Back to Journals » OncoTargets and Therapy » Volume 13
Oncogenic Role of NUPR1 in Ovarian Cancer
Authors Yu J , Zhu H, Li R, Jiang Q, Luan W, Shi J , Liu P
Received 13 June 2020
Accepted for publication 28 October 2020
Published 30 November 2020 Volume 2020:13 Pages 12289—12300
DOI https://doi.org/10.2147/OTT.S262224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Jiangtao Yu,1,2,* Haiyan Zhu,2,3,* Rui Li,1 Qi Jiang,2,4 Wenqing Luan,1 Juanjuan Shi,1 Peishu Liu1
1Department of Obstetrics and Gynecology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250012, People’s Republic of China; 2Department of Gynecology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325027, People’s Republic of China; 3Department of Gynecology, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai 200126, People’s Republic of China; 4Department of Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peishu Liu
Department of Obstetrics and Gynecology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan 250012, Shandong, People’s Republic of China
Tel +86 18560081988
Email [email protected]
Background: Nuclear protein 1 (NUPR1) plays a critical role in the development and progression of various types of human cancers. However, the role and mechanism of NUPR1 in ovarian cancer have not been elucidated. The purpose of this study was to investigate the effect of NUPR1 on ovarian cancer in vivo and in vitro.
Materials and Methods: Through the pretreatment of ovarian cancer cell lines, including A2780 and SKOV3 cells, the expression of NUPR1 was detected by RT-PCR and Western blot assays. When NUPR1 was overexpressed and knocked down in A2780 cells and overexpressed in SKOV3 cells, the MTT assays, colony formation assays and EdU assays were used to detect cell proliferation. Furthermore, cell invasion and migration ability were detected with the transwell assays. Cell cycle and apoptosis of A2780 cells after small interfering RNA-NUPR1 (siRNA-NUPR1) were detected by flow cytometry assays. Finally, the effect of NUPR1 gene silencing on the growth of ovarian cancer was evaluated by tumor xenograft experiment in vivo.
Results: The expression of NUPR1 protein in A2780 cells was significantly higher than that in ovarian surface epithelium (OSE) cells (P < 0.05). The results showed that downregulation of NUPR1 gene expression significantly inhibited the proliferation, migration and invasion ability of A2780 cells, and increased apoptosis of A2780 cells, which expressed relatively high levels of NUPR1. And the expression of apoptosis-related proteins caspase 3, caspase 9 and Bax was upregulated when NUPR1 was knocked out, while the expression of anti-apoptotic proteins of Bcl-2 and Bcl-xl was downregulated. At the same time, the opposite results were observed when NUPR1 was overexpressed in A2780 and SKOV3 cells. Notably, the effect of NUPR1 overexpression in A2780 cells could be partially or completely eliminated by treatment with the AKT inhibitor LY294002. In addition, NUPR1 knockdown could effectively inhibit tumor growth of mice in vivo.
Conclusion: In summary, NUPR1 has a carcinogenic effect in ovarian cancer, and the oncogenic effect of NUPR1 in ovarian cancer may be achieved by the AKT pathway.
Keywords: NUPR1, ovarian cancer, AKT, proliferation, migration, invasion
Plain Language Summary
NUPR1, also known as P8 and COM1, was first discovered by Mallo in the acute stage of pancreatitis more than 20 years ago. NUPR1 was highly expressed in a variety of benign and malignant tumors, but it was also low in some tumors. However, no one has studied the expression of NUPR1 in ovarian cancer. Our study focuses on the expression of NUPR1 in ovarian cancer in vivo and in vitro. We silenced NUPR1 in A2780 cells and overexpressed NUPR1 in A2780 and SKOV3 cells, and then tested the proliferation, invasion and migration ability. We found that the silencing of NUPR1 could inhibit the proliferation, invasion and migration of A2780 cells, and the overexpression of NUPR1 could promote the proliferation, invasion and migration ability of A2780 and SKOV3 cells. In A2780 cells treated with AKT inhibitor LY294002, we found that LY294002 could partially or completely inhibit the effects of the NUPR1 overexpression on the proliferation, invasion and migration ability of A2780 cells. NUPR1 gene silencing inhibited the growth of tumor xenograft in nude female mice. Therefore, we conclude that NUPR1 is upregulated in ovarian cancer and the oncogenic effect of NUPR1 in ovarian cancer may be achieved by the AKT pathway.
