Back to Journals » Cancer Management and Research » Volume 11
Oncometabolites as biomarkers in thyroid cancer: a systematic review
Authors Khatami F , Payab M , Sarvari M, Gilany K , Larijani B, Arjmand B, Tavangar SM
Received 25 September 2018
Accepted for publication 19 November 2018
Published 25 February 2019 Volume 2019:11 Pages 1829—1841
DOI https://doi.org/10.2147/CMAR.S188661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Fatemeh Khatami,1 Moloud Payab,2 Masoumeh Sarvari,3 Kambiz Gilany,3–5 Bagher Larijani,6 Babak Arjmand,7 Seyed Mohammad Tavangar1,8
1Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 2Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 3Metabolomics and Genomics Research Center, Endocrinology and Metabolomics Molecular Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 4Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran; 5Integrative Oncology Department, Breast Cancer Research Center, Motamed Cancer Institute, Acercr, Tehran, Iran; 6Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 7Cell Therapy and Regenerative Medicine Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; 8Department of Pathology, Dr. Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Introduction: Thyroid cancer (TC) is an important common endocrine malignancy, and its incidence has increased in the past decades. The current TC diagnosis and classification tools are fine-needle aspiration (FNA) and histological examination following thyroidectomy. The metabolite profile alterations of thyroid cells (oncometabolites) can be considered for current TC diagnosis and management protocols.
Methods: This systematic review focuses on metabolite alterations within the plasma, FNA specimens, and tissue of malignant TC contrary to benign, goiter, or healthy TC samples. A systematic search of MEDLINE (PubMed), Scopus, Embase, and Web of Science databases was conducted, and the final 31 studies investigating metabolite biomarkers of TC were included.
Results: A total of 15 targeted studies and 16 untargeted studies revealed several potential metabolite signatures of TC such as glucose, fructose, galactose, mannose, 2-keto-d-gluconic acid and rhamnose, malonic acid and inosine, cholesterol and arachidonic acid, glycosylation (immunoglobulin G [IgG] Fc-glycosylation), outer mitochondrial membrane 20 (TOMM20), monocarboxylate transporter 4 (MCT4), choline, choline derivatives, myo-/scyllo-inositol, lactate, fatty acids, several amino acids, cell membrane phospholipids, estrogen metabolites such as 16 alpha-OH E1/2-OH E1 and catechol estrogens (2-OH E1), and purine and pyrimidine metabolites, which were suggested as the TC oncometabolite.
Conclusion: Citrate was suggested as the first most significant biomarker and lactate as the second one. Further research is needed to confirm these biomarkers as the TC diagnostic oncometabolite.
Keywords: biomarkers, oncometabolites, thyroid cancer, TC, systematic review
Introduction
Thyroid cancer (TC) is the most common endocrine-related tumor in the past decades, and its incidence has been increasing all over the world.1–4 The starting point of TC is the thyroid nodule formation detectable by ultrasonography (US) evaluations.5,6 Thyroid nodules are mostly benign, and the current gold standard discriminative tool between TC and benign thyroid nodules (BTNs) is a cytopathologic analysis of percutaneous fine-needle aspiration (FNA) specimens.7 FNA is a simple test that samples a small amount of tissue from the thyroid with a very thin (or “fine”) needle.8–11 Histopathological report of FNA has a weak point of indeterminate results, negative predictive value, and high cost.12,13 Hence, there is an extreme need to find molecular markers either to support FNA or to take the place of FNA.14–17
Increasing evidence indicates that tumor-associated mutations represent key factors resulting in different profiles of the cancerous cells’ genomics, epigenomics, transcriptomics, proteomics, and metabolomics.18–20,117,118 Metabolomics is an extensive-scale study of small molecules (>1,000 Da), generally popular as metabolites, within cells, biofluids, tissues, or organisms.21–23 The major dissimilarities between cancerous cells and their counterpart noncancerous cells are their metabolites, which are called “oncometabolites”.24 For the first time, it was revealed in 1927 that tumors display a unique metabolic phenotype, and their glucose level is up to 200 times more than that of normal cells.25 Despite ignorance of oncometabolite impact on cancer diagnosis and management by 1970s, oncometabolites were rediscovered in the past decades.26 Oncometabolites are intrinsic metabolites that either start or continue tumor growth and metastasis. The primary oncometabolite was 2-hydroxyglutarate (2HG), which was recognized as a main metabolite with much higher concentrations in gliomas than normal cells.27 Main oncometabolites can be classified into six hallmarks: 1) those involved in glucose and amino acid uptake, 2) use of adaptable modes of nutrient gaining, 3) use of glycolysis/tricarboxylic acid (TCA) cycle and NADPH production, 4) augmented demand for nitrogen, 5) modifications in metabolite-driven gene regulation, and 6) metabolic contacts with the microenvironment. In fact, limited tumors show all six hallmarks together, and each one can be an indicator of tumor and can guide scientists to the exact tumor classification and higher efficient tumor management policies.28 There are nine oncometabolites in different types of TCs: 2HG, glucose, fumarate, succinate, sarcosine, glutamine, asparagine, choline, and lactate.29 Recently, some studies on the metabolomics analysis of FNA specimens of thyroid nodules have suggested the benefit of oncometabolites approach as the potential application for the cooperative diagnosis tool of TC.30–33 Several metabolic pathways linking it to the TCA, pentose phosphate pathway, and lipid metabolism are candidate biomarkers to discriminate between normal and cancer cells (Figure 1).
