Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Omentin as an Independent Predictor of Metabolic Syndrome and Obesity Among Adolescents in Northeast China
Authors Sun X, Li T, Tian Y, Ren S, Li L , Li P
Received 3 September 2022
Accepted for publication 15 November 2022
Published 15 December 2022 Volume 2022:15 Pages 3913—3922
DOI https://doi.org/10.2147/DMSO.S388620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xiaoshi Sun, Tianlian Li, Yumeng Tian, Shuying Ren, Ling Li, Ping Li
Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, People’s Republic of China
Correspondence: Ping Li, Email [email protected]
Purpose: We investigated the association of omentin with metabolic syndrome (MetS), MetS components, and obesity in adolescents.
Methods: A total of 742 middle-school students from Liaoyang City were enrolled in this cross-sectional study using the stratified cluster sampling method. Clinical information and blood samples were collected, and serum omentin levels were measured using enzyme-linked immunosorbent assay.
Results: Mean plasma omentin levels were lower in male than in female participants (88.25 (interquartile range 63.02– 133.61) vs 99.46 (interquartile range 69.08– 188.35) ng/L, P = 0.004). The participants were divided into four groups according to the quartile (Q) values of omentin from low to high. With increasing omentin levels from Q1 to Q4, the age of adolescents and the proportion of males gradually increased (P < 0.05), whereas the body mass index (BMI) (P < 0.05) and prevalence of MetS (P > 0.05) tended to decrease. Omentin levels were significantly and negatively correlated with waist circumference and BMI (correlation coefficients of ˗0.099 and ˗0.115, respectively). Regression analysis showed that omentin level was independently associated with the risk of MetS (Odds ratio, OR = 0.639, 95% confidence interval, CI (0.432, 0.945)), which was attributed to the association with central obesity (OR = 0.775, 95% CI (0.605, 0.993)) among MetS components. Increased omentin levels also indicated a reduced risk of obesity (OR = 0.700, 95% CI (0.563, 0.870)).
Conclusion: Omentin is an independent predictor of MetS and obesity among adolescents in northeast China.
Keywords: metabolic syndrome, omentin, adolescents, obesity, insulin resistance
Introduction
Metabolic syndrome (MetS) is a pathological state characterized by a series of common metabolic disorders, including insulin resistance, hyperlipidemia, abdominal obesity, and hypertension.1 The main pathophysiological cause of MetS is insulin resistance induced by cellular metabolic dysregulation,2 which subsequently triggers or aggravates functional damage in β-cells and prevents β-cell compensation for elevated blood glucose levels caused by insulin resistance, eventually leading to the onset of type 2 diabetes.3 MetS, as well as the accompanying components of obesity, hypertension, and dyslipidemia, collectively serve as major risk factors for cardiovascular disease.4,5 The prevalence of MetS among adolescents ranges from 4.5% to 8.4% in the United States.6,7 In China, the prevalence of MetS is from 27% in 2011,8 and 32.97% in 2021.9 MetS and its elements are significantly associated with high coronary artery disease risk.10 As a developing country, China’s increasing risk of MetS in adolescents, as well as the increasing mental and psychological stress, is attributed to obesity, unhealthy lifestyles, and external environmental factors.5 These adolescents represent a large future population of patients with type 2 diabetes and cardio-cerebrovascular diseases. Therefore, the early identification and intervention of adolescent MetS is important for improving the physical health of these populations.11 However, unlike in the adult population, an absence of routine physical examinations among adolescents compromises our ability to provide early warnings of metabolic disorders. Hence, finding biomarkers related to the early prediction and identification of adolescent MetS will improve the early diagnosis of these diseases.
Omentin is a recently identified adipokine that has gained extensive attention for its association with different diseases. For example, omentin is associated with respiratory diseases including asthma, obstructive sleep apnea, pulmonary hypertension, acute respiratory distress syndrome, and chronic obstructive pulmonary disease.12 Omentin is also closely associated with various diseases related to insulin resistance, eg, type 2 diabetes,13 gestational diabetes,14,15 polycystic ovary syndrome,16 and adult MetS,17 indicating its potential as a promising interventional target for metabolic disorders.18 Under the trend of obesity among teenagers,19,20 a study with a small sample size of adolescents revealed that omentin is closely associated with childhood and adolescent obesity.21 Moreover, children and adolescents with obesity have an increased future risk of MetS.22–24 Therefore, we performed a cross-sectional study to analyze the correlation between serum omentin levels and MetS, MetS components, and the degree of obesity in adolescents (aged 11–16) in northeast China. The aim of this research was to gain an in-depth understanding of the pathophysiological role of omentin in metabolic disorders in adolescents. The findings of this study provide additional epidemiological strategies for enhancing the early warning, diagnosis, and treatment of adolescent MetS.
