focal relapse.1 Orbital and ocular lesions associated with leukemia may result from primary/direct leukemic infiltration of ocular tissues or secondary/indirect involvement following systemic leukemic changes.2
Primary leukemic involvement occurs under three clinical pictures: orbital infiltration including myeloid sarcoma, orbital hemorrhages and proptosis; neuro‐ophthalmic signs of central nervous system (CNS) leukemia including cranial nerve palsies, optic disc infiltration, and papilledema; as well as anterior segment uveal infiltration. The secondary changes are the result of indirect involvement from nonviable or dysplastic cells, hematological abnormalities (anemia, thrombocytopenia, hyperviscosity), or iatrogenic complications (steroids, chemotherapy, bone marrow transplantation, irradiation, and immunosuppression).1–3
Ocular involvement in acute leukemia is frequent and can compromise the further functional prognosis. It can also contribute to indicate the survival prognosis of systemic leukemia.1–3 Therefore, many authors have recommended a detailed ophthalmic evaluation in all patients with acute leukemia.1–3 Despite being the third most common site of extramedullary lesions, there have been few reports focusing on the rate of ophthalmic manifestations related to acute leukemia at the time of diagnosis.1–4 Furthermore, this area has not been investigated in North African population.
Hence, our study aimed to describe ocular findings in newly diagnosed patients with acute leukemia and to assess their correlation with demographic, clinical, and biological features of the disease. Further, we investigated whether ocular lesions could predict leukemia prognosis.
Methods
A prospective study was conducted at the Hedi Rais Institute of ophthalmology of Tunis, Tunisia. Newly diagnosed patients with acute leukemia were recruited between the 1st of January 2019 and the 31st of July 2020. The study adhered to the tenets of the Declaration of Helsinki. The study was approved by the Ethics Committee of Aziza Othmana Hospital. A written informed consent was obtained from all participants above 18 years-old and the parent or legal guardian of younger patients.
A hematologist-oncologist diagnosed acute leukemia based on bone marrow aspiration results and supportive immunohistochemical evidence. The exclusion criteria were diabetes, hypertension, acquired immunodeficiency syndrome, and pre-existing ocular diseases. We also excluded patients with acute leukemia whose general condition prevented them from being transferred for ophthalmic examination and patients with a short follow-up < 3 months.
Data gathered included detailed demographic information, leukemia characteristics (type, subtype and treatment), and hematological parameters. All patients underwent a complete ophthalmic evaluation: best-corrected visual acuity (BCVA), ocular motility, anterior segment, intraocular pressure, vitreous, and fundus examination. Visual impairment was defined as a BCVA less than 20/200. All patients went through fundus photographs and spectral-domain optical coherence tomography (SD-OCT). Fluorescein angiography was performed in patients with leukemic retinopathy. Evaluation was performed at presentation and every two months during one year.
The ophthalmic manifestations were classified into:
We defined two groups based on the initial ophthalmic findings:
Results
During the period study, 52 consecutive patients with newly diagnosed acute leukemia were hospitalized in the hematology department. Among them, two patients did not meet the inclusion criteria and three critically ill patients could not be transferred to the ophthalmology department. Of the 47 patients who underwent initial ophthalmic examination, one patient passed away two months after the enrollment. Hence, data analysis was performed for 92 eyes of 46 newly diagnosed acute leukemia patients.
Twenty-nine patients (63%) were diagnosed with acute myeloid leukemia (AML) and 17 (37%) with acute lymphoblastic leukemia (ALL). The mean age at presentation was 32.1±15.3 years (range: 2–58 years). Seven patients (15.2%) belonged to the pediatric population (less than 16 years old). There were 28 male patients (60.8%) and 18 females (39.2%), defining the sex-ratio (M/F) at 1.55. The average follow-up was 9.1 months (range: 3–12 months). There was no statistically significant difference in the mean follow-up between the 2 groups (9.02 and 9.34 in group 1 and 2, respectively; p=0.645).
Ocular manifestations revealed the hematologic disorder in four patients (9%). The presenting signs were: loss of vision in two patients (4.3%), bilateral periorbital ecchymosis in one patient (2.2%), and unilateral exophthalmos in one child (2.2%). Table 1 shows the demographic characteristics of the population study. Table 2 details the distribution of the subtypes of acute leukemia.
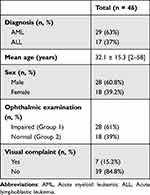 |
Table 1 Demographic Features of the Study Population |
 |
Table 2 Distribution of the Subtypes of Acute Leukemia |
Clinical findings
Ophthalmic evaluation was performed prior to initiation of systemic chemotherapy in only 21 patients (46%). Twenty-five patients (54%) went through critical condition at the time of diagnosis preventing them from pre-treatment eye examination. The average duration between the initiation of therapy and the ophthalmologic assessment was 3±3.62 days (range: one to seven days).