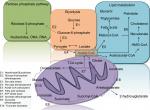  | Figure 1 Several metabolic pathways in normal and cancer cells. Abbreviation: TCA, tricarboxylic acid. |
Here, we present the first meticulous summary of the entire available primary research to evaluate the potential of oncometabolites as the discriminative molecular marker between TC and BTNs.
Research design and methods
Search strategy
The study was conducted according to International prospective register of systematic reviews PROSPERO code: CRD42018088928 (http://www.Crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018088928). All related literature searches from four main databases including MEDLINE (PubMed), Scopus, Embase, and Web of Science for relevant articles were retrieved from January 1, 1998, to end of March 2018 with the key words grouping of “metabolomics”, “metabonomics”, “oncometabolites”, “metabolic profiling”, combined with “Thyroid Neoplasm”, “Thyroid Carcinoma”, “Thyroid Adenoma”, “Thyroid Nodules”, and “Thyroid Cancer” (Supplementary materials). To minimize selection bias, two independent investigators (BA and MS) autonomously checked titles, abstracts, and available full-text articles for application. Further articles were recognized by checking the reference lists from the selected studies. Disagreements were fixed by agreement and discussion with a third researcher (KG).
Eligibility criteria
All nominated studies were reviewed by two authors independently and according to their title and abstract were categorized as the included one or excluded one. The inclusion criteria were as follows: 1) participants included thyroid patients with TC; 2) the control population was specified (eg, patients with BTN, goiter patients, or healthy subjects); 3) all metabolomics detection techniques such as HPLC, ultra performance liquid chromatography (ULC), mass spectrometry (MS), tandem mass spectrometry (TMS), and nuclear magnetic resonance (NMR) spectroscopy were selected; and 4) metabolites were examined in plasma, serum, urine, or FNA specimens. Research studies were excluded if they 1) analyzed metabolite profiles in animals (in vivo studies), 2) analyzed metabolite profiles in cell culture (in vitro studies), or 3) did not contain a suitable control group.
Data extraction and analysis
All data on population distinctiveness and indicative oncometabolites were entered in Excel. FK had performed the data completion steps, which was confirmed by another researcher (MP). Due to the inadequate quantity of studies related to TC and metabolomics, and the extensive methodological heterogeneity and the significant dissimilarities in study population characteristics, an assessable meta-analysis of the data was not applicable.
The quality assessment tools
Here, we used Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) and The Newcastle–Ottawa Scale (NOS) assessment tools to assess the methodological quality of the selected research articles. QUADAS and NOS were used to evaluate quality issues particular for “-omics” (QUADOMICS) more than the quality assessment of studies involving in systematic reviews.34,35 Each research article that scored 12/16 or more on the QUADOMICS tool together with 6/8 or more on NOS were considered as “high quality”, while each research article that scored 11/16, 5/8, or less were considered as “low quality”.
Results
Study selection and characteristics
The selection algorithm and results of study selection are presented in Figure 2. A total of 806 articles were retrieved after duplication deletion, including 374 articles from PubMed, 293 from Scopus, 83 from Web of Science, and 56 from Embase. After deleting the review, in vivo/in vitro studies, and book or conference paper with no available full-text articles, the final 31 articles were chosen for further considerations. A total of 15 studies with targeted metabolites (Table 1) and 16 studies with untargeted metabolites methods (Table 2) were selected. Two studies with targeted metabolites were removed because of low quality after quality assessment.
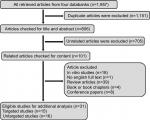  | Figure 2 Flow diagram of study selection for the current systematic review. |
The sample size of the study population was different from one case report that discussed 138 TC cases. Five studies were conducted in USA, 12 in China, five in Korea, two in Poland, two in Italy, one in Japan, one in Nepal, and one in the Netherlands. Both case/control and case report/series were included in the studies. In most case/control studies, the metabolites were compared between TC as the case group with healthy individuals and BTN or goiter patients as the controls. Exceptionally in two studies, the case/control was based on menopause TC and non-menopause TC. One study in Nepal evaluated the oncometabolites in differentiated thyroid carcinoma (DTC) with an increasing risk of cardiovascular disease. The oncometabolites included amino acids such as isoleucine, leucine, valine, lactate, threonine, alanine, uracil, lysine, glutamate, methionine, aspartate, choline, phosphocholine, glycerophosphocholine, taurine, myo-inositol, glycine, phosphoethanolamine, inosine, thyrosine, hypoxanthine, formate, succinate, and uridine; carboxylic acids such as acetate, citrate, fumarate, and lactate; monosaccharides such as glucose and glycosylation; estrogen metabolites such as 16 alpha-OH E1/2-OH E1 and catechol estrogens (2-OH E1); lipids such as total cholesterol, triglycerides, HDL, LDL, and VLDL; fibrinogen; calcitonin; and carcinoembryonic antigen (CEA). The unique phospholipids of bilayer membrane (phosphatidylcholine, phosphatidylcholine, and sphingomyelin) in addition to thyroid hormones, free triiodothyronine (fT3), free thyroxine (fT4), and thyroid-stimulating hormone (TSH) were also included in the list of metabolites. Some studies used the oncometabolites for TC diagnosis and some for follow-up and management of TC patients.
