Back to Journals » OncoTargets and Therapy » Volume 13
Omega-3PUFA Attenuates MNU-Induced Colorectal Cancer in Rats by Blocking PI3K/AKT/Bcl-2 Signaling
Authors Huang Z , Liu CA, Cai PZ, Xu FP, Zhu WJ, Wang WW, Jiang HP
Received 6 December 2019
Accepted for publication 24 February 2020
Published 5 March 2020 Volume 2020:13 Pages 1953—1965
DOI https://doi.org/10.2147/OTT.S241298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Zhe Huang,1,2,* Chun-An Liu,3,* Peng-Zhu Cai,2 Fei-Peng Xu,2 Wen-Jing Zhu,2 Wei-Wei Wang,2 Hai-Ping Jiang1
1The First Affiliated Hospital, Jinan University, Guangzhou 510000, People’s Republic of China; 2Department of Gastrointestinal Surgery, The Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, People’s Republic of China; 3Department of General Surgery, Ji’an Central Hosipital, Ji’an 343000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hai-Ping Jiang
The First Affiliated Hospital, Jinan University, Guangzhou 510000, People’s Republic of China
Tel +86-13392692225
Email [email protected]
Background: Omega 3 polyunsaturated fatty acid (Omega-3PUFA) is one of the essential nutrients for human body involved in intracellular metabolic regulation and cell signaling. Previous studies have shown that Omega-3PUFA is involved in the pathogenesis of digestive system tumors, including colorectal cancer (CRC), however, the effects of Omega-3PUFA on CRC has not been fully elucidated. In the current study, we evaluated whether Omega-3PUFA can alleviate N-methyl-N-nitrosourea(MNU) induced CRC in a rat model and illustrated the potential mechanism.
Methods: The effects of Omga-3PUFA on MNU-induced colorectal cancer in rats were analyzed by in vivo experiments. The viability, apoptosis, colony formation and invasion of CRC cells treated with Omga-3PUFA were detected by CCK8, flow cytometry, clone formation assay and transwell invasion assay. The expression of apoptosis-related proteins in CRC cells treated with Omga-3PUFA was detected by Western blotting. Finally, after adding PI3K activator, the viability, apoptosis and protein expression of CRC cells treated with Omga-3PUFA were detected by CCK8, flow cytometry and Western blotting.
Results: Our results showed that Omega-3PUFA attenuated MNU-induced CRC in rats and inhibited AKT/Bcl-2 signaling in rats. In addition, Omega-3PUFA inhibited CRC cell proliferation and induces CRC cell apoptosis. Moreover, Omega-3PUFA inhibited CRC cell colony formation and invasion, and inhibited PI3K/AKT/Bcl-2 signaling in CRC cells. Furthermore, The effects of Omega-3PUFA on cell proliferation and apoptosis were inhibited by blocking PI3K/AKT signaling.
Conclusion: Omega-3PUFA can attenuate MNU-induced colorectal cancer in rats by blocking PI3K/AKT/Bcl-2 signaling, which suggests that Omega-3PUFA may be a potent agent for CRC treatment.