Introduction
As an overexpressed gene, nuclear protein 1 (NUPR1/p8/Com1) was first described by Mallo in the acute stage of pancreatitis more than 20 years ago.1 NUPR1 was a small nuclear protein associated with various stress stimuli.2,3 Many previous studies had shown that many factors or molecules could lead to overexpression of NUPR1, such as endothelin and angiotensin,4 tumor necrosis factor α,5 demyelinating agents,6 lipopolysaccharides (LPS),7 CCl48 and cannabinoids.9 There are two isoforms of NUPR1 gene, including a isoform and b isoform, respectively. Containing 100 amino acids in length, NUPR1a is one of two proteins produced by human NUPR1 gene, which no function have been reported, and NUPR1b, composed of 82 amino acid polypeptides, which had been studied in all previous literature.10 NUPR1b is a small molecule with a molecular mass of 8872.7 Da. This article is also a study about NUPR1b, the following NUPR1 is the NUPR1b by default. Gene sequencing showed that NUPR1 contained nuclear targeting signals (NLS)11 and a N-terminal PEST region.12
Some studies had found that NUPR1 had abnormal expression in many benign diseases, such as acute pancreatitis,13 failing heart,5 diabetic mesangial cell hypertrophy12 and so on. NUPR1 was an important factor about sensitizing of astrocytes in serum-free medium to oxidative stimuli.14 Many studies had confirmed the role and status of overexpression of NUPR1 in various malignant tumors, including breast cancer,15,16 pancreatic cancer,17 brain tumors and metastases,18 thyroid neoplasms,19 pituitary tumor cell.20,21 Moreover, the overexpression of NUPR1 might be positively correlated with the progression and poor prognosis in most tumors. But a study had shown that NUPR1 expression was negatively correlated with the invasion and progression of tumor, because they found that NUPR1 was underexpressed in prostate cancer.22 Those meant that NUPR1 might have a context-dependent role in cancer development.
In short, NUPR1 is a complex small molecule with many different functions. It plays different roles in various benign and malignant tumors, but its role in ovarian cancer has not been elucidated. In this study, the expression and function of NUPR1 in ovarian cancer will be described in detail. In addition, we verified that NUPR1 might affect the proliferation and invasion of ovarian cancer through the AKT signaling pathway.
Materials and Methods
Ethics Statement
The animal experiment in this study follows the Laboratory Animals-Guideline of welfare and ethics (China, 2018). The ethics committee of the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China) and the experimental animal ethics review committee of Cheeloo College of Medicine of Shandong University (Jinan, China) approved the study.
Cell Lines and Culture Conditions
OSE cells and the human ovarian cancer cell lines, including A2780 and SKOV3 cells, were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). RPMI 1640 Medium, 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, 100 U/mL penicillin and a humidified incubator with 5% CO2 at 37°C were necessary for the process of culturing above three cell lines.
Inhibitors and Antibodies
The chemical LY294002 with DMSO as solvent was purchased from Selleck, TX, USA. The primary antibodies were purchased from Cell Signaling Technology (MA, USA), including AKT, phosphorylated AKT (p-AKT), caspase 3, caspase 9 and β-actin. The NUPR1 antibody was purchased from Abcam (Cambridge, UK). The primary antibodies of Bcl-2, Bax, Bcl-xl, CDK4 and CDK6 were purchased from Santa Cruz (CA, USA).
RNA Interference Experiments
We cultured and reverse-transfected A2780 cells with 6-well plates (30 × 104 cells per well), using LipofectamineTM 2000 (Invitrogen) as a medium, and adding 0.05 μM of control small interfering RNA (siRNA-NC) or siRNA directed against NUPR1, respectively. The RPMI 1640 medium without streptomycin and penicillin was used instead of the culture medium in 6 h after transfection. And after 24 h of transfection, the A2780 cells were used for subsequent experiments.
Plasmid Constructs
NUPR1 plasmid and the lentivirus vector Ubi-MCS-3FLAG-SV40-EGFP-IRES-Puro (11.6 kb) were designed by the GeneChem (Shanghai, China). After the transfection and virus package, the puromycin dihydrochloride (2 μg/mL, Amresco, USA) was used to generate A2780 and SKOV3 cells that stably overexpressing NUPR1.