Amongst all selected studies in this systematic review, two studies were considered metabolite profile of premenopausal women. A targeted study conducted in Manipal Teaching Hospital of Nepal suggested that the hypercoagulable state atherogenic lipid profile is the different metabolite correlated with an increasing risk of cardiovascular disease in DTC patients. Other studies considered the different metabolite profiles as the discriminative tool of thyroid malignancy.
Discussion
Cancer studies highlighted the fact that cancer cells are common in biological capabilities such as constant proliferative signaling, growth suppressor’s avoidance, resistance to cell death, replicative immortality, high angiogenesis, reprogrammed energy metabolism, immune-mediated destruction, invasion, and metastasis.11,64,65,119 Metabolic reprogramming orchestrates cancer cell properties, so “cancer metabolism” became an important research topic for cancer management. The first study on cancer metabolism in 1924 suggested that the cancer phenotype for glucose metabolism is unique one and with higher ability of glucose uptake and lactate production is typical in several tumors.66 These pathways are named as “aerobic glycolysis” or the “Warburg effect”, which has the effect on the extracellular fluid around tumor tissue and change it to acidic pH.67–69 Glucose is the critical source of carbon that helps in the maintenance of cancer cell anabolism, TCA anaplerosis, aerobic glycolysis, hexokinase II activation, and modified signal transduction.70,71 Glucose was the most frequent metabolite elevated in most cancers26,72,73 and has been used as the oncometabolite of TCs in both targeted42,44 and untargeted studies.50,54,56 Analysis of the serum metabolic alterations among PTC, benign thyroid tumor, and healthy controls suggested that glucose metabolism cannot be the only important metabolite because metabolism of lipids, amino acids, and nucleic acids is important as well.50 Moreover, it was shown that the mRNA quantity of metabolic enzyme-coding genes resulting in different glucose, fructose, galactose, mannose, 2-keto-D-gluconic acid and rhamnose, malonic acid and inosine, cholesterol and arachidonic acid significantly increased in PTC.56 These studies were confirmed by detecting 31 different metabolites related to amino acid, lipid, glucose, vitamin metabolism, and diet/gut microbiota interaction.74
Metabolome analysis of amino acid profile is under consideration for biomarkers of thyroid malignancy. The plasma-free amino acid (PFAA) profiles of breast cancer, gastric cancer, and TC patients and investigation of their diagnostic potential were shown in the study by Gu et al.75 Carnitine, trimethylamine N-oxide (TMAO), proline, glutamine, and asparagine were known as the most significant metabolites of 392 metabolites in TC.49 In serum specimens of papillary TC patients, the amount of metabolites like valine, leucine, isoleucine, lactic acid, alanine, glutamic acid, lysine, glycine, whereas the lipids, choline, tyrosine decreased.76 Similarly alanine, creatine, glutamine, tyrosine, and valine in both serum and urine of TC patients were diagnosed by H NMR-based method.77
Glycosylation is one of the most frequent posttranslational modification reactions, and almost half of all proteins in eukaryotes are glycosylated.11,78 Some findings revealed the potential of IgG glycosylation as a biomarker for inflammation, metabolic health, and cancers.79,80 In TC, it was suggested that human IgG Fc-glycosylation profiling could be linked with age, sex, female sex hormones, and TC risk.38 IgG glycosylation in addition to glycans, glycome and glycoproteome are important in controlling thyroid cancer development and progression.81,82 The translocase of outer mitochondrial membrane 20 (TOMM20), a marker of oxidative phosphorylation, and monocarboxylate transporter 4 (MCT4), a marker of glycolysis, are candidate metabolites for aggressive behavior of TC.39
High levels of lactate and choline and low levels of citrate, glutamine, and glutamate in malignant thyroid nodules were reported by Ryoo et al33 and suggested them as the discriminative biomarker for determining the preoperative metabolomic profiles of thyroid nodules. Lactate is often augmented in several malignancies including head and neck cancers.30,31,83,84 High lactate level is the sign of glycolytic pathway increasing in response to hypoxia or ischemia in tumor tissues.85–87 Lactogenesis, an important step for the production of lactate, is started and triggered by gene mutations (the Warburg effect), so deregulated lactate metabolism and signaling are the critical elements in carcinogenesis.88 Lactate was established as an important factor in terms of cancer cell mobility and immune suppressor molecule that promote the tumor evasion as well.89,90 Lactate was found to be the most promising metabolite for discrimination of lymph node metastasis from nonmetastatic TC.48 Two studies confirmed that reduced levels of fatty acids and elevated levels of several amino acids (phenylalanine, tyrosine, lactate, serine, cystine, lysine, glutamine/glutamate, taurine, leucine, alanine, isoleucine, and valine) in papillary thyroid microcarcinoma (PTMC) and rise of phenylalanine, taurine, and lactate and a reduction of choline and choline derivatives, myo- and scyllo-inositol in the malignant tumors vs to the benign ones.59,62