Materials and Methods
Study Population
Students of junior and senior high schools in Liaoyang, a city with a medium level of economic development in northeast China, were recruited in this study using stratified cluster sampling from December 2010 to January 2011. We also collected fasting blood samples to measure the levels of omentin, fasting plasma glucose (FPG), fasting insulin (FINS), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), total cholesterol (TC), and serum uric acid. A total of 742 students with complete information were included in the statistical analysis. None of the participants had a history of anemia, diabetes, hypertension, or drug therapy. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Shengjing Hospital, China Medical University (2010PS676K).
Prior to collecting the blood samples, a trained physician measured the height, weight, waist circumference (WC), hip circumference, and blood pressure (systolic blood pressure [SBP] and diastolic blood pressure [DBP]) of the participants. After sitting relaxed for more than 10 min, blood pressure was measured twice using a desktop mercury sphygmomanometer, with an interval of 2 min between measurements, and the average SBP and DBP values were recorded. All participants fasted for ≥10 h overnight, and fasting venous blood was obtained at 07:00–09:00 the next morning. As soon as the blood samples were collected, they were sent to the laboratory of Liaoyang Diabetes Hospital for centrifugation (within 1 h). After centrifugation, FPG was determined within 2 h (via the Glucose Oxidase method, Olympus 400; Olympus Optical Company, Tokyo, Japan). Routine enzymatic methods were used to determine LDL-C, HDL-C, TG, TC, and uric acid levels in the serum. FINS was measured by radioimmunoassay (China Institute of Atomic Energy, Beijing, P.R. China) using plasma stored at ˗80 °C. An enzyme-linked immunosorbent assay kit (R&D, Minneapolis, USA) was used to measure plasma omentin levels, with intra-assay and inter-assay coefficients less than 9% and 15%, respectively. The samples were tested at the central laboratory of Shengjing Hospital, China Medical University. The body mass index (BMI) was calculated by body weight/height2 (kg/m2). Steady-state homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR= fasting blood glucose (mmol/L) × FINS (U/mL)/22.5.
Diagnostic Criteria and Definition
Metabolic Syndrome for Adolescents
MetS was defined according to International Diabetes Federation 2007 guidelines.25 These guidelines state that, for an adolescent to be diagnosed as MetS, the individual must have central (abdominal) obesity (≥90th percentile, criteria proposed by the Capital Institute of Pediatrics),26 as well as two or more of the following clinical features: hypertriglyceridemia (TG ≥ 1.7 mmol/L), low HDL-C (< 1.03 mmol/L for individuals 10–15 years of age and boys ≥16 years of age or <1.29 mmol/L for girls ≥ 16 years of age), hypertension (SBP ≥ 130 or DBP ≥ 85 mmHg), or fasting hyperglycemia (FPG ≥5.6 mmol/L) L, or a previous diagnosis of type 2 diabetes.
Overweight/Obesity
According to the Group of China Obesity Task Force, the terms obesity and overweight were defined in adolescents as BMI ≥ 95th percentile and BMI ≥ 85th percentile, respectively.27
Statistical Analysis
Statistical analyses were conducted using SPSS 27.0 for Windows (SPSS, Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to verify whether the variables conformed to the normal distribution. Normally distributed continuous variables were presented as the mean ± standard error, whereas non-normally distributed variables were presented as the median (interquartile range). Non-normally distributed variables were log-transformed to normality prior to statistical analysis. Categorical data were shown as percentages and compared using a chi-square test. Analysis of variance or Kruskal–Wallis H-tests were used to test for differences between groups. After adjusting for confounding factors using a one-way generalized linear model, multiple groups of data were compared. Partial correlation analysis was used to evaluate the correlation between omentin levels and MetS-related risk factors after adjusting for confounding factors. Logistic binary regression analysis was used to evaluate associations of omentin levels with MetS and MetS-related risk factors. Linear regression analysis was used to analyze the relationship between omentin level and BMI. P < 0.05 was considered statistically significant.