Transfection of Lentivirus
To generate lentivirus with low-expressing NUPR1 gene, the RNA interference sequence for NUPR1 (GACUCCUGCCUUGGUUACATT) was designed by using the manufacturer’s RNAi Design programme, and then those series had been verified by some functional experiments. The negative control group was a control group without homology with the human genome including the structure (TTCTCCGAACGTGTCACGT). The lentivirus vector was generated by cloning the sequence into GV493 (GeneChem, Shanghai, China). We used the MOI (volume = [cell number × MOI]/virus titer, MOI of A2780 = 50) to determine the volume of adenovirus. The cells were digested with trypsin and suspended in normal medium and seeded in 6-well plates at a density of 30 × 104 cells/well overnight to adhere. When ovarian cancer cell lines transfected adenovirus were cultured in opti-MEM medium for 6 h, the opti-MEM medium was replaced by normal medium. To clarify the effect of AKT kinase inhibitor in A2780 cells, the A2780 cells were first infected with adenovirus for 6 h and then treated with 10 μM LY294002 for 24 h before collecting for the following assays.
RT-qPCR (Reverse Transcription Quantitative PCR)
The relative expression level of mRNA was analyzed by using the 2−ΔΔCT method. The concentration and purity of RNA were detected by a spectrophotometer (Termo Fisher Scientific Inc., MA, USA) after total RNA of cells was extracted. Then the RNA with the reaction system (3000 ng/20 μL) was reverse transcribed into cDNA. The StepOne™ PCR amplifier (Applied Biosystems, USA) was used to achieve the PCR reaction, and the reaction system was a 10 μL reaction system containing SYBR-green. The primer sequences of NUPR1 were 5′-GGAAAGGTCGCACCAAGAGAG-3′ (forward) and 5′-ACCAGTTTCCTCTCGTGCCC-3′ (reverse). The primer sequences of ß-actin, as a control group, were 5′-CATGTACGTTGCTATCCAGGC-3′ (forward) and 5′-CTCCTTAATGTCACGCACGAT-3′ (reverse). We quantified the relative mRNA level of NUPR1 using the 2−ΔΔCT method based on the control ß-actin and plotted in Graphpad Prism 7.0.
Western Blot
Following the protein extraction procedure, all cells were washed with 1 × PBS for 3 times, scraped down and placed in EP tubes, centrifuged at 12,000 rpm for 15 mins, and then added the mixture of RIPA lysis buffer with 1% sodium fluoride and 1% phenylmethanesulfonyl fluoride (PMSF) to lysates. Protein concentration was detected with BCA method. We used 12% polyacrylamide gel to isolate proteins and transferred them onto a polyvinylidene fluoride (PVDF) membrane. PVDF membranes were placed in 5% skimmed milk powder for 1 h at room temperature and incubated with primary antibody overnight at 4 °C. The PVDF membranes were washed 3 times with 1 x TBS and incubated with an appropriate concentration of secondary antibody for 1 h at room temperature. Chemiluminescent substrates (Termo Fisher Scientific Inc., MA, USA) and Image J software were used to detect and analyze bands, respectively. ß-actin was used as the internal control.
MTT Assay
When the human ovarian cancer cell lines adhered to the wall, those cells were interfered with siRNA for 24 h or transfected with lentivirus for 8 ~ 9 days. Then, the 2000 A2780 or SKOV3 cells were cultured in each 96-well plates in RPMI 1640 Medium, 10% FBS with 5% CO2 at 37°C. From the next day, after MTT was added to each well without replacing the medium for 4 h, 100 μL of DMSO was added to dissolve the formazan. A microplate reader (Tecan Group Ltd., Männedorf, Switzerland) was used to detect the absorbance of each well at 490 nm or 550 nm at the end of incubation.
Transwell Assay
Transwell chambers with 8 µm pore filter were used for cell migration and invasion assays. Before the start of a formal assay, bottom of the pore filters should be treated with or without Matrigel (60 µL, the ratio of Matrigel to serum-free medium was 1:9, BD Biosciences, USA). A total of 2 × 104 A2780 cells in 200 µL of serum-free medium were added to the upper chamber in the migration tests, meanwhile, the number of cells was 14 × 104 in the invasion tests. 1 × 104 and 6 × 104 SKOV3 cells with NUPR1 overexpression were used for migration and invasion assays, respectively. Seven hundred and fifty microliter culture medium containing 10% FBS was added to the lower chamber. These cells were incubated at 37°C for 36 h, and then the non-invading and non-migrating cells were completely removed. The cells invaded and migrated to the bottom of the chamber were immobilized with 4% paraformaldehyde and stained with 0.5% crystal violet. Experiments were performed in triplicate. The stained cells were observed and counted under a microscope, and the average number was calculated.