At presentation, 28 patients (60.8%) had an impaired ophthalmic examination (group 1). Among them, only 7 (25%) patients had visual complaints. Ophthalmic involvement was seen in three pediatric patients (6.5%) and four adult patients (8.7%) (p=0.407). Leukemic ophthalmopathy was bilateral in 23 patients (50%).
In group 1, 17 patients (37%) had a decreased BCVA in at least one eye. The average BCVA was 20/32 (range: from 20/400 to 20/20) (Figure 1).
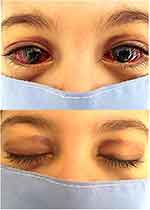
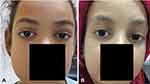
Orbit and anterior segment were involved in 13 eyes (13.5%). We noted subconjunctival hemorrhage in six eyes (6.5%), bilateral periorbital ecchymosis in two patients (4.3%), bilateral ptosis in one patient (2.2%) and exophthalmos in one eye (1.1%) (Figure 2).
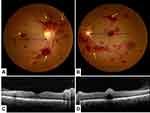
Leukemic retinopathy was observed in 23 patients (50%). Retinal hemorrhages were the most prevalent ocular finding (44/51 eyes; 86.3% of all ophthalmic manifestations). The observed lesions were: superficial and deep retinal hemorrhages in 25 eyes (27.2%), white-centred retinal hemorrhages in 17 eyes (17.7%), sub-hyaloid hemorrhage with foveal involvement in 2 eyes (2.2%), vascular dilation and tortuosity in 16 eyes (16.7%), papilledema in 10 eyes (10.9%), cotton wool spots in 3 eyes (3.1%) and areas of pigment epithelial damage in 4 eyes (4.2%) (Table 3) (Figure 3).
 |
Table 3 Clinical Eye Findings in the Study Population |
Tomographic findings
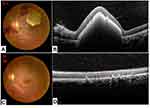
Retinal hyperreflectivity with posterior shading corresponding to retinal hemorrhage was the most common tomographic sign (27.2%) (Figures 3–4). A serous retinal detachment occurred in four eyes (4.3%) of three patients (6.5%), hemorrhagic detachment of the pigment epithelium (Figure 4), inner segment-outer segment line rupture, and macular pseudo-hole in one eye each.
Fluorescein angiography findings
Thirteen patients (28.3%) underwent fluorescein angiography. It revealed hypo-fluorescence with mask effect in 10 eyes (10.4%), early fluorescein retention with late diffusion corresponding to papillary edema in 10 eyes (10.4%), and pigeon nest image with mask effect in two eyes with sub-hyaloid hemorrhage (2.2%).
Ocular involvement subtypes
Hence, direct tumor infiltration was observed in 13 eyes (14.1%) of seven patients.
Optic disc edema occurred in ten eyes of ten patients (10.9%). In seven cases (7.6%), the cerebral MRI was within normal limits and the lumbar puncture indicated leukemic involvement. The remaining three patients were critically ill and expired before cerebral imaging could be performed.
Direct leukemic infiltration resulted in exophthalmos in an 11-year-old child. MRI showed hypervascular intraorbital process enclosing the optic nerve and invading the rectus muscles and the lacrimal gland. Orbital biopsy was performed; and histopathology disclosed a myeloid sarcoma. (Figure 5)
Secondary involvement lesions were noted in 27 eyes (29.3%) while ocular manifestations which etiopathogenesis is not fully understood occurred in 21 eyes (22.8%). Table 4 demonstrates the distribution of different eye findings based on involvement type and disease subgroups.
 |
Table 4 Comparison of Ophthalmic Manifestations in Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia |
Correlation study
In the current study, adults were more likely to develop the retinal disorder than children (23/39 and 1/7 in adult and pediatric groups, respectively, chi-2 = 4.75, p=0.029, Phi correlation=−0.321). Patients diagnosed with AML developed significantly more secondary ophthalmic manifestations (21/29 and 7/17 in AML and ALL patients, respectively, chi-2=4.39, p=0.036, Phi correlation=0.308), especially the retinal hemorrhages (19/29 and 5/17 in AML and ALL patients, respectively, chi-2=5.59, p=0.017, Phi correlation=0.34).