Results
Clinical Characteristics of Participants
A total of 742 adolescents in Liaoyang City were included in this study. The clinical characteristics of the participants are shown in Table 1. Male participants had higher WC, waist to hip ratio (WHR), BMI, SBP, FPG and uric acid levels than female participants but lower values of DBP and TC (P < 0.05). Plasma omentin levels were markedly lower in males than in females (88.25 (63.02, 133.61) vs 99.46 (69.08, 188.35) ng/L, P = 0.004) (Figure 1).
 |
Table 1 Clinical Characteristics of the Participants According to Sex |
 |
Figure 1 Serum omentin levels in male and female participants. |
Relationship Between Omentin and Clinical Parameters
As shown in Table 2, the participants were divided into four groups according to their quartile values of omentin from low to high (Q1‒Q4). As omentin levels increased from group Q1 to Q4, the age of adolescents and the proportion of males gradually increased, whereas the BMI decreased, even when adjusting for age and sex (P < 0.05). The prevalence of MetS tended to decrease with increasing omentin; however, this difference was not statistically significant (P > 0.05). Uric acid levels were lowest in Q3; however, this difference was not significant after adjusting for sex and age. We observed no difference in blood pressure, blood glucose, or other MetS risk factors among the four groups.
 |
Table 2 Clinical Characteristics of the Participants According to Their Omentin Levels |
Relationship Between Omentin and MetS Risk Factors
Partial correlation analysis, after adjusting for age and sex, showed that omentin levels were significantly negatively correlated with WC and BMI (correlation coefficient (r) of ˗0.099 and ˗0.115, respectively, all P < 0.001; Table 3).
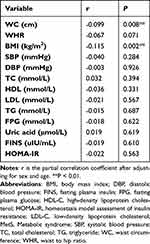 |
Table 3 Partial Correlation Between Omentin Level and MetS Risk Factors |
Association of Omentin with MetS and MetS Components
According to the binary logistic regression analysis (Table 4), increased omentin levels were associated with a reduced risk of MetS. This association was significant even after adjusting for sex and age (Odds ratio, OR = 0.639, 95% confidence interval, CI (0.432, 0.945)), and was predominantly attributed to central obesity (OR = 0.775, 95% CI (0.605, 0.993)), which was the only one of five MetS components that was significantly associated with omentin.
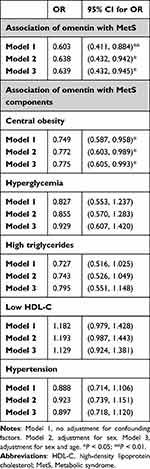 |
Table 4 Association of Omentin Level with MetS and MetS Components |
Association of Omentin with Overweight Status and Obesity
In binary logistic regression analysis (Table 5), we defined the group of participants that were overweight or obese as the independent variable, and defined the group with normal BMI as the dependent variable. Omentin was highly associated with overweight status/obesity. Increased levels of omentin indicated a reduced risk of overweight status/obesity (OR = 0.700, 95% CI (0.563, 0.870)). This association was also confirmed by linear regression analysis (regression coefficient (B) = −0.625, 95% CI (˗1.021, ˗0.230)).
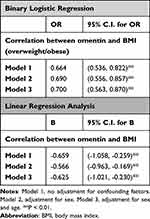 |
Table 5 Association of Omentin Level with BMI |
Discussion
According to our results, omentin levels were associated with MetS in adolescents from northeast China and were most closely associated with central obesity among all MetS components. Moreover, among cardiovascular risk factors other than MetS, the level of omentin was significantly negatively correlated with the degree of obesity/overweight status. The above evidence suggests that the level of omentin can serve as a predictor of MetS and obesity in adolescents.