Phospholipids are esters of glycerol, fatty acids, phosphoric acid, and other alcohols. Nearly, all frequent phospholipids are phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidylserine. Evidence showed that phosphatidylcholine, the major phospholipid element of eukaryotic membranes, like choline metabolites resulting from its metabolism, has an important role in cancer proliferation and survival.91 In thyroid malignancies, the increased multiplication and proliferation of cancer cells are linked to the increased choline contents even in FNA specimen.47,57,92 Choline was in the list of discriminative oncometabolites of TC with lymph node metastasis from non-metastatic one.48 These findings are contradictory with a study in which the content of lipids, choline, and tyrosine decreased in malignant TC compared to that in the benign one.31,50
Another metabolite that increased as the result of glycolysis in TC is glycine. It could be one of the essential metabolites in tumorigenesis and mitochondrial synthesis, and consumption of glycine was suggested as a discriminative metabolites triggering the cancer cell growth and development.33 Glycine dehydrogenase enzyme (GLDC), which cleavages glycine and mediates folate cycle charging, is highly expressed in tumor-promoting cells.93–95
Oncometabolomic analysis revealed that citrate uptake largely affected cancer cell metabolism through citrate-dependent metabolic pathways, and the extracellular citrate is provided to cancer cells through a plasma membrane-specific variant of the mitochondrial citrate transporter (pmCiC).96 In the study by Ryoo et al,57 it was suggested that citrate was the most powerful discriminator oncometabolite for diagnosis of TC. Previous studies also indicated that ATP citrate lyase, essential for cell proliferation, is upregulated in some human malignancies such as lung, colorectal, and ovarian cancers.97,98 The inhibition of ATP citrate lyase can block the proliferation of multiple tumor cell lines.99
The role of isocitrate dehydrogenase (IDH) mutations and d-2-HG accumulation in malignancy has increased recently.100 2-HG has been considered as oncometabolites and epigenetic modifiers in different malignancies such as gliomas,101–103 myelogenous leukemia,104 and renal cancer.105 Non-synonymous variants of IDH1 gene have been detected in thyroid carcinomas, and in PTC, the increased levels of 2-HG were reported.106–109 However, it has not been considered as an oncometabolite in TCs.
There are three main forms of estrogen in the human body: estradiol, estrone, and estriol. These forms of estrogen together with estrogen receptor and other estrogen metabolites (16 alpha-OH E1/2-OH E1 and catechol estrogens [2-OH E1]) are more commonly associated with cancer risk.110,111 For checking the possible outcome of estrogens in premenopausal female TC, the concentrations of 14 estrogens were assessed in the urine of patients with PTC preoperatively and postoperatively, and it was confirmed that low mean value of 16α-OH E1/2-OH E1 was observed in preoperative patients, and it was considerably dissimilar to the ratio of postoperative TC cases. The increase of 2-hydroxylation in estrogen metabolism may have a noteworthy relationship with the risk of TC formation in females.46 Moreover, it was shown that higher exposure to estrogens can increase the risk for TC, and 38 urinary estrogen metabolites were checked by Zahid et al, they suggested the unbalanced estrogen metabolism and formation of estrogen-DNA adducts as the role player in the initiation of TC.36 Supporting information indicated to anti-estrogenic dietary supplement function of 3,3′-diindolylmethane (DIM) to help reduce the risk of developing thyroid proliferative disease (TPD).43
In addition, the synthesis of purines and pyrimidine is upregulated in cancer cells, and the catalyzing enzymes of this pathway including thymidylate synthase and inosine synthetase 2 are subjected to Myc-induced upregulation.112,113 Glutamine is a nitrogen source for multiple steps of both purine and pyrimidine synthesis.114 Glutamine is a critical nutrient indispensable for cancer cell growth and is the new therapeutic target in cancers.115,116 In preoperative percutaneous FNA specimens of TC, it was shown that glutamine and glutamate are presented with lower relative concentrations.50,57 These results were generally in agreement with a previous finding obtained using surgical specimens.59 Pathway analysis indicated the “alanine, aspartate and glutamate metabolism” and “inositol phosphate metabolism” as the most relevant pathways in thyroid carcinogenesis.74 However, gastric cancer cells was promoted by cysteine, but inhibited by alanine and glutamic acid because alanine and glutamic acid induced apoptosis of gastric cancer cells.45 Follicular adenomas exhibit a unique metabolic profile with several oncometabolite profiles including glutamine.63
Conclusion
Because of the complexity of thyroid carcinogenesis, a wide range of oncometabolites is suggested as TC diagnostic markers. Potential biomarkers common to all thyroid lesions were mainly fatty acids, amino acids, cell membrane phospholipids, estrogen metabolites (16 alpha-OH E1/2-OH E1 and catechol estrogens(2-OH E1), purine and pyrimidine metabolites, citrate, glucose, mannose, pyruvate, and 3-hydroxybutyrate glycosylation (IgG Fc-glycosylation), TOMM20, MCT4, choline, choline derivatives, myo-/scyllo-inositol, and lactate. Among all metabolites, citrate was suggested as the first most significant oncometabolite and lactate as the second one in thyroid malignancies.