Anemia and thrombocytopenia were highly associated with the occurrence of retinal hemorrhages (mean hemoglobin rate at 6.7 ± 2 versus 8.3 ± 3.1 g/dl, unpaired t = 2.13, p=0.038 and platelet count at 45,585.7 ± 52,398.5 versus98625± 112 056.7 cells/mm3, unpaired t = 2.17, p=0.035, in group 1 and 2 respectively). Anemia was also significantly correlated with visual impairment (mean hemoglobin rate at 6.2 ± 2.1 versus 8.7 ± 2.8 g/dl with a cut-off BCVA of 20/200; unpaired t=2.17, p=0.003).
The presence of direct leukemic infiltration lesions was significantly associated with hyperleukocytosis (mean WBC count at 140 155 ± 168,354 (n=8) versus 39,467 ± 69,765 cells/mm3 (n=38) in patients with and without direct infiltration, respectively; unpaired t = 2.79, p = 0.007). However, there was no significant correlation between primary lesions and mortality rate (3 among 8 deaths during the study period, chi-2=2.72, p = 0.098, Phi correlation = 0.24).
There was no significant association between hematological parameters and white-centred retinal hemorrhage or cotton wool spots (all p=ns). No statistically significant association was found between eye damage and failure of chemotherapy induction (p=0.693) neither with hematological relapse (p=0.378). Finally, survival did not differ significantly regarding the presence or absence of ophthalmic manifestations (Log Rank test: chi-square = 0.079, p = 0.778). Figure 6 represents the Kaplan-Meier survival curves of leukemic patients with and without ophthalmic manifestations.
 |
Figure 6 Kaplan-Meier survival curves of leukemic patients in both groups. |
At last follow-up, ophthalmic signs resolved in 13 patients treated with chemotherapy and transfusion (46.4% of group 1). In 15 patients (53.6% of group 1), we did not observe ocular lesions regression despite medical treatment. Among them, functional visual impairment persisted in six eyes of six patients (21.4% of group 1). It was associated with macular retinal hemorrhage in three cases (10.7% of group 1), sub-hyaloid hemorrhage in two patients (7.1% of group 1), and a hemorrhagic pigment epithelial detachment in one patient (3.6% of group 1).
Discussion
We performed an ophthalmic evaluation in 46 Tunisian patients with recently diagnosed acute leukemia and found ocular involvement in 61% of patients. Gawai et al reported a similar rate (63%).5 Some recent studies reported a lower rate, ranging between 25 and 48% of the studied sample.3,6–8 On the flip side, other authors found it to be as high as 90%.1 Study sampling criteria (age, delay of examination, or type of hemopathy) could explain this disparity between studies.
The incidence of ophthalmic manifestations might also change if there is a distinction between leukemic cells primary infiltration and secondary ocular changes. Primary involvement is a rare finding in most studies. Indeed, it was reported in 2.3% and 7.3% of newly diagnosed patients with acute leukemia in Iranian and Indian populations, respectively.4,9 However, in the current study, direct tumor infiltration was far more prevalent, affecting 15.2% of our patients. This higher rate is consistent with other African studies and could be attributable to late diagnosis and management due to limited health care resources.10
Furthermore, autopsy documentations highlighted that primary involvement would be underestimated. According to Leonardy et al, leukemic cells infiltrate 31.1% of tissue sections of the eyes.11
Regarding neuro‐ophthalmic signs of CNS leukemia, we noted disc edema in 21.7% of our patients. Disc edema is either attributed to cellular infiltration of the nerve itself12 or increased intracranial pressure due to hemorrhagic tendency and renal dysfunction.13 In the current study, seven out of ten patients with disc edema underwent lumbar puncture which disclosed leukemic involvement. Neuro-ophthalmic manifestations of leukemia may reveal the diagnosis, indicate a recurrence, or point to a CNS involvement. Due to their correlation with poor prognosis, an appropriate change in therapeutic regimen is often required.14
Leukemic cells can infiltrate all orbital structures.15 In the series of Gawai et al, the most common primary orbital manifestation was exophthalmos (42.85%).5 He also reported bilateral lacrimal gland involvement in one patient. In our study, primary orbital involvement affected two patients (4.3%): bilateral ptosis in one patient, and exophthalmos in an 11 year-old-child. The latter was associated with myeloid sarcoma.