Omentin is a novel hydrophilic adipokine of 313 amino acids (35 kDa) produced by visceral adipose tissue, containing a secretory signal sequence and a fibrinogen-related domain.28 It has been demonstrated that omentin can regulate the inflammatory state, vasomotor, and endothelial functions, as well as the proliferation, apoptosis, and differentiation of cells, via various molecular mechanisms.12 Furthermore, omentin exhibits many other functions, eg, anti-atherosclerotic, anti-inflammatory, antioxidant, anti-apoptotic, and antimicrobial effects.18 It can also inhibit the tumor necrosis factor-α-induced activation of nuclear factor kappa B, which is the central mechanism underlying the onset of insulin resistance.29 Omentin modulates insulin sensitivity by upregulating the expression of adiponectin, promotes the insulin-mediated uptake of glucose by adipocytes, and mutually regulates the expression of other cytokines and inflammatory factors,28,30,31 thereby playing an important role in the onset and development of diabetes and MetS.32
The above-mentioned biological effects of omentin explain its close association with insulin resistance-related diseases, eg, MetS and obesity. A study conducted in the United States found significantly higher omentin levels in the serum and gluteal adipose tissue of patients in the earliest stage of MetS than in those of normal individuals.33 Moreover, a study involving 201 adult males in Japan found that the serum concentration of omentin in adults decreased with an increase in the number of metabolic risk factors.34 Serum levels of omentin are also associated with the risk of MetS in both male and female participants,35,36 which has been confirmed by studies on patient populations with different diseases, eg, hypertension and psoriasis.37,38 A well-designed interventional study showed that eight weeks of vigorous cycling exercise can increase the level of omentin among high-risk populations for MetS,39 suggesting that an increased omentin concentration is often indicative of an improved metabolic state; however, whether such a change is a cause or effect of metabolic improvement remains unclear and requires further fundamental research.
Contrary to the above findings, a previous study on young males showed no association between omentin concentration and MetS; however, as this study only involved 38 participants, we suggest that further investigations of a larger sample size are required to be conclusive. A study conducted on patients with prostate cancer also indicated no association between the level of omentin and the risk of MetS;40 however, this finding may be attributable to the presence of more confounding factors in the study because of the poorer physical condition of cancer patients. Unlike the above-mentioned studies on adults, other diseases are less common in children and adolescents, with fewer confounding factors affecting the level of omentin, thereby allowing for a more objective investigation of the relationship between omentin level and MetS. For example, a study conducted on obese children in Turkey showed that children with MetS have significantly lower serum levels of omentin than children without MetS.41 Additionally, a study conducted in China also found that the level of omentin in children with MetS was significantly elevated following a six-month lifestyle intervention.42
In addition to MetS, obesity/overweight is also an important risk factor for cardiovascular disease. Indeed, our data showed a significant correlation between BMI and omentin levels, which is consistent with previous findings in the adult population. For example, a Spanish study with a small sample size showed that obese adults had lower plasma concentrations of omentin than adults with normal weight; however, omentin concentrations increased with weight loss after a four-month dietary intervention.43 Another study conducted in the United States revealed that serum levels of omentin in the obese and overweight population was significantly negatively correlated with BMI, WC, serum leptin level, and HOMA-IR, which indicates that these factors may also be markers of adipose-tissue metabolism integrating insulin sensitivity and blood pressure.21,44,45 Furthermore, a study on obese children and adolescents showed significantly lower omentin levels in obese children than in children with a normal weight,21 which then returned to normal upon weight loss following a lifestyle intervention. In another study, overweight/obese children also displayed improved omentin levels after undergoing a short-term (four-week) standardized lifestyle intervention program.46 These reversible changes suggest that omentin is also a promising serological marker for monitoring the outcome of obesity interventions.47
Common problems faced by previous clinical studies on omentin in children and adolescents include: (1) a small sample size; (2) most of the recruited participants were obese patients; (3) relatively few studies on Han Chinese populations. To date, there are still no reported large-scale epidemiological studies on Han Chinese adolescents. Therefore, the strong points of this study are to elucidate the correlation between omentin and the risk of MetS and obesity by recruiting research participants from a large adolescent population in northeast China via the stratified cluster sampling method. Our study confirms that omentin can be used as an effective serological marker for MetS and obesity in adolescents. However, this study has the following limitations: (1) our cross-sectional study does not help speculate on the causal relationship between omentin and the risk of MetS or obesity; (2) this study has certain geographical limitations, as the recruitment of research participants was confined to northeast China.