Abbreviations
BC, breast cancer; BN, benign nodule; BTA, benign thyroid adenoma; BTN, benign thyroid nodules; CEA, carcinoembryogenic antigen; CPMG, Carr-Pure-Me boom-Gill sequence; DESI-MS, desorption electrospray ionization mass spectrometry; DTC, differentiated thyroid carcinoma; FA, follicular adenoma; FTC, follicular thyroid cancer; GC, gastric cancer; GC-TOF-MS, gas chromatography time-of-flight mass spectrometry; HI, healthy individual; HRMAS, high-resolution magic angle spinning; LC-DIA-MS, liquid chromatography–data independent-mass spectrometry; LNMBC, lymph node with metastatic breast cancer; LNMP, lymph node with metastatic PTC; MNG, multinodular goiters; MTC, medullary thyroid cancer; NAT, normal adjacent tissue; NLN, normal lymph node; NMR, nuclear magnetic resonance; NN, non-neoplastic nodule; NOEPR, nuclear over Hauser effect spectroscopy with P resaturation; NTC, noncancerous thyroid tissue; PTC, papillary thyroid carcinoma; TCP, thyroid cancer patients; TMS, tandem mass spectrometry; TPD, thyroid proliferative disease; ULC, ultra performance liquid chromatography; UPLC−QTOFMS, ultra-performance liquid chromatography−quadruple time-of-flight mass spectrometry; UTC, undifferentiated thyroid carcinoma.
Acknowledgments
The authors thank the Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiology Biomarkers & Prevention. 2009;18(3):784–791. | ||
Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164(6):1481–1485. | ||
Larijani B, Shirzad M, Mohagheghi MA, et al. Epidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR). Asian Pac J Cancer Prev. 2004;5(1):36–39. | ||
Haghpanah V, Soliemanpour B, Heshmat R, et al. Endocrine cancer in Iran: based on cancer registry system. Indian J Cancer. 2006;43(2):80. | ||
Moon WJ, Baek JH, Jung SL, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12(1):1–14. | ||
Larijani B, Mohagheghi MA, Bastanhagh MH, et al. Primary thyroid malignancies in Tehran, Iran. Med Princ Pract. 2005;14(6):396–400. | ||
Cooper DS, Doherty GM, Haugen BR. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. | ||
Cibas ES, Ali SZ, NCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665. | ||
Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. | ||
Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006;14(4):422–425. | ||
Sanii S, Saffar H, Tabriz HM, Qorbani M, Haghpanah V, Tavangar SM. Expression of matrix metalloproteinase-2, but not caspase-3, facilitates distinction between benign and malignant thyroid follicular neoplasms. Asian Pac J Cancer Prev. 2012;13(5):2175–2178. | ||
Proietti A, Borrelli N, Giannini R, et al. Molecular characterization of 54 cases of false-negative fine-needle aspiration among 1347 papillary thyroid carcinomas. Cancer Cytopathol. 2014;122(10):751–759. | ||
Haddadi-Nezhad S, Larijani B, Tavangar SM, Nouraei SM. Comparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodules. Endocr Pathol. 2003;14(4):369–374. | ||
Khatami F, Larijani B, Tavangar SM. Circulating Tumor BRAF Mutation and Personalized Thyroid Cancer Treatment. Asian Pac J Cancer Prev. 2017;18(2):293. | ||
Khatami F, Tavangar SM. Liquid biopsy in thyroid cancer: new insight. Int J Hematol Oncol Stem Cell Res. 2018;12(3):234–247. | ||
Tavangar SM, Monajemzadeh M, Larijani B, Haghpanah V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singapore Med J. 2007;48(8):744–747. | ||
Mohammadi-Asl J, Larijani B, Khorgami Z, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol. 2011;28(4):1123–1128. | ||
Kildegaard HF, Baycin-Hizal D, Lewis NE, Betenbaugh MJ. The emerging CHO systems biology era: harnessing the “omics revolution for biotechnology. Curr Opin Biotechnol. 2013;24(6):1102–1107. | ||
Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar SM, Expression AP. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol. 2009;4(1):41. | ||
Gilany K, Minai-Tehrani A, Amini M, Agharezaee N, Arjmand B. The challenge of human spermatozoa proteome: a systematic review. J Reprod Infertil. 2017;18(3):267. | ||
Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–459. | ||
Gilany K, Jafarzadeh N, Mani-Varnosfaderani A, et al. Metabolic fingerprinting of seminal plasma from non-obstructive Azoospermia patients: positive versus negative sperm retrieval. J Reprod Infertil. 2018;19(2):109–114. | ||
Agharezaee N, Marzbani R, Rezadoost H, Koukhaloo SZ, Arjmand B, Gilany K. Metabolomics: a bird’s eye view of infertile men. Tehran University Medical Journal. 2018;75(12):860–868. | ||