Secondary involvements are the most common manifestation of acute leukemia (43.8% in Koshy et al study,9 29.3% in our study), retinal hemorrhages being the most prevalent expression (88.8% of all ocular findings in Mirashi et al study,4 86.3% in our study). Involving nerve fiber layer, deeper layers, sometimes centered by a white component, or extending into the subretinal space or retro-hyaloid area, retinal hemorrhages seem to reflect the general clinical picture.12 In our study, retinal hemorrhages were particularly associated with AML similarly to studies by Koshy et al,9 Reddy et al3 and Omoti et al.16
As in other studies,4,17,18 anemia and thrombocytopenia appeared to significantly increase the occurrence of retinal hemorrhages (respectively p=0.038 and p=0.035). It is suggested that local factors such as anatomical vascular retinal arrangement or high intraocular pressure would contribute to retinal hemorrhage development.19 Soman et al noticed that the hemoglobin level improvement from 5 to 7 g/dL reduced the risk of having sub-hyaloid hemorrhages, and maintaining platelet counts above 50,000 cells/mm3 decreased the risk of ophthalmic manifestations.2
In our paper, adults were more likely to have leukemic retinopathy than children. This finding was similar to the results of Reddy and Orhan studies.3,6 The possible age-related vascular fragility predisposing to more retinal damages could explain this difference.14
Although retinopathy is the most commonly reported clinical expression, the choroid seems to be the most frequently involved site in autopsy-based studies.1,7,13 In our cohort, we recorded serous retinal detachment in four eyes of three patients and pigment epithelial detachment in one eye.
As for other ocular manifestations where primary or secondary attribution remains unclear, pseudo Roth hemorrhages are usually associated with acute leukemia but not pathognomonic.20 White-centred retinal hemorrhages histological analysis showed the white component as platelet-fibrin aggregates, leukemic cells, debris, or septic emboli.1 Cotton-wool spots can either be indicative of direct leukemic infiltration or represent clusters of nonviable or dysplastic cells occluding arterioles.1 These lesions were frequently described in patients with anemia.21 In our study, we found no significant association between hemoglobin level and cotton-wool spots.
The current research showed a unilateral macular pseudo-hole in a 42-year-old male patient with AML. To the best of our knowledge, this association has not been described in the literature. Yet, other publications reported vitreo-macular traction resulting in macular hole and macular pseudo hole in chronic myeloid leukemia.22,23 Hence, we cannot exclude the hypothesis that macular pseudo-hole could be a coexisting sight-threatening lesion regardless of acute leukemia.
Several studies have evaluated the relationship between the presence of ocular manifestations and survival rates. Mirashi et al have documented that ophthalmic involvement was associated with higher rates of death on the first day after diagnosis.4 The ophthalmic presentation seems to be a poor prognostic sign.4,21,24,25 A study comparing the 5-year survival rate between patients with and without ophthalmic manifestations has concluded a significantly lower survival for the group of ocular involvement.26 In the current study, the mortality rate did not significantly differ between the two groups. However, the short duration of follow-up can explain this difference. Another limitation of our work is the monocentric character, which could not allow having a larger sample.
Owing to leukemia’s poor vital prognosis, hematologists, internists, and pediatricians tended to neglect ophthalmic manifestations. With evolving diagnostic and therapeutic advances, the survival of patients has considerably improved which led to an increase in the variability of ophthalmic presentations. In view of the high rate of asymptomatic ocular involvement in our study (21/28 patients; 75%) consistent with literature review, we highly recommend ophthalmic screening in acute leukemia especially in AML and in patients with anemia, thrombocytopenia or hyperleukocytosis. Therefore, the role of ophthalmologists should be upgraded among members of leukemia management team.
Disclosure
Dr Hend Benneji reports personal fees from abbvie, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Talcott KE, Garg RJ, Garg SJ. Ophthalmic manifestations of leukemia. Curr Opin Ophthalmol. 2016;27(6):545–551. doi:10.1097/ICU.0000000000000309
2. Soman S, Kasturi N, Srinivasan R, Vinod KV. Ocular manifestations in leukemias and their correlation with hematologic parameters at a tertiary care setting in South India. Ophthalmol Retina. 2018;2(1):17–23.
3. Reddy SC, Jackson N, Menon BS. Ocular involvement in leukemia – a study of 288 cases. Ophthalmologica. 2003;217(6):441–445. doi:10.1159/000073077
4. Mirshahi R, Ghassemi F, Koochakzadeh L, et al. Ocular manifestations of newly diagnosed acute leukemia patients. J Curr Ophthalmol. 2022;34:100–105.
5. Gawai D, Jhavar S, Patil S. Orbital and ocular manifestations of acute and chronic leukemia. Int J Health Sci. 2016;6(9):4.
6. Orhan B, Malbora B, Akça Bayar S, Avcı Z, Alioğlu B, Özbek N. Ophthalmologic findings in children with leukemia: a single-center study. Türk Oftalmoloji Dergisi. 2016;46(2):62–67.
