Back to Journals » Cancer Management and Research » Volume 12
Oncological Outcome of Combining Cytoreductive Prostatectomy and Metastasis-Directed Radiotherapy in Patients with Prostate Cancer and Bone Oligometastases: A Retrospective Cohort Study
Authors Xue P, Wu Z, Wang K, Gao G, Min Z, Yan M
Received 8 July 2020
Accepted for publication 4 September 2020
Published 23 September 2020 Volume 2020:12 Pages 8867—8873
DOI https://doi.org/10.2147/CMAR.S270882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Peng Xue,1 Ziyu Wu,2 Kunpen Wang,1 Guojun Gao,3 Min Zhuang,4 Miao Yan4
1Department of Urology, First People Hospital of Lianyungang, Lianyungang, Jiangsu Province, People’s Republic of China; 2Department of Urology, NO 2 Hospital of Huaian, Huaian, Jiangsu Province, People’s Republic of China; 3Department of Urology, The Affiliated Hospital of Weifang Medical College, Weifang, Shandong Province, People’s Republic of China; 4Department of Oncology, First People Hospital of Lianyungang, Lianyungang, Jiangsu Province, People’s Republic of China
Correspondence: Miao Yan
Department of Oncology, First People Hospital of Lianyungang, Zhenghua Road 6, Lianyungang, Jiangsu Province 222002, People’s Republic of China
Tel +86051885605016
Email [email protected]
Background: The current standard of care for metastatic prostate cancer (mPCa) is androgen deprivation therapy (ADT) with or without anti-androgen and chemotherapy. The aim of this study was to evaluate the efficacy and safety of a multimodal approach including local primary tumor therapy, metastasis-directed therapy (MDT), and hormonal therapy in patients with oligometastatic prostate cancer (PCa).
Methods: We reviewed data of patients with PCa and bone oligometastases at diagnosis treated in three institutions with ADT followed by cytoreductive surgery with or without metastases-directed radiotherapy. Oligometastases were defined as the presence of five or fewer metastatic lesions with the absence of visceral metastases. In this retrospective cohort study, 58 patients underwent cytoreductive radical prostatectomy and ADT. Of these, 26 patients (45%) received stereotactic body radiation therapy (SBRT) to all metastatic sites as a MDT. Oncological outcomes were analyzed using the Kaplan–Meier method.
Results: The median follow-up period was 46.2 months. Of the 58 patients, the 3-year castration-resistant prostate cancer (CRPC)-free survival and cancer-specific survival was 75.9% and 91.4%, respectively. Pre- or post-treatment predictive factors for progression to CRPC, including prostate-specific antigen (PSA) level at diagnosis ≥ 20 ng/mL, Gleason grade groups 5, clinical T stage cT3b-4, PSA nadir level of ≥ 0.05 ng/mL, and no MDT with SBRT, were significantly associated with progression to CRPC. Subgroup analysis showed that the MDT group had significantly better CRPC-free survival than the non-MDT group with Gleason grade groups 1– 4 (HR=0.228; 95% CI= 0.056– 0.926). A total of 3.4% of the patients had grade 2 acute genitourinary toxicities and 5.2% had grade 2 acute gastrointestinal toxicities. No late grade > 2 adverse events were observed.
Conclusion: This multi-center, retrospective cohort study revealed the feasibility of combining cytoreductive prostatectomy and metastasis-directed radiotherapy for newly-diagnosed oligometastatic PCa. This treatment strategy has the potential to delay the progression to CRPC.