Keywords: Omega-3PUFA, colorectal cancer, PI3K/AKT signaling, apoptosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors in the digestive system.1 The incidence of CRC in men is the third most common malignant tumor in the world, and the incidence rate in women is the second. Moreover, the incidence of CRC in most countries is on the rise.2 Colorectal cancer often has been in the middle and late stage of the disease when the typical clinical signs and symptoms appear. Although there has been much progress in treatment, it fail to improve significantly 5-year survival, which still below 30%. More and more researchers are paying attention to the prevention and early treatment of CRC.3
One of the hot areas of multidisciplinary cross-disciplinary research is “diet, nutrition and cancer”. Polyunsaturated fatty acids (PUFAs) have become a hot spot in medical research as an “immune nutrient”.4 As one of the essential nutrients in the human body, polyunsaturated fatty acids, in addition to providing basic energy, are also involved in the formation of cell membrane phospholipids, and participate in the body’s anti–inflammatory, intracellular metabolic regulation, and intercellular signaling.5–7 Studies have explored the relationship between fatty acid composition and tumor-related factors in colorectal cancer tissues. It is found that the metabolism of PUFA may play an important role in the development of inflammation-driven tumorigenesis in colorectal cancer and may be considered as a potential marker of prognosis.8
Omega-3PUFA, a class of PUFAs which the human body cannot synthesize and must obtain from food, are of particular interest due to their potential roles in the development of various cancers.9–11 Studies have shown that Omega-3PUFA can improve the survival rate of colon cancer patients by improving their quality of life.12 Studies have also shown that Omega-3PUFA may prevent colorectal tumor formation through their anti–inflammatory properties.13 On the one hand, omega-3 PUFA as an important nutrient has a preventive effect on the occurrence of CRC. Studies have shown that omega-3 PUFAs have a potential role in preventing CRC through DNA mismatch repair.14 On the other hand, omega-3 PUFA acts as a chemotherapy and radiotherapy adjuvant to inhibit tumor growth. Recent studies have shown that Omega-3 PUFA inhibited tumor growth by promoting the synthesis of 5-methylcytosine in CRC rats.15 Although Omega-3PUFA are involved in the development and progression of CRC, but specific molecular mediators that regulate this process have not been identified.
In this study, we evaluated the role of Omega-3PUFA in the MNU-induced CRC rats and explored its molecular mechanisms in vitro. We found Omega-3PUFA attenuated MNU-induced CRC in rats. In addition, Omega-3 PUFA promoted CRC cell apoptosis, inhibited cell colony formation, invasion and PI3K/AKT/Bcl-2 signaling. Most importantly, PI3K activators inhibited the effects of Omega-3 PUFA on CRC cell proliferation and apoptosis. Our research suggests that blocking PI3K/AKT signaling by dietary Omega-3 PUFA may represent a novel therapeutic strategy for colorectal cancer in humans.
Materials and Methods
Animal Experiments
Six-week-old female Sprague Dawley rats (average weight of 150–180 g) were maintained in an air-conditioned animal facility under constant temperature and humidity with a 12 h day-night cycle and food and water ad libitum. Sixty SD female rats with normal breeding were allocated at random into two groups (30/group): the control group and the Omega-3PUFA group.
The rats of two groups received an intra-rectal instillation of MNU solution (0.5 mL, 10 mg/kg) three times a week for 4 weeks. Rats in the Omega-3PUFA intervention group were intragastrically given Omega-3PUFA (2 g.kg−1) once a day for 4 weeks (the control groups of rats were given the same amount of normal saline instead of Omega-3PUFA). All drugs (MNU and Omega-3PUFA) were diluted with physiological saline.
All procedures and animal handling were carried out according to the guidelines for the care and use of laboratory animals in China, and approved by the Animal Care and Use Committee of Guangdong Medical University.
Cell Culture and Treatment
Human CRC cell lines HCT116 and SW480 were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China). All cell lines were maintained in a humidified incubator containing 5% CO2 at 37°C in DMEM growth medium (10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin).
Primary rat colorectal cancer cells (RCCC) were isolated from the colorectal cancer tissues of previous model rats. The isolation and culture of RCCC were performed as previously described.16 Rat intestinal cancer tissues were carefully stripped, shredded (1 mm3) and washed. 10 mL of 0.1% collagenase and hyaluronidase combined digestion solution was added to the tissues and digested at 37 ° C for 25 min. After washing, the digested cells are seeded in complete culture medium, and the fibroblasts are removed by differential adherence method. Cells adhere to 80−90% and are digested and passaged.
For Omega-3PUFA treatment, HCT116, SW480 and RCCC cells were treated with 10, 20, 40 and 80 μg/mL Omega-3PUFA, and the cytotoxicity was then analyzed. 40 μg/mL doses of Omega-3PUFA was selected to treat the cells in later experiments. To promote the phosphorylation of PI3K, HCT116, SW480 and RCCC cells were pretreated with 10 μM PI3K activator (740Y-P) for 1h before exposure to Omega-3PUFA.