Our capacity for early identification and intervention is incredibly important because adolescents with MetS or obesity will become a large future population of patients with diabetes and cardiovascular diseases. The diagnosis of MetS can only be confirmed through various examinations, including blood glucose, blood lipid and other blood examinations and physical indicators of height and weight. Omentin helps to screen out the high-risk groups of adolescents, so that we can do early intervention and in-depth examination. The goal of research on MetS or obesity is to reduce the risk of cardio-cerebrovascular diseases. Some studies have shown that omentin has a protective effect against cardiovascular diseases, whereby an increased level of circulating omentin is conducive to preventing cardiovascular disease.48 A study conducted in China on patients with MetS showed that omentin levels were independently and negatively correlated with coronary artery disease and angiographic severity.49 Hence, omentin has been proposed as an alternative diagnostic tool to ensure the optimal management of patients with coronary artery disease.49–52 The predictive capacity of omentin levels during adolescence for cardiovascular diseases in adulthood should be confirmed via large-scale prospective studies.
Conclusion
In summary, our cross-sectional study on the clinical characteristics and omentin concentrations of 742 adolescents (aged 12–16) residing in the Liaoyang region of northeast China revealed that a decrease in the level of omentin in adolescents is an independent predictor of MetS and obesity and, among all MetS components, is most closely associated with central obesity. We predict that omentin, as a link between MetS, obesity, and cardiovascular diseases, will become a simple and effective serological marker for predicting and monitoring the intervention of these diseases. Moreover, omentin may become a future pharmacotherapeutic target, which should be further investigated via prospective and fundamental studies.
Abbreviations
MetS, metabolic syndrome; Q, quartile; FPG, fasting plasma glucose; FINS, fasting insulin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; WC, waist circumference; WHR, waist to hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostasis model assessment of insulin resistance; OR, odds ratio; CI, confidence interval.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Shengjing Hospital, China Medical University.
Consent to Participate
We conducted a questionnaire survey to obtain informed consent of the students and their guardians to participate in the study.
Funding
This work was supported by Fund for the Natural Science Foundation of Liaoning Province in China (grant number 2020-MS-153).
Disclosure
The authors declare no competing interests in this work.
References
1. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi:10.1016/S0140-6736(05)66378-7
2. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131–1141. doi:10.1056/NEJMra1011035
3. Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001–4008. doi:10.1172/JCI129188
4. Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. 2017;140(2). doi:10.1542/peds.2017-1603
5. Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. doi:10.3390/ijms23020786
6. Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148. doi:10.1016/j.numecd.2010.05.006
7. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31(3):587–589. doi:10.2337/dc07-1030
8. Li R, Li W, Lun Z, et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. doi:10.1186/s12889-016-2870-y
9. Xiong Y, Zhang Y, Zhang F, Wu C, Qin F, Yuan J. Prevalence and associated factors of metabolic syndrome in Chinese middle-aged and elderly population: a national cross-sectional study. Aging Male. 2021;24(1):148–159. doi:10.1080/13685538.2021.1998432