Cell Proliferation Test of EdU Assay
After the 1 × 104 treated A2780 or SKOV3 cells were cultured in RPMI 1640 medium, 10% FBS with 5% CO2 at 37°C in 96-well plates for 24 h, then EdU (100 μL, 50 μM) were added to each well and incubated with the cells for 2 h. The cell proliferation was observed with the EdU Kit (RIBOBIO, China) according to the manufacturer’s instructions.
Colony Formation Assay
The effect of NUPR1 overexpression or silence on the proliferation of A2780 cells and NUPR1 overexpression on the proliferation of SKOV3 cells were analyzed by clone formation assays. About 500 A2780 or SKOV3 cells were cultured in a 6-well plate containing 10% FBS, RPMI 1640 for 10 days at 37 °C with 5% CO2. The colonies were washed twice with 1 × PBS, fixed for 5 mins with 4% paraformaldehyde, stained for 30 mins with Giemsa, washed twice with ddH2O, and then colonies were manually counted.
Cell Cycle Analysis
A2780 cells treated with RNA interference targeting NUPR1 were used for cell cycle analysis. The cells were digested by trypsin and fixed with 75% ethanol overnight at 4 °C, resuspended in 20 mg/mL propidium iodide (PI), and DNA content was detected by a flow cytometer.
Detection of Apoptosis
The apoptosis rate of A2780 cells was detected by a flow cytometry and Cellquest Pro software. After treated with siRNA for 72 h, A2780 cells were digested with trypsin without EDTA, and washed 3 times with 1 × PBS and centrifuged 3 times at 1000 rpm, respectively, then suspended to 100 × 104 cells/mL in 400 μL binding buffer. The A2780 cells were dyed in dark with 5 μL fluorescein isothiocyanate (FITC) Annexin-V and 5 μL PI for 30 mins. Then we collected apoptotic cells by flow cytometry and analyzed the apoptotic rate by Cellquest Pro software.
Tumor Xenograft Experiment
To confirm the effect of NUPR1 on ovarian cancer in vivo, 1000 × 104 A2780 cells with low expression of NUPR1 were suspended in 100 μL PBS and were injected subcutaneously into the armpit of the 4-week-old nude female mice, randomly divided into 2 groups, 5 mice in each group. Cells transfected with sh-NC were used as the negative control group. The mice were observed every day after the injection. Tumor size was measured every 3 days when the tumor began to form on the 12th day after injection. Sizes of tumors were measured (Volume = [length × width2]/2) with vernier caliper. Mice were sacrificed at 27 days after injection and tumors were removed and weighted. Part of the tumors was fixed in 4% formaldehyde for immunohistochemistry and the other parts were used for Western blot assays. The research was approved by the experimental animal ethics review committee of Cheeloo College of Medicine of Shandong University (Approval number: 19,069).
Statistical Analyses
The data were analyzed through the Graphpad Prism software program (version 7.0; GraphPad Software, Inc., La Jolla, CA, USA). Results were expressed as means S.E.M. or means S.D. Statistical significance was tested using Student’s t-test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). All the experimental data were collected from three independent experiments. The P-values with statistical significance were set as <0.05.
Results
Expression of NUPR1 in OSE Cells and Ovarian Cancer Cells
The protein and total RNA of OSE cells, A2780 and SKOV3 cells were extracted by standard protein extraction procedure and RNA extraction procedure, respectively, and then the total RNA was reverse transcribed into cDNA. As shown in Figure S1A, the expression of NUPR1 protein in A2780 and SKOV3 cells was significantly higher than that in OSE cells (P < 0.05). A2780 cells had higher expression of NUPR1 protein than SKOV3 cells (P < 0.05). The mRNA level of NUPR1 in A2780 and SKOV3 cells were significantly higher than that in OSE cells (P < 0.05) (Figure S1B).