Cell Counting Kit-8 (CCK8) Assays
Cell viability was determined using CCK8 assay according to the manual (MedChemExpress, USA). First, HCT116, SW480 or RCCC cells were seeded at a suitable density and treated with Omega-3PUFA (10, 20, 40 and 80 µg/mL) for 24h. The CCK8 solution (10 μL) was then added to the cells and incubated for 3 h. Finally, the absorbance (450 nm) was read by a microplate reader.
Plant Clone Formation Assays
HCT116, SW480 or RCCC cells (400 cells/well) were seeded in a 6-well plate and cultured in DMEM (containing 10% FBS and 20 µg/mL Omega-3PUFA) for two weeks, and then the plate was fixed and stained with 0.1% crystal violet solution. Photographs were taken under the microscope and colonies with more than 50 cells were counted. The ability of cell clone formation was represented by the number of cell clones.
Apoptosis Detection
Cells were seeded and treated with Omega-3PUFA or Omega-3PUFA+740Y-P for 24 h. The cells were washed and re-suspended in Annexin V-APC binding buffer. The cells were then incubated with 10 μL of Annexin V-APC and 5 µL of 7-AAD for 15 min at room temperature protected from light. The apoptosis rate of the cells was measured by flow cytometry.
Histopathological Analysis and Immunohistochemistry
Tumor tissues of all rats fixed in paraformaldehyde and embedded in paraffin were sectioned to a thickness of 5 μm. Then, the tissue slides were deparaffinized, rehydrated and stained with hematoxylin and eosin (H&E).
For immunohistochemical detection of p-AKT, Bcl-2 and cleaved caspase3, the tissue slides were subjected to antigen retrieval, and the endogenous peroxidase was inactivated by treatment with H2O2 (3%). The slides were then washed and incubated with Biotin-labeled goat anti-mouse IgG, followed by staining with the DAB substrate kit.
Western Blot Analysis
Proteins collected from cell lysates were separated by SDS-PAGE and PVDF membranes. Subsequently, blots were incubated with the primary antibodies (p-PI3K, PI3K, p-AKT, AKT, BCL2, 1:300; Cell Signaling Technology, USA) and the secondary antibody (1:300; Abcam, USA). The blots were analyzed using a gel imaging system (BIO-RAD, USA). The bands grayscale values were quantified by ImageJ2X software.
Statistical Analysis
All experiments were replicated at least three times. Results are presented as mean ± SD (standard deviation). Comparisons between two groups were performed using the unpaired two-tailed t-test and between multiple groups using ANOVA followed by Dunnett’s post hoc test. For all analyses, a value of p<0.05 was considered statistically significant.
Result
Omega-3PUFA Attenuates MNU-Induced CRC in Rats
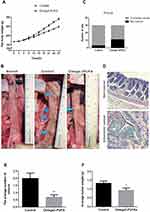
MNU-induced CRC is a well-established experimental animal model. Rats that were treated with MNU (control group) or the combination of MNU and Omega-3PUFA (Omega-3PUFA group) were evaluated. The body weight of the Omega-3 PUFA group was significantly increased from day 12 compared with the control group (Figure 1A). Compared with normal rats, the rats in the control group and the Omega-3 PUFA group showed nodular-like tumor tissue at about 2–9 cm from the anus (Figure 1B). Moreover, the incidence of colorectal cancer in the experimental group was 63.33% (19/30), which was significantly lower than that in the control group (Figure 1C). In addition, HE staining results showed that the pathology of the colorectal cancer tissues was adenoma, and the tumor cells were round or elliptical, showing invasive growth and pathological mitotic figures (Figure 1D). Notably, the average number and weight of tumors in the Omega-3 PUFA group were significantly lower than those in the control group (Figure 1E and F). These results indicate that Omega-3 PUFA inhibits MNU-induced colorectal cancer in rats.