Collins RRJ, Patel K, Putnam WC, Kapur P, Rakheja D. Oncometabolites: a new paradigm for oncology, metabolism, and the clinical laboratory. Clin Chem. 2017;63(12):1812–1820. | ||
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. | ||
Zhou Z, Ibekwe E, Chornenkyy Y. Metabolic alterations in cancer cells and the emerging role of Oncometabolites as drivers of neoplastic change. Antioxidants. 2018;7(1):16. | ||
Ward PS, Patel J, Wise DR et al. The Common Feature of Cancer-Associated IDH1 and IDH2 Mutations is a Neomorphic Enzyme Activity Converting α-ketoglutarate to the Oncometabolite 2-Hydroxyglutarate. Cancer Cell. 2010; 17(3):225-234. | ||
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. | ||
Wishart DS, Mandal R, Stanislaus A, Ramirez-Gaona M. Cancer metabolomics and the human metabolome database. Metabolites. 2016;6(1):10. | ||
Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. Eur J Radiol. 2007;64(3):414–418. | ||
Miccoli P, Torregrossa L, Shintu L, et al. Metabolomics approach to thyroid nodules: a high-resolution magic-angle spinning nuclear magnetic resonance-based study. Surgery. 2012;152(6):1118–1124. | ||
Tian Y, Nie X, Xu S, et al. Integrative metabonomics as potential method for diagnosis of thyroid malignancy. Sci Rep. 2015;5:14869. | ||
Ryoo I, Kwon H, Kim SC, et al. Metabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: Potential application for the preoperative diagnosis of thyroid cancer. Sci Rep. 2016;6(1):30075. | ||
Bae JM. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health. 2016;38:e2016014. | ||
Lumbreras B, Porta M, Márquez S, Pollán M, Parker LA, Hernández-Aguado I. QUADOMICS: an adaptation of the Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of “-omics”-based technologies. Clin Biochem. 2008;41(16–17):1316–1325. | ||
Zahid M, Goldner W, Beseler CL, Rogan EG, Cavalieri EL. Unbalanced estrogen metabolism in thyroid cancer. Int J Cancer. 2013;133(11):n/a–9. | ||
Villanueva J, Nazarian A, Lawlor K, Yi SS, Robbins RJ, Tempst P. A sequence-specific exopeptidase activity test (SSEAT) for “functional” biomarker discovery. Mol Cell Proteomics. 2008;7(3):509–518. | ||
Chen G, Wang Y, Qiu L, et al. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteomics. 2012;75(10):2824–2834. | ||
Curry JM, Tassone P, Cotzia P, et al. Multicompartment metabolism in papillary thyroid cancer. Laryngoscope. 2016;126(10):2410–2418. | ||
de Groot JW, Kema IP, Breukelman H, et al. Biochemical markers in the follow-up of medullary thyroid cancer. Thyroid. 2006;16(11):1163–1170. | ||
Do SI, Kim HS, Kim K, et al. Predictive value of sphingosine kinase 1 expression in papillary thyroid carcinoma. Anticancer Res. 2017;37(10):5399–5405. | ||
Mittal A, Poudel B, Pandeya DR, Gupta SP, Sathian B, Yadav SK. Metabolic changes enhance the cardiovascular risk with differentiated thyroid carcinoma – a case control study from Manipal Teaching Hospital of Nepal. Asian Pac J Cancer Prev. 2012;13(5):2335–2338. | ||
Rajoria S, Suriano R, Parmar PS, et al. 3,3’-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: a pilot study. Thyroid. 2011;21(3):299–304. | ||
Shang X, Zhong X, Tian X. Metabolomics of papillary thyroid carcinoma tissues: potential biomarkers for diagnosis and promising targets for therapy. Tumour Biol. 2016;37(8):11163–11175. | ||
Gu Y, Chen T, Fu S, et al. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med. 2015; 13(1):35. | ||
Lee SH, Kim KM, Jung BH, Chung WY, Park CS, Chung BC. Estrogens in female thyroid cancer: alteration of urinary profiles in pre- and post-operative cases. Cancer Lett. 2003;189(1):27–32. | ||
Ishikawa S, Tateya I, Hayasaka T, et al. Increased expression of phosphatidylcholine (16:0/18:1) and (16:0/18:2) in thyroid papillary cancer. PLoS One. 2012;7(11):e48873. | ||
Seo JW, Han K, Lee J, et al. Application of metabolomics in prediction of lymph node metastasis in papillary thyroid carcinoma. PLoS One. 2018;13(3):e0193883. | ||
Zhou J, Li Y, Chen X, Zhong L, Yin Y. Development of data-independent acquisition workflows for metabolomic analysis on a quadrupole-orbitrap platform. Talanta. 2017;164:128–136. | ||
Zhao WX, Wang B, Zhang LY, Yan SY, Yang YH. Analysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonance. Int J Clin Exp Med. 2015;8(10):18013. | ||