10. Alshammary AF, Alharbi KK, Alshehri NJ, Vennu V, Ali Khan I. Metabolic syndrome and coronary artery disease risk: a meta-analysis of observational studies. Int J Environ Res Public Health. 2021;18(4):1773. doi:10.3390/ijerph18041773
11. DeBoer MD. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients. 2019;11(8):1788. doi:10.3390/nu11081788
12. Zhou Y, Zhang B, Hao C, et al. Omentin-a novel adipokine in respiratory diseases. Int J Mol Sci. 2017;19(1):73. doi:10.3390/ijms19010073
13. Herder C, Kannenberg JM, Niersmann C, et al. Independent and opposite associations of serum levels of omentin-1 and adiponectin with increases of glycaemia and incident type 2 diabetes in an older population: KORA F4/FF4 study. Eur J Endocrinol. 2017;177(4):277–286. doi:10.1530/EJE-17-0100
14. Tsiotra PC, Halvatsiotis P, Patsouras K, et al. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157–166. doi:10.1016/j.peptides.2018.01.005
15. Franz M, Polterauer M, Springer S, et al. Maternal and neonatal omentin-1 levels in gestational diabetes. Arch Gynecol Obstet. 2018;297(4):885–889. doi:10.1007/s00404-018-4652-5
16. Orlik B, Madej P, Owczarek A, Skałba P, Chudek J, Olszanecka-Glinianowicz M. Plasma omentin and adiponectin levels as markers of adipose tissue dysfunction in normal weight and obese women with polycystic ovary syndrome. Clin Endocrinol. 2014;81(4):529–535. doi:10.1111/cen.12381
17. Liu R, Wang X, Bu P. Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract. 2011;93(1):21–25. doi:10.1016/j.diabres.2011.03.001
18. Zhao A, Xiao H, Zhu Y, et al. Omentin-1: a newly discovered warrior against metabolic related diseases. Expert Opin Ther Targets. 2022;26(3):275–289. doi:10.1080/14728222.2022.2037556
19. Alshammary AF, Khan IA. Screening of obese offspring of first-cousin consanguineous subjects for the angiotensin-converting enzyme gene with a 287-bp Alu sequence. J Obes Metabol Syndr. 2021;30(1):63–71. doi:10.7570/jomes20086
20. Alharbi KK, Al-Sheikh YA, Alsaadi MM, et al. Screening for obesity in the offspring of first-cousin consanguineous couples: a Phase-I study in Saudi Arabia. Saudi J Biol Sci. 2020;27(1):242–246. doi:10.1016/j.sjbs.2019.09.001
21. Zengi S, Zengi O, Kirankaya A, Kucuk SH, Kutanis EE, Yigit O. Serum omentin-1 levels in obese children. J Pediatr Endocrinol Metab. 2019;32(3):247–251. doi:10.1515/jpem-2018-0231
22. Gupta N, Goel K, Shah P, Misra A. Childhood obesity in developing countries: epidemiology, determinants, and prevention. Endocr Rev. 2012;33(1):48–70. doi:10.1210/er.2010-0028
23. Pacheco LS, Blanco E, Burrows R, Reyes M, Lozoff B, Gahagan S. Early onset obesity and risk of metabolic syndrome among Chilean adolescents. Prev Chronic Dis. 2017;14:E93. doi:10.5888/pcd14.170132
24. Christian Flemming GM, Bussler S, Körner A, Kiess W. Definition and early diagnosis of metabolic syndrome in children. J Pediatr Endocrinol Metab. 2020;33(7):821–833. doi:10.1515/jpem-2019-0552
25. Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents - An IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306. doi:10.1111/j.1399-5448.2007.00271.x
26. Ma GS, Ji CY, Ma J, et al. 中国7~18岁学龄儿童青少年腰围界值点研究 [Waist circumference reference values for screening cardiovascular risk factors in Chinese children and adolescents aged 7–18 years]. Zhonghua liu xing bing xue za zhi. 2010;31(6):609–615. Chinese.
27. Group of China Obesity Task Force. 中国学龄儿童青少年超重、肥胖筛查体重指数值分类标准 [Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents]. Zhonghua liu xing bing xue za zhi. 2004;25(2):97–102. Chinese.
28. Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–1261. doi:10.1152/ajpendo.00572.2004
29. Reinehr T, Roth CL. Inflammation markers in type 2 diabetes and the metabolic syndrome in the pediatric population. Curr Diab Rep. 2018;18(12):131. doi:10.1007/s11892-018-1110-5
30. Watanabe T, Watanabe-Kominato K, Takahashi Y, Kojima M, Watanabe R. Adipose tissue-derived omentin-1 function and regulation. Compr Physiol. 2017;7(3):765–781.