Keywords: oligometastases, prostate cancer, stereotactic body radiotherapy, androgen deprivation therapy, radical prostatectomy, metastasis-directed therapy
Introduction
ADT alone was historically indicated for palliative treatment in patients with PCa and skeletal metastases.1 However, resistance to ADT is inevitable. Modern cohorts of mPCa patients treated with ADT alone have a median time to progression and overall survival of 11 and 42 months, respectively.2 Oligometastasis has been defined as an intermediate state of malignancy spread between localized disease and widespread systemic metastasis,3 in which patients develop only a limited number of metastatic lesions (defined as 1–5 lesions in most studies).4 With improvements in functional imaging modalities, oligometastatic PCa is being diagnosed with increasing frequency. More recently, several studies have shown that selected patients with limited nonvisceral metastases might benefit from local treatment of the primary tumor despite established metastatic spread.5–10 However, a strategy for aggressive cytoreductive treatment of the primary tumor is not currently standard for synchronous oligometastatic PCa.
Analyses by the Phase III SWOG 8894 trial showed that mPCa patients who had previously undergone radical prostatectomy (RP) decreased disease-related morbidity compared with those who had not undergone earlier RP (HR=0.77; 95% CI=0.53–0.89).11 The benefit for cytoreductive surgery in patients with a low metastatic burden leads to the next question of whether additional radiotherapy to metastatic sites could improve progression-free or overall survival. Although growing evidence suggests that MDT and/or local therapy targeted to the primary tumor may be of benefit in patients with oligometastatic PCa,12 no definite consensus has been reached on the adequate management approach. In the present study, we retrospectively evaluated the safety and efficacy of the combination of cytoreductive surgery, metastases-directed radiotherapy, and long-term ADT in newly-diagnosed prostate cancer patients with bone oligometastases. We also investigated whether the combination of cytoreductive surgery and metastases-directed radiotherapy had better outcomes than cytoreductive surgery alone.
Patients and Methods
Study Design
A multi-institutional retrospective study evaluated the survival benefit and potential prognostic factors of newly-diagnosed oligometastatic PCa treated with a combination of cytoreductive radical prostatectomy, metastasis-directed SBRT, and ADT from November 2012 to September 2016. Oligometastatic PCa was defined as the presence of five or fewer metastatic lesions with the absence of visceral metastases on Tc99m bone scan, with or without suspicious pelvic or retroperitoneal lymph node involvement at preoperative imaging. Inclusion criteria included: 1) Biopsy confirmed diagnosis of prostate adenocarcinoma; 2) M1b disease with the presence of 1–5 visible bone metastases (by Tc-99m MDP bone scan, CT, or MRI); 3) not received radiotherapy and chemotherapy in hormone-sensitive phase; 4) adequate organ function; 5) ECOG performance status 0–1; 6) pretreatment total testosterone > 200 ng/dL; and 7) written informed consent.
Cytoreductive Surgery, Metastases-Directed Radiotherapy, and Hormone Therapy
Open or laparoscopic radical prostatectomy with concurrent pelvic lymph node dissection (PLND) was performed by experienced urologic surgeons and was the first treatment intervention, preceding the metastasis-directed radiotherapy. In addition, patients underwent post-operative adjuvant radiotherapy for the pathological findings of positive surgical margin. ADT was achieved through luteinizing hormone-releasing hormone analogs, or through maximal androgen blockade (luteinizing hormone releasing hormone analogs combined with an antiandrogen drug). Patients initiate leuprolide acetate depot (3.75 mg intramuscularly every 28 days) and bicalutamide (50 mg/day) immediately (within 0–3 days) after RP for a duration of approximately 24 months. In cases of metastases-directed radiotherapy, patients undergo SBRT to all radiographically visible sites of bone metastasis using a three-dimensional conformal radiation therapy technique within 2 months of ADT initiation. Dosing for SBRT were 18 Gy/1, 30 Gy/3, and 40 Gy/5 fractions. A biologically equivalent dose (BED) to tumor of >100 Gy was calculated with a α/β of 3.