Omega-3 PUFA Inhibits AKT/Bcl-2 Signaling in MNU-Induced Colorectal Cancer Rats
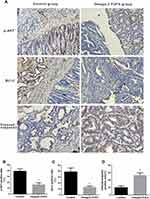
To further elucidate the molecular mechanism by which Omega-3 PUFA inhibits MNU-induced colorectal cancer in rats, we examined the expression of proteins involved in tumorigenesis and development in tumor tissues. The results of immunohistochemistry showed that the expression of p-AKT (Figure 2A and B) and Bcl-2 (Figure 2A and C) protein in the tumor tissue of the Omega-3 PUFA group was significantly lower than that of the control group. Moreover, the expression of cleaved caspase3 protein in the tumor tissue of the Omega-3 PUFA group was significantly increased (Figure 2A and D). These results show that Omega-3 PUFA inhibits AKT/Bcl-2 signaling in MNU-induced colorectal cancer rats.
Omega-3PUFA Inhibits CRC Cell Proliferation and Induces CRC Cell Apoptosis
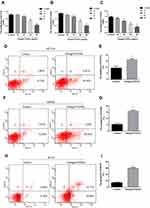
Based on the inhibitory effect of Omega-3 PUFA on MNU-induced colorectal cancer in rats, we further explored the effect of Omega-3 PUFA on colorectal cancer cells in vitro. The effect of Omega-3 PUFA on cell proliferation was observed by CCK8 analysis. The results of Figure 3A–C show that Omega-3 PUFA significantly inhibited HCT116, SW480 and RCCC cell proliferation at concentrations above 20 μg/mL. Based on this observation, the experiments in cultured HCT116, SW480 and RCCC were conducted using 40 μg/mL of Omega-3PUFA for 24 h.
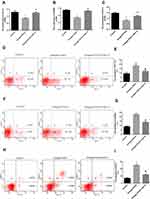
Inhibition of cell proliferation is usually caused by apoptosis. To test whether Omega-3PUFA induce apoptosis in HCT116, SW480 and RCCC cells, we examined the apoptosis rate using Annexin V-APC and 7-AAD double staining flow cytometry analysis. After 24 h of treatment, we found that Omega-3PUFA caused significant apoptosis of HCT116, SW480 and RCCC cells as compared with control group (Figure 3D–I). These results show that Omega-3PUFA inhibits CRC cell proliferation and induces CRC cell apoptosis in vitro.
Omega-3 PUFA Inhibits CRC Cell Colony Formation and Invasion
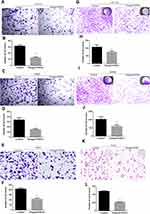
Based on the inhibitory effect of Omega-3 PUFA on the apoptosis of colorectal cancer cells, we further explored the effect of Omega-3 PUFA on colony formation and invasion of colorectal cancer cells. The results of plate clone formation assay showed that the number of clones of HCT-116, SW480 and RCCC cells in the Omega-3 PUFA group was significantly reduced compared with the control group (Figure 4A–F). In addition, the results of transwell assay showed that the number of invasion of HCT-116, SW480 and RCCC cells in the Omega-3 PUFA group was significantly lower than that in the control group (Figure 4G–L). These results show that Omega-3 PUFA inhibits CRC cell colony formation and invasion in vitro.