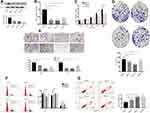
NUPR1 Knockdown Inhibited Invasion, Migration and Proliferation in A2780 Cells
Through pre-experiments and related literature reports, we speculated that NUPR1 may play a role in the proliferation and metastasis of ovarian cancer. To confirm our idea, we knocked down the NUPR1 expression in A2780 cells through methods mediated by RNAi (Figure 1) and lentivirus transfection (Figure 2). Four siRNAs for NUPR1 were designed, respectively, including siRNA-337, siRNA-414, siRNA-850 and siRNA-negative control (siRNA-NC) (Table 1). The series of siRNA-414 was considered as the significant impact in A2780 cells compared with other interference series by MTT assays, colony formation assays, transwell assays. Therefore, this paper selected siRNA-414 to design sh-NUPR1. The protein level and mRNA level of NUPR1 in the treatment group were significantly lower than the control group, respectively (P < 0.05) (Figures 1A and B and 2A and B). And we could find that the cell cycle regulatory proteins, including Bcl-2, Bcl-xl, CDK4, CDK6, were downregulated by silencing NUPR1 (P < 0.05). But apoptosis-related proteins (caspase 3, caspase 9, Bax) increased with the silencing NUPR1 in A2780 cells (P < 0.05). MTT assays showed that the proliferation of A2780 cells was slower in the siRNA-NUPR1 groups or sh-NUPR1 group than the siRNA-NC group or short hairpin RNA-NC group (sh-NC group) (72 h and 96 h, P < 0.01) (Figures 2C and 3C). The proliferation of siRNA-interfered or sh-NUPR1-infected A2780 cells were significantly lower than that of siRNA-NC or sh-NC-infected A2780 cells according to the colony formation assays and the EdU assays (P < 0.05) (Figures 1D and 2D and F), suggesting that the silencing of NUPR1 could significantly suppress the proliferation of A2780 cells. The results of the cell invasion and migrate assays showed that the siRNA-NC-interfered cells or sh-NC-infected cells exhibited faster invasion or migration than sh-NUPR1-interfered cells or siRNA-NUPR1-infected cells, respectively (P < 0.05) (Figures 1E and 2E). These finding revealed that the silencing of NUPR1 suppressed invasion and migration of A2780 cells. To further explore the effects of downregulation of NUPR1 on apoptosis and proliferation of A2780 cells, the apoptosis and cell cycle of arrested cells were evaluated by flow cytometry. As shown in Figure 1F and G, the apoptosis rate of downregulation of NUPR1 significantly decreased in the siRNA-NC group than the siRNA-NUPR1 groups (P < 0.01), and we found that cell viability and the specific upregulation arrested cells in S phase of the cell cycle in vitro were reduced when NUPR1 was downregulated. These data showed that apoptotic cell death resulting from downregulation of NUPR1 in A2780 cells might cause suppression of cell proliferation.
|
Table 1 siRNA Sequences for NUPR1 |
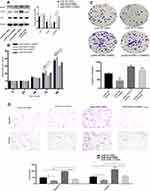
Overexpression of NUPR1 in Ovarian Cancer
The effect of overexpressed NUPR1 was confirmed by detecting the change in the function of A2780 and SKOV3 cells to further study the effect of NUPR1 in ovarian cancer cells. The plasmid of NUPR1 was purchased from the GeneChem (Shanghai, China) and a negative control-CON 238 (Ubi-MCS-3FLAG-SV40-EGFP-IRES-Puro) was designed by the GeneChem, pCMV-NUPR1-238 was named as the control group. The protein level and mRNA level of overexpressed NUPR1 in SKOV3 and A2780 cells increased significantly than those of the control group (P < 0.05) (Figures 3A and B and S2A and B). Through further research, we could find that the cell cycle regulatory proteins, including Bcl-2, Bcl-xl, CDK4, CDK6, were influenced by NUPR1 overexpression. Specifically, protein expression of Bcl-2, Bcl-xl, CDK4, CDK6 were adjusted with the change of NUPR1. However, caspase 3, caspase 9, Bax and other apoptosis-related proteins showed the opposite trend. MTT assays were performed after 24 h, 48 h, 72 h and 96 h after cell adhesion, the results showed that the cell proliferation was faster after overexpression of NUPR1 (72 h and 96 h, P < 0.01) (Figures 3C and S2C). The colony formation assays and EdU assays also verified this phenomenon (respectively, P < 0.05 and P < 0.01), (Figures 3D and F and S2D and F). These results also suggested that the overexpression of NUPR1 might promote the proliferation of A2780 and SKOV3 cells. The overexpression of NUPR1 markedly increased cell migration and invasion than the control group (pCMV-NUPR1-238) (Figures 3E and S2E).