Follow-Up
The duration of the follow-up was calculated from the date of the surgery. The nadir of PSA was defined as the absolute lowest level that PSA drops after initial treatment. CRPC was diagnosed while biochemical or radiologic progression was confirmed in the presence of castration levels of plasma testosterone <1.7 nmol/L. Biochemical progression was defined as three consecutive rises in PSA levels at a minimum of 1-week intervals, resulting in two 50% increases over the nadir, as well as PSA level was more than 2.0 ng/mL. Radiological progression was defined as the appearance of new bone lesions on bone scan, progression of existing metastases, or locally recurrent advanced disease. CRPC-free survival was calculated from the start of cytoreductive surgery.
Statistical Analysis
Fisher’s exact test was used to assess the association between categorical variables, and unpaired t-test was used to assess differences between group means for continuous measurements. Kaplan–Meier methods were used to estimate CRPC free survival rates and the differences were compared with the log-rank statistic. Multivariate time-to-event analysis was performed with the use of a Cox proportional-hazards model. Differences were regarded as statistically significant at P<0.05. All P-value determinations were 2-sided. All analyses were performed with the SPSS software (version 22.0), and GraphPad Prism, version 7 (GraphPad Software, Inc., La Jolla, CA).
Results
Characteristics of Study Cohorts
A total of 58 patients with locally resectable tumors underwent cytoreductive surgery were matched in this retrospective study. Thirty-two patients received RP plus ADT and 26 patients underwent the combination of RP, ADT, and metastasis directed SBRT. In addition, five patients (8.6%) received 3 months of neoadjuvant ADT, and seven (12.1%) patients underwent post-operative fractionated radiotherapy because of positive margins.
The median follow-up for all patients was 46.2 months (range=15–65). Table 1 shows the demographic and clinical characteristics of the 58 patients. Twenty-six patients (44.8%) received metastasis-directed SBRT. Fourteen patients (24.1%) progressed to CRPC and five patients (8.6%) died of prostate cancer during follow-up.
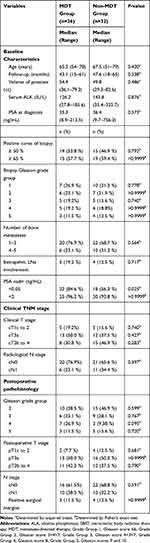 |
Table 1 Descriptive Characteristics of 58 Consecutive Patients Newly Diagnosed with Oligometastatic Prostate Cancer Between 2012 and 2016 |
ADT and Subsequent Therapy for Disease Progression
Regarding the status of ADT, the mean duration of ADT was 32.6±13.6 months during follow-up. Thirty-two patients (55.2%) discontinued ADT after the administration of 24-month adjuvant ADT. Of those 32 patients, seven patients who experienced biochemical failure resumed the long-term salvage ADT. The other 25 patients (15 patients in MDT group and 17 patients in non-MDT group) (P=0.794) did not progress and were free from ADT at the point of analysis. Meanwhile, 23 of these patients achieved a serum PSA of <0.05 ng/mL 24 months after recovery of serum testosterone ≥150 ng/dL. Continuous ADT was adopted in 26 patients because of progression to CRPC during adjuvant ADT (n=7) and patients’ willingness (n=19). ADT were continued to maintain serum testosterone castration levels in all patients after clinical progression. Additional antineoplastic therapies for progression were as follows: five (8.6%) patients received chemotherapy with docetaxel, eight (13.8%) patients switched from bicalutamide to abiraterone acetate plus prednisone, and four (6.9%) received flutamide or estramustine. The percentage of patients who received zoledronic acid was 20.7% in the study.