Omega-3PUFA Inhibits PI3K/AKT/Bcl-2 Signaling in CRC Cells
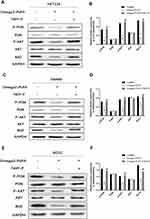
To elucidate the molecular mechanism by which Omega-3 PUFA promotes apoptosis in colorectal cancer cells, we examined the expression of apoptotic proteins in colorectal cancer after Omega-3 PUFA treatment. We investigated whether PI3K/AKT/Bcl-2 is involved in Omega-3 PUFA-induced CRC apoptosis. We observed that omega-3 PUFA induced a significant decrease in p-PI3K, p-AKT and Bcl-2 expression in HCT-116 (Figure 5A and B), SW480 (Figure 5C and D) and RCCC (Figure 5E and F) cells, while PI3K and AKT protein expression did not change significantly. In addition, 740Y-P (PI3K activator) significantly promoted protein expression of p-PI3K, p-AKT and Bcl-2 in HCT-116 (Figure 5A and B), SW480 (Figure 5C and D) and RCCC (Figure 5E and F) cells. These results suggest that Omega-3PUFA inhibits PI3K/AKT/Bcl-2 signaling in CRC cells.
Omega-3PUFA Regulates CRC Cell Proliferation and Apoptosis by Blocking PI3K-AKT-Bcl-2 Signaling
We sought to determine the role of PI3K/AKT signaling in Omega-3 PUFA-induced apoptosis in HCT116 and SW480 cells. The results of CCK8 showed that Omega-3 PUFA significantly inhibited the proliferation of HCT-116, SW480 and RCCC cells. Compared with Omega-3 PUFA group, the proliferation of HCT-116, SW480 and RCCC cells in Omega-3 PUFA +740Y-P group was significantly increased (Figure 6A–C). Flow cytometry results showed that Omega-3 PUFA significantly promoted the apoptosis of HCT-116, SW480 and RCCC cells. Compared with Omega-3 PUFA group, the apoptosis of HCT-116 and SW480 cells in Omega-3 PUFA +740Y-P group was significantly decreased (Figure 6D–I). These results suggest that Omega-3PUFA promotes CRC cell apoptosis and inhibits cell proliferation by inhibiting PI3K/AKT/Bcl-2 signaling.
Discussion
Colorectal tumor is a common malignant tumor in the digestive system, and it is the third largest tumor worldwide after lung cancer and breast cancer.17 Colorectal cancer is mostly sporadic and the pathogenesis is unclear. 70−90% of colorectal cancer is related to dietary factors. Studies have shown that diet optimization will help prevent and treat most colorectal cancers.18
Polyunsaturated fatty acids (PUFA), as one of the essential nutrients in the human body, not only provide basic energy, but also participate in the body’s anti–inflammatory, intracellular metabolic regulation, and intercellular signal transduction, which has become a hot research topic in medicine and nutrition.19–21 As an important biologically active PUFA, Omega-3PUFA has been shown to be involved in the pathogenesis of digestive system tumors, including colorectal cancer.22–24 Despite advances in elucidating the prevention and treatment of cancer by Omega-3PUFA, the molecular mechanisms by which Omega-3PUFA regulates colorectal cancer are not fully understood. Consistent with previous research, this study of animal models showed that gavage of OME significantly inhibited the development of MNU-induced colorectal cancer in rats. Our research showed that OME significantly inhibited PI3K/AKT signaling and promoted colorectal cancer cell apoptosis.
The suppression of colonic aberrant crypt foci formation has been established as a short-term assay to screen candidate compounds for chemo-preventive activity in colon carcinogenesis studies in rats.25 MNU is a direct alkylating agent that does not require metabolic activation and is therefore an effective local carcinogen.26,27 MNU-induced rat colorectal cancer models are widely used to elucidate researches involved in colorectal cancer carcinogenesis (initiation and progression) and chemo-preventive mechanisms.28 In this study, we have found that MNU-induced colorectal cancer strategies are very effective in rats because 90% of the rats (27/30) used in this study developed visible colorectal tumors induced by MNU. In addition, we found that the incidence of MNU-induced colorectal cancer in rats was significantly reduced after gavage of Omega-3PUFA. Moreover, the average tumor number and tumor weight of the rats in the Omega-3 PUFA group were significantly lower than those in the control group. These results indicate that Omega-3 PUFA inhibits MNU-induced colorectal cancer in rats.