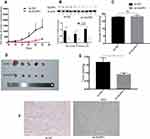
NUPR1 May Play Its Biological Function in Ovarian Cancer Through the AKT Pathway
To confirm that NUPR1 affected the biological function of ovarian cancer cells by influencing the AKT pathway, A2780 cells with overexpressed NUPR1 were treated by LY294002, an AKT pathway nonspecific inhibitor. In this part, the pCMV-NUPR1-238 group was the reference of the pCMV-NUPR1 group. Notably, the treatment with LY294002 (10 μM) could completely or partially eliminate the effect of NUPR1 overexpression on the proliferation (Figure 4B and C), migration and invasion (Figure 4D) in A2780 cells. These data showed that the AKT pathway was a necessary condition for NUPR1 to play a role in ovarian cancer.
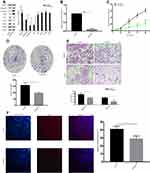
Effect of NUPR1 Knockout on the Growth of Ovarian Cancer in vivo
To determine the biological behavior of NUPR1 in ovarian cancer, we evaluated the tumorigenesis of knockout A2780 cells in nude mice. According to Figure 5C, there was no significant statistical difference in body weight between the two groups on the 27 days after tumorigenesis (P > 0.05). The tumor growth curve results as shown in Figure 5A demonstrate that the growth rate of A2780 cells transfected with sh-NUPR1 was significantly slower than that of sh-NC group (P < 0.05). On the 27 days after injection the weight of tumor in the sh-NUPR1 group was significantly lower than that in the sh-NC group (P < 0.05) (Figure 5D and E). The level of NUPR1 protein in sh-NUPR1 group was significantly lower than that in sh-NC group (P < 0.05), (Figure 5B), suggesting that the low expression of NUPR1 was effective in mice, which was consistent with our expectation. The expression of NUPR1 was also determined in the sh-NUPR1 group and sh-NC group by immunohistochemistry staining. As shown in Figure 5F, the sh-NUPR1 group had a lower level of NUPR1 protein than the sh-NC group. All the data indicated that the low expression of NUPR1 could inhibit the growth of ovarian cancer in vivo.
Discussion
Extensive metastasis and invasion of ovarian cancer have a great influence on the poor prognosis of patients with ovarian cancer. To explore the target molecules were conducive to improve the diagnosis and treatment of ovarian cancer, and provided a theoretical basis for future drug treatment. In the study, we elucidated the effects of NUPR1 silencing and overexpression on the biological behavior of ovarian cancer cells. It was also discussed that NUPR1 might play its biological function in ovarian cancer through the AKT pathway.
According to previous literature, NUPR1 plays a role in carcinogenesis in many tumors, such as breast cancer,15,16 pancreatic cancer,17,23 brain tumor,18 thyroid neoplasm,19 and pituitary tumor.20,21 siRNA-NUPR1 inhibited the proliferation and tumorigenicity of pancreatic cancer cells.24 However, it had also been reported that NUPR1 had a low expression in prostate cancer, the overexpression of NUPR1 inhibited the growth of prostate cancer in vivo.22,25 Here were some contradictory reports on the role of NUPR1 in tumor cell proliferation. A study confirmed NUPR1 was overexpressed in most pancreatic cancer specimens,26 while another study considered NUPR1 could inhibit the growth of pancreatic cancer cells.27 One explanation for these two seemingly contradictory theories was that one study was conducted in vivo and the other in vitro and those different microenvironments in vivo and in vitro resulted in different biological functions of NUPR1. Cano et al also found that the function of NUPR1 was different or even opposite in different microenvironment.3 In the study, we confirmed the existence of the high expression of NUPR1 in ovarian cancer cell lines. Both in vitro cellular experiments and in vivo tumorigenesis experiments confirmed that NUPR1 might be an oncogene in the process of occurrence and development of ovarian cancer. Consistent with these findings, we first found that NUPR1 knockout could block the proliferation and migration of ovarian cancer cells as indicated by MTT assays (Figure 2C), colony formation assays (Figure 2D) and transwell assays (Figure 2E). The opposite results were found in A2780 and SKOV3 cells when NUPR1 was overexpressed (Figures 3C–E and S2C–E). This finding might not be surprising that NUPR1 played an oncogenic role in ovarian cancer cells, suggesting that NUPR1 exerted different functions in ovarian cancer cells compared to prostate cancer. Further study was needed to explore the underlying mechanism of NUPR1 regulating cell motility.