Zhang J, Feider CL, Nagi C, et al. Detection of metastatic breast and thyroid cancer in lymph nodes by desorption electrospray ionization mass spectrometry imaging. J Am Soc Mass Spectrom. 2017;28(6):1166–1174. | ||
Yao Z, Yin P, Su D, et al. Serum metabolic profiling and features of papillary thyroid carcinoma and nodular goiter. Mol Biosyst. 2011;7(9):2608–2614. | ||
Xu Y, Zheng X, Qiu Y, Jia W, Wang J, Yin S. Distinct metabolomic profiles of papillary thyroid carcinoma and benign thyroid adenoma. J Proteome Res. 2015;14(8):3315–3321. | ||
Wojtowicz W, Zabek A, Deja S, et al. Serum and urine 1H NMR-based metabolomics in the diagnosis of selected thyroid diseases. Sci Rep. 2017;7(1):9108. | ||
Choi MH, Moon JY, Cho SH, Chung BC, Lee EJ. Metabolic alteration of urinary steroids in pre- and post-menopausal women, and men with papillary thyroid carcinoma. BMC Cancer. 2011;11(1):342. | ||
Chen M, Shen M, Li Y, et al. GC-MS-based metabolomic analysis of human papillary thyroid carcinoma tissue. Int J Mol Med. 2015;36(6):1607–1614. | ||
Ryoo I, Kwon H, Kim SC, et al. Metabolomic analysis of percutaneous fine-needle aspiration specimens of thyroid nodules: potential application for the preoperative diagnosis of thyroid cancer. Sci Rep. 2016;6(1):30075. | ||
Shen CT, Zhang Y, Liu YM, et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin Endocrinol. 2017;87(6):844–852. | ||
Torregrossa L, Shintu L, Nambiath Chandran J, et al. Toward the reliable diagnosis of indeterminate thyroid lesions: a HRMAS NMR-based metabolomics case of study. J Proteome Res. 2012;11(6):3317–3325. | ||
Guo L, Wang C, Chi C, et al. Exhaled breath volatile biomarker analysis for thyroid cancer. Transl Res. 2015;166(2):188–195. | ||
Liu S, Zhao G, Li J, et al. Association of polybrominated diphenylethers (PBDEs) and hydroxylated metabolites (OH-PBDEs) serum levels with thyroid function in thyroid cancer patients. Environ Res. 2017;159:1–8. | ||
Lu J, Hu S, Miccoli P, et al. Non-invasive diagnosis of papillary thyroid microcarcinoma: a NMR-based metabolomics approach. Oncotarget. 2016;7(49):81768. | ||
Deja S, Dawiskiba T, Balcerzak W, et al. Follicular adenomas exhibit a unique metabolic profile. 1H NMR studies of thyroid lesions. PLoS One. 2013;8(12):e84637. | ||
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12(50):1131–1137. | ||
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. | ||
Volk T, Jähde E, Fortmeyer HP, Glüsenkamp KH, Rajewsky MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer. 1993;68(3):492–500. | ||
Delli Castelli D, Ferrauto G, Cutrin JC, Terreno E, Aime S. In vivo maps of extracellular pH in murine melanoma by CEST-MRI. Magn Reson Med. 2014;71(1):326–332. | ||
Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R. Biology of glucose metabolization in cancer cells. J Oncol Sci. 2017;3(2):45–51. | ||
Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. J Exp Med. 2012;209(2):211–215. | ||
Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14(4):263–276. | ||
Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. | ||
Shen C-T, Zhang Y, Liu Y-M, et al. A distinct serum metabolic signature of distant metastatic papillary thyroid carcinoma. Clin Endocrinol. 2017;87(6):844–852. | ||
Gu Y, Chen T, Fu S, et al. Perioperative dynamics and significance of amino acid profiles in patients with cancer. J Transl Med. 2015;13(1):35. | ||
Zhao WX, Wang B, Zhang LY, Yan SY, Yang YH. Analysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonance. Int J Clin Exp Med. 2015;8(10):18013–18022. | ||
Wojtowicz W, Zabek A, Deja S, et al. Serum and urine 1H NMR-based metabolomics in the diagnosis of selected thyroid diseases. Sci Rep. 2017;7(1):9108. | ||
Miyoshi E, Ito Y, Miyoshi Y. Involvement of aberrant glycosylation in thyroid cancer. J Oncol. 2010;2010(2):1–7. | ||
Plomp R, Ruhaak LR, Uh HW, et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci Rep. 2017;7(1):12325. | ||
Zhang D, Chen B, Wang Y, et al. Disease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseases. Sci Rep. 2016;6(1):25957. | ||
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. | ||
Ząbczyńska M, Kozłowska K, Pocheć E. Glycosylation in the thyroid gland: vital aspects of glycoprotein function in thyrocyte physiology and thyroid disorders. Int J Mol Sci. 2018;19(9):2792. | ||
Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–353. | ||
Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr Med Chem. 2004;11(16):2195–2204. | ||
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. | ||
Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci. 2015;9(408):22. | ||
Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164(6):1875–1882. | ||
San-Millán I, Brooks GA. Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38(2):119–133. | ||
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol. 2016;7(Pt 2):52. | ||
Jiang B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017;4(1):25–27. | ||
Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol. 2013;48(1):20–38. | ||
Li Y, Chen M, Liu C, et al. Metabolic changes associated with papillary thyroid carcinoma: a nuclear magnetic resonance-based metabolomics study. Int J Mol Med. 2018;41(5):3006–3014. | ||
Kwon H, Oh S, Jin X, An YJ, Park S. Cancer metabolomics in basic science perspective. Arch Pharm Res. 2015;38(3):372–380. | ||
Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. | ||
Zhang WC, Shyh-Chang N, Yang H, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148(1–2):259–272. | ||
Mycielska ME, Dettmer K, Rümmele P, et al. Extracellular citrate affects critical elements of cancer cell metabolism and supports cancer development In Vivo. Cancer Res. 2018;78(10):2513–2523. | ||
Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–321. | ||
Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72(15):3709–3714. | ||
Ren JG, Seth P, Ye H, et al. Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci Rep. 2017;7(1):4537. | ||
Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27(4):599–608. | ||
Ye D, Guan KL, Xiong Y. Metabolism, activity, and targeting of D- and L-2-hydroxyglutarates. Trends Cancer. 2018;4(2):151–165. | ||
Ward PS, Cross JR, Lu C, et al. Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene. 2012;31(19):2491–2498. | ||
Choi C, Ganji SK, Deberardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–629. | ||
Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207(2):339–344. | ||
Shim EH, Livi CB, Rakheja D, et al. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov. 2014;4(11):1290–1298. | ||
Rakheja D, Boriack RL, Mitui M, Khokhar S, Holt SA, Kapur P. Papillary thyroid carcinoma shows elevated levels of 2-hydroxyglutarate. Tumour Biol. 2011;32(2):325–333. | ||
Hemerly JP, Bastos AU, Cerutti JM. Identification of several novel non-p.R132 IDH1 variants in thyroid carcinomas. Eur J Endocrinol. 2010;163(5):747–755. | ||
Ward PS, Lu C, Cross JR, et al. The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J Biol Chem. 2013;288(6):jbc. M112:3804–3815. | ||
Alimoghaddam K, Shariftabrizi A, Tavangar SM, et al. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyelocytic leukemia. Leuk Lymphoma. 2006;47(1):81–88. | ||
Miller VM. Estrogen metabolomics: a physiologist’s perspective. Hypertension. 2010;56(5):816–818. | ||
Alakwaa FM, Chaudhary K, Garmire LX. Deep learning accurately predicts estrogen receptor status in breast cancer metabolomics data. J Proteome Res. 2018;17(1):337–347. | ||
Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19(1):32–37. | ||
Mannava S, Grachtchouk V, Wheeler LJ, et al. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7(15):2392–2400. | ||
Cory JG, Cory AH. Critical roles of glutamine as nitrogen donors in purine and pyrimidine nucleotide synthesis: asparaginase treatment in childhood acute lymphoblastic leukemia. In Vivo. 2006;20(5):587–589. | ||
Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2(10):881–898. | ||
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. | ||
Khatami, F., Larijani, B., Heshmat, R., Keshtkar, A., Mohammadamoli, M., Teimoori-Toolabi, L., Nasiri, S. and Tavangar, S.M., 2017. Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancer. Plos One, 12(9), p.e0184892. | ||
Natanzi, M.M., Pasalar, P., Kamalinejad, M., Dehpour, A.R., Tavangar, S.M., Sharifi, R., Ghanadian, N., Rahimi-Balaei, M. and Gerayesh-Nejad, S., 2012. Effect of aqueous extract of Elaeagnus angustifolia fruit on experimental cutaneous wound healing in rats. Acta Medica Iranica, 50(9), pp.589–596. | ||
Omidfar K, Moinfar Z, Sohi AN, Tavangar SM, Haghpanah V, Heshmat R, Kashanian S, Larijani B. Expression of EGFRvIII in thyroid carcinoma: immunohistochemical study by camel antibodies. Immunological Investigations. 2009 Jan 1;38(2):165–80. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.