Survival Analysis
For the whole cohort (58 patients), the 3-year CRPC-free survival rate and cancer specific survival were 75.9% and 91.4%, respectively. To provide predictive factors for survival outcomes of oligometastatic PCa patients in the present study, the Log rank test was performed for survival data using several well-recognized risk-factors (Gleason score, PSA level at diagnosis, PSA nadir, clinical T stage, alkaline phosphatase (ALK), and positive biopsy core rate). Result suggested pre-treatment predictive values including PSA at diagnosis ≥20 ng/mL, clinical T stage (cT3b to 4) and Gleason grade group 5 had significant prognostic features for CRPC free survival (Table 2). In addition, PSA nadir ≥0.05 ng/mL was significantly associated with increased risk of CRPC (HR=24.46; 95% CI=6.800–87.95). Multivariate analysis suggested that Gleason grade group 5 (OR=4.46, 95% CI=1.59–6.78) and PSA nadir ≥0.05 ng/mL (OR=3.88, 95% CI=1.62–9.17) were associated with progression to CRPC.
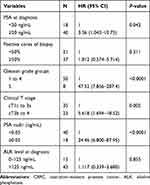 |
Table 2 Log Rank Test of CRPC-Related Risk Factors in Whole Cohort |
Metastasis-Directed SBRT
Patients with metastasis-directed SBRT had a significantly better CRPC-free survival than those without the therapy (HR=0.411, 95% CI=0.178–0.950, Figure 1A). The median cancer-specific survival was not reached in the cohort. Combined therapy in our study did not confer a survival advantage over cytoreductive surgery alone (HR=0.5855, 95% CI=0.116–2.949, Figure 1B). Table 1 shows the differences of the clinical feature between oligometastatic Pca patients with and without metastases-directed SBRT. Patients had significantly higher probability of achieving a unequivocal PSA nadir <0.05 ng/mL after radiotherapy to all visible sites of metastases than those without radiation therapy (84.6% vs 56.3%, P=0.025). Acute grade 1–2 gastrointestinal (GI) and genitourinary (GU) toxicities were observed in 3.4 and 5.2% of patients respectively. No acute Grade 3 RTOG toxicity was recorded. No late grade >2 adverse events were observed.
 |
Figure 1 Kaplan–Meier estimates of time to (A) castration resistance prostate cancer, (B) prostate cancer-specific mortality between MDT group and non-MDT group. |
Subgroup Analysis
This study was limited by a small sample size, subgroup analyses were performed separately for men with Gleason grade groups 1–4, PSA 0–100 ng/mL, clinical stage of primary tumor T1c-3a, and radiological stage of regional lymph node N0. Figure 2 shows metastases-directed SBRT had significantly better CRPC-free survival than no radiotherapy in patients with Gleason grade groups 1–4 (HR=0.228; 95% CI=0.056–0.926).
Discussion
In this report, we restricted our assessment to newly-diagnosed PCa patients with bone oligometastases who were treated with a combination of cytoreductive RP, metastasis-directed SBRT, and long-term ADT. We also analyzed the risk factors related to CRPC using univariate analysis. Pre-treatment PSA level, clinical T stage, and Gleason grade groups of the RP specimen were found to be significantly associated with progression to CRPC. Furthermore, patients who received metastasis-directed SBRT had a lower PSA nadir level compared with those who did not, and consequently had longer median time to progression to CRPC. Meanwhile, SBRT involves safe and accurate delivery of ionizing radiation to metastatic lesions with acceptable toxicities. These results are in line with the data reported by the most recent literature.13,14 We suggest that cytoreductive RP in combination with metastasis-directed SBRT is a feasible cytoreductive treatment strategy and has the potential to postpone disease progression to CRPC in PCa patients with synchronous bone oligometastases.