In order to elucidate the molecular mechanism of the effect of Omega-3 PUFA on colorectal cancer in rats, we explored the effect of Omega-3 PUFA on the proliferation of colorectal cancer cell lines HCT116 and SW480 in vitro. The results showed that Omega-3 PUFA significantly inhibited cell proliferation and promoted apoptosis.
Apoptosis is a highly regulated process that contributes to the control of cell number during development and the maintenance of many adult tissues.29 The B-cell lymphoma-2 (Bcl-2) protein family contains pro-apoptotic proteins and anti-apoptotic proteins. The anti-apoptotic protein Bcl2 is localized on the outer membrane of mitochondria and plays an important role in promoting cell survival and inhibiting pro-apoptotic proteins.30 Studies have shown that Bcl2 gene damage is related to the occurrence and development of various cancers such as lung cancer, breast cancer and colorectal cancer.31–33 Caspase9 protein is a member of the cysteine-aspartic protease (caspase) family and can be activated by interacting with caspase-3. Caspase9 can be activated in the mitochondrial apoptotic pathway and becomes its active form cleaved-caspase9, which plays an important role in the execution of apoptosis.34 Studies have shown that PUFA promotes the apoptosis of colorectal cancer cells by inhibiting the expression of Bcl-2 and promoting the activation of caspase9.35 In this study, we found that Omega-3PUFA inhibits the expression of Bcl-2 in colorectal cancer in vitro and in vivo, and promotes cleaved-caspase9 expression. More importantly, we found that Omega-3PUFA significantly inhibited p-AKT protein levels in rat colorectal cancer tissues and colorectal cancer cell lines.
The invasion of tumor cells is a manifestation of the malignant behavior of tumor cells.36 Colorectal cancer cells have higher biological characteristics of invasion and metastasis. The main factor affecting the treatment effect of colorectal cancer patients is distant metastasis, and liver metastasis is the most common.37 Generally, tumor suppressor drugs can not only promote tumor cell apoptosis, but also inhibit tumor cell invasion.38,39 In this study, we found that Omega-3PUFA significantly inhibited the invasion of colorectal cancer cells. These results indicate that Omega-3 PUFA may be used for the treatment of highly malignant colorectal cancer.
Phosphatidylinositol-3-kinases (PI3K) is an intracellular phosphatidylinositol kinase and also has serine/threonine (Ser/Thr) kinase activity.40 A variety of growth factors and cell-conduction complexes, including FGF, VEGF, and insulin, can initiate the activation of PI3K.41–43 AKT is the most important signaling molecule downstream of PI3K. The activation of PI3K can further promote the phosphorylation of AKT protein.44 Activated AKT regulates cell functions, including metabolism, growth, proliferation, survival, transcription, and protein synthesis, by regulating downstream factors such as various enzymes, kinases, and transcription factors.45,46 Studies have shown that PI3K/AKT signaling plays an important role in promoting cell survival and inhibiting colorectal cancer cell apoptosis.37 In this study, we found that Omega-3 PUFA inhibits PI3K/AKT signaling and promotes colorectal cancer cell apoptosis. Moreover, AKT activators inhibited Omega-3 PUFA-induced apoptosis and down-regulation of Bcl-2 expression, supporting PI3K/AKT signaling as a key pathway for Omega-3 PUFA-activated apoptosis in CRC cells.
The expansion of this study should include molecular mechanisms that regulate PI3K/AKT signaling in cells, such as the effects on factors such as extracellular FGF, VEGF, and cell surface receptors. In addition, more in vivo effects should be considered, such as the study of a wider range of inflammatory factors in serum and intestinal tissue, as well as the effects on liver and kidney function.
In summary, we demonstrate that oral administration of Omega-3 PUFA promotes apoptosis of colorectal cancer cells, thereby inhibiting the development and progression of CRC induced by MNU. We provide evidence that PI3K/AKT signaling is involved in the potential mechanism of Omega-3 PUFA-induced apoptosis in colorectal cancer cells. Our findings are a step toward understanding the pathogenesis and treatment of CRC.