The PI3K/AKT pathway plays an extremely important role in regulating cell survival, metabolism, proliferation, invasion, angiogenesis and tumor transformation and growth. Previous clinical studies had shown that the PI3K pathway was repeatedly activated in ovarian cancer.28 LY294002 is the first small molecule that can be synthesized to inhibit the PI3K/AKT pathway. In the study, overexpression of NUPR1 increased the protein level of p-AKT in ovarian cancer cells (Figure 4A). Therefore, we proposed that the biological functions of NUPR1 in ovarian cancer cells by activating the AKT pathway. Proliferation and migration of A2780 cells induced by NUPR1 overexpression could be blocked by LY294002 (Figure 4B–D). In short, NUPR1 might play a role in promoting tumor growth in ovarian cancer cells through the AKT pathway according to the above data.
Conclusion
NUPR1 could promote the proliferation, invasion and migration of ovarian cancer cells, but the AKT inhibitor LY294002 could partially or completely eliminate this effect. The tumorigenesis of NUPR1 silenced in A2780 cells also supported the view that NUPR1 was an oncogene in ovarian cancer. This conclusion might provide theoretical support for NUPR1 targeted treatment of ovarian cancer.
Ethics Approval
The Experimental Animal Ethics Committee of Cheeloo College of Medicine of Shandong University approved the animal experiment (Approval number: 19,069).
Acknowledgment
Thank the staff of gynecological oncology Laboratory of Qilu Hospital Cheeloo College of Medicine, Shandong University for their support to our work. The experiment was also funded by the Natural Science Foundation of Zhejiang province, China (project number: Y19H160128) and Wenzhou Science and Technology Planning Project, Wenzhou, China (project number: 2019Y0568).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Mallo GV, Fiedler F, Calvo EL, et al. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997;272(51):32360–32369. doi:10.1074/jbc.272.51.32360
2. Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer Metastasis Rev. 2009;28(1–2):225–232. doi:10.1007/s10555-009-9183-x
3. Cano CE, Hamidi T, Sandi MJ, Iovanna JL. Nupr1: the Swiss-knife of cancer. J Cell Physiol. 2011;226(6):1439–1443. doi:10.1002/jcp.22324
4. Goruppi S, Bonventre JV, Kyriakis JM. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. EMBO J. 2002;21(20):5427–5436. doi:10.1093/emboj/cdf535
5. Goruppi S, Patten RD, Force T, Kyriakis JM. Helix-loop-helix protein p8, a transcriptional regulator required for cardiomyocyte hypertrophy and cardiac fibroblast matrix metalloprotease induction. Mol Cell Biol. 2007;27(3):993–1006. doi:10.1128/mcb.00996-06
6. Plant SR, Wang Y, Vasseur S, et al. Upregulation of the stress-associated gene p8 in mouse models of demyelination and in multiple sclerosis tissues. Glia. 2006;53(5):529–537. doi:10.1002/glia.20297
7. Jiang Y-F, Vaccaro MI, Fiedler F, Calvo EL, Iovanna JL. Lipopolysaccharides induce p8 mRNA expression in vivo and in vitro. Biochem Biophys Res Commun. 1999;260(3):686–690. doi:10.1006/bbrc.1999.0953
8. Taïeb D, Malicet C, Garcia S, et al. Inactivation of stress protein p8 increases murine carbon tetrachloride hepatotoxicity via preserved CYP2E1 activity. Hepatology. 2005;42(1):176–182. doi:10.1002/hep.20759
9. Carracedo A, Lorente M, Egia A, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9(4):301–312. doi:10.1016/j.ccr.2006.03.005
10. Urrutia R, Velez G, Lin M, et al. Evidence supporting the existence of a NUPR1-like family of helix-loop-helix chromatin proteins related to, yet distinct from, AT hook-containing HMG proteins. J Mol Model. 2014;20(8):2357. doi:10.1007/s00894-014-2357-7