Although cytoreductive RP is not usually advocated for metastatic PCa due to the conventional view that the biological nature of metastasis could not be reversed by aggressive local therapies, growing evidence suggests that a subset of patients with a low number of nonvisceral metastases might have a favorable oncological profile and thus might benefit from resection of the primary tumor.5–10 A multi-institutional retrospective study of 113 patients with biopsy-proven PCa, minimal bone metastases (three or fewer hot spots on bone scan), no or minimal visceral metastases, and absence of extensive lymph node metastases reported 5-year OS of 80% over a median follow-up of 53.6 months after cytoreductive RP with extended pelvic lymphadenectomy.15 In our oligometastatic cohort, the predictive value of pre-treatment including PSA at diagnosis <20 ng/mL, clinical T stage (cT1c to 3a), and Gleason grade group 1–4 were significantly associated with longer CRPC-free survival. Our result inferred that patients selection criteria for cytoreductive surgery should depend on not only the definition of oligometastatic disease but also the other prognostic factors and risk-stratification models. Moreover, time to development of CRPC was used as an endpoint, as castration-resistant free-survival is known to influence overall survival of patients with CRPC.16
Given the complex patterns of metastatic seeding involving crosstalk between primary lesions and metastatic sites, physicians began to choose a comprehensive systemic and tumor directed therapeutic strategy for patients with newly-diagnosed oligometastatic PCa. A retrospective cohort study of a multimodal treatment strategy including ADT, high-dose-rate prostate brachytherapy, external beam radiotherapy, and SBRT to bone metastases was conducted in 40 men with synchronous oligometastatic PCa.13 Patients with metastasis-directed radiotherapy had a much better CRPC-free survival than those without the therapy (HR=0.319, 95% CI=0.116–0.877) and did not significantly increase the incidences of GI and GU toxicities. This suggests that control of the existing metastases is important in mPCa. Parikh et al17 conducted a single-arm Phase II clinical trial in which they concluded that the combination of aggressive local, metastasis-directed, and hormonal therapy represent a path forward for the development of definitive curative intent therapy for a mPCa patient population where palliation has been the norm.
Emerging literature has shown that the PSA nadir after prostate surgery or radiotherapy is well correlated with prognostic values of survival.18–20 In the present study, the proportion of patients who achieved a PSA level of less than 0.05 ng/mL after metastases-directed SBRT was significantly higher than those without radiation therapy. This result suggests that ablative radiotherapy to all bone metastatic lesions, in addition to cytoreductive surgery, could achieve lower PSA nadir values and consequently improve CRPC-free survival in patients with PCa and bone oligometastases.
There are several limitations of the current study in addition to its retrospective nature. First, this is a highly selected patient cohort of small sample size and limited duration of follow-up. We analyzed the related risk factors for CRPC but not prostate cancer-specific mortality because of the small number of events. Ongoing prospective randomized trials would be helpful to evaluate the role of a multimodal therapy combining metastasis-directed and local therapy in newly-diagnosed oligometastatic PCa.17 Second, Tc-99m MDP bone scan was used in the screening process and response assessment outcomes may lack sufficient sensitivity compared to using positron emission tomography (PET) imaging with prostate specific membrane antigen (PSMA).21 Third, the lack of a control group of oligometastatic patients who received ADT alone prevented us from comprehensively assessing the oncologic benefit associated with a tumor directed therapeutic strategy.
Conclusions
We concluded that the combination of cytoreductive surgery and metastasis-directed SBRT was a feasible approach and had better CRPC-free survival rates than cytoreductive surgery alone for patients with PCa and bone oligometastases. However, a comprehensive systemic and aggressive tumor directed therapeutic strategy in synchronous oligometastatic disease remains an experimental phase, and further data are needed to confirm the benefits of this strategy.
Disclosure
The authors report that there is no actual or potential conflict of interest in relation to this article.