7. Hafeez MU, Ali MH, Najib N, et al. Ophthalmic manifestations of acute leukemia. Cureus. 2019;11(1):e3837. doi:10.7759/cureus.3837
8. Bitirgen G, Belviranli S, Caliskan U, Tokgoz H, Ozkagnici A, Zengin N. Ophthalmic manifestations in recently diagnosed childhood leukemia. Eur J Ophthalmol. 2016;26(1):88–91. doi:10.5301/ejo.5000647
9. Koshy J, John MJ, Thomas S, Kaur G, Batra N, Xavier WJ. Ophthalmic manifestations of acute and chronic leukemias presenting to a tertiary care center in India. Indian J Ophthalmol. 2015;63:659–664. doi:10.4103/0301-4738.169789
10. Eze BI, Ibegbulam GO, Ocheni S. Ophthalmic manifestations of leukemia in a tertiary hospital population of adult Nigerian Africans. Middle East Afr J Ophthalmol. 2010;17:325–329. doi:10.4103/0974-9233.71599
11. Leonardy NJ, Rupani M, Dent G, Klintworth GK. Analysis of 135 autopsy eyes for ocular involvement in leukemia. Am J Ophthalmol. 1990;109(4):436–444. doi:10.1016/S0002-9394(14)74610-0
12. Bagheri S, Armstrong GW, Vavvas DG. ”Retinopathy Associated with Blood Disorders”. Albert and Jakobiec’s Principles and Practice of Ophthalmology. Cham: Springer International Publishing; 2020:1–21.
13. Sharma H, Majumder PD, Rao C, Biswas J. A case of Acute Myeloid Leukemia masquerading as unilateral exudative detachment. Am J Ophthalmol Case Rep. 2016;4:47–49.
14. Alrobaian MA, Henderson AD. Neuro-Ophthalmic Manifestations of Acute Leukemia. J Neuro Ophthalmol. 2021;41(4):e584–e590. doi:10.1097/WNO.0000000000001071
15. Charif Chefchaouni M, Belmekki M, Hajji Z, et al. Ophthalmic manifestations of acute leukemia. J Fr Ophtalmol. 2002;25(1):62–66.
16. Omoti AE, Omoti CE, Momoh RO. Ocular disorders in adult leukemia patients in Nigeria. Middle East Afr J Ophthalmol. 2010;17:165–168. doi:10.4103/0974-9233.63081
17. Suresh K, Sampath R. Ocular manifestations in hematological disorders. Sri Ramachandra J Med. 2011;4:1–4.
18. Dhasmana R, Prakash A, Gupta N, Verma SK. Ocular manifestations in leukemia and myeloproliferative disorders and their association with hematological parameters. Ann Afr Med. 2016;15:97–103. doi:10.4103/1596-3519.188887
19. Valade S, Lemiale V, Mariotte E, Vincent F. Syndrome d’hyperviscosité: mise au point pour les réanimateurs. Méd Intensive Réa. 2018;27(4):317–323. doi:10.3166/rea-2018-0046
20. Ben Abdesslem N, Zaafrane N, Ben Abderazek A, et al. White-centered retinal hemorrhage revealing acute leukemia: a case report. Ann Med Surg. 2022;77:103632. doi:10.1016/j.amsu.2022.103632
21. Abu el-Asrar AM, al-Momen AK, Kangave D, Harakati MS, Ajarim DS. Correlation of fundus lesions and hematologic findings in leukemic retinopathy. Eur J Ophthalmol. 1996;6(2):167–172. doi:10.1177/112067219600600213
22. Bhargava R, Kumar P. Visual disturbance as presenting feature and macular hole as complication of chronic myeloid leukemia. J Clin Ophthalmol Eye Disord. 2018;2(1):1021.
23. Yassin MA, Ata F, Mohamed SF, et al. Ophthalmologic manifestations as the initial presentation of chronic myeloid leukemia: a review. Surv Ophthalmol. 2022;67(2):530–543. doi:10.1016/j.survophthal.2021.07.001
24. Russo V, Scott IU, Querques G, Stella A, Barone A, Delle Noci N. Orbital and ocular manifestations of acute childhood leukemia: clinical and statistical analysis of 180 patients. Eur J Ophthalmol. 2008;18(4):619–623. doi:10.1177/112067210801800420
25. Grishina E, Mamontov A. Ophthalmic manifestations of leukemia. Almanac Clin Med. 2016;44:587–591. doi:10.18786/2072-0505-2016-44-5-587-591
26. Ohkoshi K, Tsiaras WG. Prognostic importance of ophthalmic manifestations in childhood leukaemia. Br J Ophthalmol. 1992;76(11):651–655. doi:10.1136/bjo.76.11.651
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.