11. Vasseur S, Vidal Mallo G, Fiedler F, et al. Cloning and expression of the human p8, a nuclear protein with mitogenic activity. Eur J Biochem. 2001;259(3):670–675. doi:10.1046/j.1432-1327.1999.00092.x
12. Goruppi S, Kyriakis JM. The pro-hypertrophic basic helix-loop-helix protein p8 is degraded by the ubiquitin/proteasome system in a protein kinase B/Akt- and glycogen synthase kinase-3-dependent manner, whereas endothelin induction of p8 mRNA and renal mesangial cell hypertrophy require NFAT4. J Biol Chem. 2004;279(20):20950–20958. doi:10.1074/jbc.M312401200
13. Päth G, Opel A, Knoll A, Seufert J. Nuclear protein p8 is associated with glucose-induced pancreatic beta-cell growth. Diabetes. 2004;53(Suppl):1S82–85. doi:10.2337/diabetes.53.2007.s82
14. Carracedo A, Egia A, Guzmán M, Velasco G. p8 upregulation sensitizes astrocytes to oxidative stress. FEBS Lett. 2006;580(6):1571–1575. doi:10.1016/j.febslet.2006.01.084
15. Ree AH, Tvermyr M, Engebraaten O, et al. Expression of a novel factor in human breast cancer cells with metastatic potential. Cancer Res. 1999;59(18):4675–4680.
16. Ree AH, Pacheco MM, Tvermyr M, Fodstad O, Brentani MM. Expression of a novel factor, com1, in early tumor progression of breast cancer. Clin Cancer Res. 2000;6(5):1778–1783.
17. Su SB, Motoo Y, Iovanna JL, et al. Overexpression of p8 is inversely correlated with apoptosis in pancreatic cancer. Clin Cancer Res. 2001;7(5):1320–1324.
18. Ree AH, Bratland A, Kroes RA, et al. Clinical and cell line specific expression profiles of a human gene identified in experimental central nervous system metastases. Anticancer Res. 2002;22(4):1949–1957.
19. Ito Y, Yoshida H, Motoo Y, et al. Expression and cellular localization of p8 protein in thyroid neoplasms. Cancer Lett. 2003;201(2):237–244. doi:10.1016/j.canlet.2003.07.002
20. Mohammad HP, Seachrist DD, Quirk CC, Nilson JH. Reexpression of p8 contributes to tumorigenic properties of pituitary cells and appears in a subset of prolactinomas in transgenic mice that hypersecrete luteinizing hormone. Mol Endocrinol. 2004;18(10):2583–2593. doi:10.1210/me.2004-0163
21. Brannon KM, Million Passe CM, White CR, et al. Expression of the high mobility group A family member p8 is essential to maintaining tumorigenic potential by promoting cell cycle dysregulation in LβT2 cells. Cancer Lett. 2007;254(1):146–155. doi:10.1016/j.canlet.2007.03.011
22. Jiang WG, Davies G, Martin TA, et al. Com-1/p8 acts as a putative tumour suppressor in prostate cancer. Int J Mol Med. 2006;18(5):981–986.
23. Giroux V, Malicet C, Barthet M, et al. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res. 2006;12(1):235–241. doi:10.1158/1078-0432.Ccr-05-1700
24. Sandi MJ, Hamidi T, Malicet C, et al. p8 expression controls pancreatic cancer cell migration, invasion, adhesion, and tumorigenesis. J Cell Physiol. 2011;226(12):3442–3451. doi:10.1002/jcp.22702
25. Jiang WG, Davies G, Kynaston H, Mason MD, Fodstad O. Does the PGC-1/PPARgamma pathway play a role in Com-1/p8 mediated cell growth inhibition in prostate cancer? Int J Mol Med. 2006;18(6):1169–1175.
26. Hamidi T, Algül H, Cano CE, et al. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest. 2012;122(6):2092–2103. doi:10.1172/jci60144
27. Malicet C, Lesavre N, Vasseur S, Iovanna JL. p8 inhibits the growth of human pancreatic cancer cells and its expression is induced through pathways involved in growth inhibition and repressed by factors promoting cell growth. Mol Cancer. 2003;237. doi:10.1186/1476-4598-2-37
28. Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137(1):173–179. doi:10.1016/j.ygyno.2015.02.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.