Acknowledgments
This work was supported by the Natural Science Foundation of China (grant No: NSFC 81800069) and Guangdong Natural Science Foundation (2018A030313559).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jideh B, Bourke MJ. Colorectal cancer screening reduces incidence, mortality and morbidity. Med J Aust. 2018;208(11):483–484. doi:10.5694/mja2.2018.208.issue-11
2. Mansouri D. Lower morbidity and improved outcomes in patients with screen-detected colorectal cancer. Rev Esp Enferm Dig. 2017;109(7):483–484. doi:10.17235/reed.2017.5107/2017
3. Bailon-cuadrado M, Perez-saborido B, Sanchez-gonzalez J, et al. Prognostic Nutritional Index predicts morbidity after curative surgery for colorectal cancer. Cir Esp. 2019;97(2):71–80. doi:10.1016/j.ciresp.2018.08.015
4. Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012;99(3–4):57–67. doi:10.1016/j.prostaglandins.2012.09.006
5. Altenburg JD, Siddiqui RA. Omega-3 polyunsaturated fatty acids down-modulate CXCR4 expression and function in MDA-MB-231 breast cancer cells. Mol Cancer Res. 2009;7(7):1013–1020. doi:10.1158/1541-7786.MCR-08-0385
6. Saedisomeolia A, Wood LG, Garg ML, et al. Anti-inflammatory effects of long-chain n-3 PUFA in rhinovirus-infected cultured airway epithelial cells. Br J Nutr. 2009;101(4):533–540. doi:10.1017/S0007114508025798
7. Sonestedt E, Ericson U, Gullberg B, et al. Do both heterocyclic amines and omega-6 polyunsaturated fatty acids contribute to the incidence of breast cancer in postmenopausal women of the Malmo diet and cancer cohort? Int J Cancer. 2008;123(7):1637–1643. doi:10.1002/ijc.23394
8. Jia HJ, Zhang PJ, Liu YL, et al. Relationship of serum polyunsaturated fatty acids with cytokines in colorectal cancer. World J Gastroenterol. 2016;22(8):2524–2532. doi:10.3748/wjg.v22.i8.2524
9. Serini S, Cassano R, Corsetto PA, et al. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int J Mol Sci. 2018;19(2):586. doi:10.3390/ijms19020586
10. Corsetto PA, Colombo I, Kopecka J, et al. Omega-3 long chain polyunsaturated fatty acids as sensitizing agents and multidrug resistance revertants in cancer therapy. Int J Mol Sci. 2017;18(12):2770. doi:10.3390/ijms18122770
11. Zhu S, Lin G, Song C, et al. RA and omega-3 PUFA co-treatment activates autophagy in cancer cells. Oncotarget. 2017;8(65):109135–109150. doi:10.18632/oncotarget.22629
12. Aglago EK, Huybrechts I, Murphy N, et al. Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large european cohort. Clin Gastroenterol Hepatol. 2019;18:654–666.e6.
13. Miccadei S, Masella R, Mileo AM, et al. Omega3 polyunsaturated fatty acids as immunomodulators in colorectal cancer: new potential role in adjuvant therapies. Front Immunol. 2016;7:486. doi:10.3389/fimmu.2016.00486
14. Song M, Nishihara R, Wu K, et al. Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst. 2015;107(4). doi:10.1093/jnci/djv007
15. Huang Q, Wen J, Chen G, et al. Omega-3 polyunsaturated fatty acids inhibited tumor growth via preventing the decrease of genomic DNA methylation in colorectal cancer rats. Nutr Cancer. 2016;68(1):113–119. doi:10.1080/01635581.2016.1115526
16. Huang H, Jiang Y, Wang Y, et al. miR-5100 promotes tumor growth in lung cancer by targeting Rab6. Cancer Lett. 2015;362(1):15–24. doi:10.1016/j.canlet.2015.03.004
17. Ghadban T, Reeh M, Bockhorn M, et al. Decentralized colorectal cancer care in Germany over the last decade is associated with high in-hospital morbidity and mortality. Cancer Manag Res. 2019;11:2101–2107. doi:10.2147/CMAR.S197865
18. Chen GC, Qin LQ, Lu DB, et al. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control. 2015;26(1):133–141. doi:10.1007/s10552-014-0492-1
19. Ilag LL. Are long-chain polyunsaturated fatty acids the link between the immune system and the microbiome towards modulating cancer? Medicines (Basel). 2018;5(3):E102.