31. Herder C, Ouwens DM, Carstensen M, et al. Adiponectin may mediate the association between omentin, circulating lipids and insulin sensitivity: results from the KORA F4 study. Eur J Endocrinol. 2015;172(4):423–432. doi:10.1530/EJE-14-0879
32. Escoté X, Gómez-Zorita S, López-Yoldi M, et al. Role of omentin, vaspin, cardiotrophin-1, TWEAK and NOV/CCN3 in obesity and diabetes development. Int J Mol Sci. 2017;18(8):1770. doi:10.3390/ijms18081770
33. Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab. 2013;98(3):E514–517. doi:10.1210/jc.2012-3673
34. Shibata R, Ouchi N, Takahashi R, et al. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012;4(1):37. doi:10.1186/1758-5996-4-37
35. Vu A, Sidhom MS, Bredbeck BC, Kosmiski LA, Aquilante CL. Evaluation of the relationship between circulating omentin-1 concentrations and components of the metabolic syndrome in adults without type 2 diabetes or cardiovascular disease. Diabetol Metab Syndr. 2014;6(1):4. doi:10.1186/1758-5996-6-4
36. Auguet T, Quintero Y, Riesco D, et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet. 2011;12:60. doi:10.1186/1471-2350-12-60
37. Cetin Sanlialp S, Nar G, Nar R. Relationship between circulating serum omentin-1 levels and nascent metabolic syndrome in patients with hypertension. J Investig Med. 2022;70(3):780–785. doi:10.1136/jim-2021-002071
38. Dağdelen D, Karadag AS, Kasapoğlu E, Wang JV, Erman H. Correlation of metabolic syndrome with serum omentin-1 and visfatin levels and disease severity in psoriasis and psoriatic arthritis. Dermatol Ther. 2020;33(6):e14378. doi:10.1111/dth.14378
39. Madsen SM, Thorup AC, Bjerre M, Jeppesen PB. Does 8 weeks of strenuous bicycle exercise improve diabetes-related inflammatory cytokines and free fatty acids in type 2 diabetes patients and individuals at high-risk of metabolic syndrome? Arch Physiol Biochem. 2015;121(4):129–138. doi:10.3109/13813455.2015.1082600
40. Borowski A, Sieminska L. Serum omentin levels in patients with prostate cancer and associations with sex steroids and metabolic syndrome. J Clin Med. 2020;9(4):1179. doi:10.3390/jcm9041179
41. Buyukinan M, Atar M, Can U, Pirgon O, Guzelant A, Deniz I. The association between serum vaspin and omentin-1 levels in obese children with metabolic syndrome. Metab Syndr Relat Disord. 2018;16(2):76–81. doi:10.1089/met.2017.0133
42. Zhang M, Tan X, Yin C, Wang L, Tie Y, Xiao Y. Serum levels of omentin-1 are increased after weight loss and are particularly associated with increases in obese children with metabolic syndrome. Acta Paediatr. 2017;106(11):1851–1856. doi:10.1111/apa.14026
43. Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab. 2010;7:27. doi:10.1186/1743-7075-7-27
44. de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–1661. doi:10.2337/db06-1506
45. Prats-Puig A, Bassols J, Bargalló E, et al. Toward an early marker of metabolic dysfunction: omentin-1 in prepubertal children. Obesity. 2011;19(9):1905–1907. doi:10.1038/oby.2011.198
46. Siegrist M, Heitkamp M, Braun I, et al. Changes of omentin-1 and chemerin during 4 weeks of lifestyle intervention and 1 year follow-up in children with obesity. Clin Nutr. 2021;40(11):5648–5654. doi:10.1016/j.clnu.2021.09.042
47. Rothermel J, Lass N, Barth A, Reinehr T. Link between omentin-1, obesity and insulin resistance in children: findings from a longitudinal intervention study. Pediatr Obes. 2020;15(5):e12605. doi:10.1111/ijpo.12605
48. Tan YL, Zheng XL, Tang CK. The protective functions of omentin in cardiovascular diseases. Clin Chim Acta. 2015;448:98–106. doi:10.1016/j.cca.2015.05.019
49. Shang FJ, Wang JP, Liu XT, et al. Serum omentin-1 levels are inversely associated with the presence and severity of coronary artery disease in patients with metabolic syndrome. Biomarkers. 2011;16(8):657–662. doi:10.3109/1354750X.2011.622789
50. Stejskal D, Vaclavik J, Smekal A, Svobodova G, Richterova R, Svestak M. Omentin-1 levels in patients with premature coronary artery disease, metabolic syndrome and healthy controls. Short communication. Biomed Pap Med Fac Univ. 2016;160(2):219–221. doi:10.5507/bp.2016.019
51. Zhou JY, Chan L, Zhou SW. Omentin: linking metabolic syndrome and cardiovascular disease. Curr Vasc Pharmacol. 2014;12(1):136–143. doi:10.2174/1570161112999140217095038
52. Askin L, Duman H, Ozyildiz A, Tanriverdi O, Turkmen S. Association between omentin-1 and coronary artery disease: pathogenesis and clinical research. Curr Cardiol Rev. 2020;16(3):198–201. doi:10.2174/1573403X16666200511085304
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