References
1. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi:10.1001/jama.294.2.238
2. James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate Cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67(6):1028–1038. doi:10.1016/j.eururo.2014.09.032
3. Joice GA, Rowe SP, Pienta KJ, et al. Oligometastatic prostate cancer: shaping the definition with molecular imaging and an improved understanding of tumor biology. Curr Opin Urol. 2017;27:533–541. doi:10.1097/MOU.0000000000000449
4. Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10. doi:10.1016/S0360-3016(03)01442-1
5. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832–838. doi:10.1016/j.juro.2014.09.089
6. Katelaris N, Murphy D, Lawrentschuk N, et al. Cytoreductive surgery for men with metastatic prostate cancer. Prostate Int. 2016;4:103–106. doi:10.1016/j.prnil.2015.11.003
7. Poelaert F, Verbaeys C, Rappe B, et al. Cytoreductive Prostatectomy for metastatic prostate cancer: first lessons learned from the multicentric prospective local treatment of metastatic prostate cancer (LoMP) trial. Urology. 2017;106:146–152. doi:10.1016/j.urology.2017.02.051
8. Steuber T, Berg KD, Røder MA, et al. Does cytoreductive prostatectomy really have an impact on prognosis in prostate cancer patients with low-volume bone metastasis? Results from a prospective case control study. Eur Urol Focus. 2017;3:646–649. doi:10.1016/j.euf.2017.06.016
9. Gandaglia G, Fossati N, Stabile A, et al. Radical prostatectomy in men with oligometastatic prostate cancer: results of a single institution series with long-term follow-up. Eur Urol. 2017;72:289–292. doi:10.1016/j.eururo.2016.08.040
10. Kim DK, Parihar JS, Kwon YS, et al. Risk of complications and urinary incontinence following cytoreductive prostatectomy: a multi-institutional study. Asian J Androl. 2018;20:9–14. doi:10.4103/1008-682X.196852
11. Thompson IM, Tangen C, Basler J, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002;168:1008–1012. doi:10.1016/S0022-5347(05)64562-4
12. Battaglia A, De Meerleer G, Tosco L, et al. Novel insights into the management of oligometastatic prostate cancer: a comprehensive review. Eur Urol Oncol. 2019;2(2):174–188. doi:10.1016/j.euo.2018.09.005
13. Tsumura H, Ishiyama H, Ken-Ichi T, et al. Long-term outcomes of combining prostate brachytherapy and metastasis-directed radiotherapy in newly diagnosed oligometastatic prostate cancer: A retrospective cohort study. Prostate. 2019;79(5):506–514. doi:10.1002/pros.23757
14. O’Shaughnessy MJ, McBride SM, Vargas HA, et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early-stage metastatic prostate cancer. Urology. 2017;102:164–72.15. doi:10.1016/j.urology.2016.10.044
15. Heidenreich A, Fossati N, Pfister D, et al. Cytoreductive radical prostatectomy in men with prostate cancer and skeletal metastases. Eur Urol Oncol. 2018;1(1):46–53. doi:10.1016/j.euo.2018.03.002
16. Bournakis E, Efstathiou E, Varkaris A, et al. Time to castration resistance is an independent predictor of castration-resistant prostate cancer survival. Anticancer Res. 2011;31:1475–1482.
17. Parikh NR, Huiza C, Patel JS, et al. Systemic and tumor-directed therapy for oligometastatic prostate cancer: study protocol for a phase II trial for veterans with de novo oligometastatic disease. BMC Cancer. 2019;19(1):291. doi:10.1186/s12885-019-5496-5
18. Tsumura H, Satoh T, Ishiyama H, et al. Prostate-specific antigen nadir after high-dose-rate brachytherapy predicts long-term survival outcomes in high-risk prostate cancer. J Contemp Brachytherapy. 2016;8(2):95–103. doi:10.5114/jcb.2016.59686
19. Yoshida T, Matsuzaki K, Kobayashi Y, et al. Usefulness of postoperative nadir prostate-specific antigen value by ultra-sensitive assay as a predictor of prostate-specific antigen relapse for pathological t3 or positive surgical margins after radical prostatectomy for prostate cancer. Int Urol Nephrol. 2012;44(2):479–485. doi:10.1007/s11255-011-0044-5
20. Nickers P, Albert A, Waltregny D, et al. Prognostic value of PSA nadir < or =4 ng/ml within 4 months of high-dose radiotherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65(1):73–77.
21. Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive (68) Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi:10.1016/j.eururo.2016.06.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