20. Michalak A, Mosinska P, Fichna J. Polyunsaturated fatty acids and their derivatives: therapeutic value for inflammatory, functional gastrointestinal disorders, and colorectal cancer. Front Pharmacol. 2016;7:459. doi:10.3389/fphar.2016.00459
21. Khaddaj-mallat R, Morin C, Rousseau E. Novel n-3 PUFA monoacylglycerides of pharmacological and medicinal interest: anti-inflammatory and anti-proliferative effects. Eur J Pharmacol. 2016;792:70–77. doi:10.1016/j.ejphar.2016.10.038
22. Nindrea RD, Aryandono T, Lazuardi L, et al. Association of dietary intake ratio of n-3/n-6 polyunsaturated fatty acids with breast cancer risk in western and asian countries: a meta-analysis. Asian Pac J Cancer Prev. 2019;20(5):1321–1327. doi:10.31557/APJCP.2019.20.5.1321
23. Rahmani B, Hamedi Asl D, Naserpour Farivar T, et al. Omega-3 PUFA alters the expression level but not the methylation pattern of the WIF1 gene promoter in a pancreatic cancer cell line (MIA PaCa-2). Biochem Genet. 2019;57(4):477–486. doi:10.1007/s10528-018-9895-0
24. Luu HN, Cai H, Murff HJ, et al. A prospective study of dietary polyunsaturated fatty acids intake and lung cancer risk. Int J Cancer. 2018;143(9):2225–2237. doi:10.1002/ijc.v143.9
25. Gueraud F, Tache S, Steghens JP, et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic Biol Med. 2015;83:192–200. doi:10.1016/j.freeradbiomed.2015.02.023
26. Ahmed HH, Aglan HA, Zaazaa AM, et al. Quercetin confers tumoricidal activity through multipathway mechanisms in A N-methylnitrosourea rat model of colon cancer. Asian Pac J Cancer Prev. 2016;17(11):4991–4998. doi:10.22034/APJCP.2016.17.11.4991
27. Perse M, Cerar A, Injac R, et al. N-methylnitrosourea induced breast cancer in rat, the histopathology of the resulting tumours and its drawbacks as a model. Pathol Oncol Res. 2009;15(1):115–121. doi:10.1007/s12253-008-9117-x
28. Ahmed HH, El-abhar HS, Hassanin EAK, et al. Ginkgo biloba L. leaf extract offers multiple mechanisms in bridling N-methylnitrosourea - mediated experimental colorectal cancer. Biomed Pharmacother. 2017;95:387–393. doi:10.1016/j.biopha.2017.08.103
29. Nasehi M, Torabinejad S, Hashemi M, et al. Effect of cholestasis and NeuroAid treatment on the expression of Bax, Bcl-2, Pgc-1alpha and Tfam genes involved in apoptosis and mitochondrial biogenesis in the striatum of male rats. Metab Brain Dis. 2019;35:183–192.
30. Anantram A, Degani M. Targeting cancer’s Achilles’ heel: role of BCL-2 inhibitors in cellular senescence and apoptosis. Future Med Chem. 2019;11(17):2287–2312. doi:10.4155/fmc-2018-0366
31. Wang J, Li X. Chamaejasmine induces apoptosis in human non-small-cell lung cancer A549 cells through increasing the Bax/Bcl-2 ratio, caspase-3 and activating the Fas/FasL. Minerva Med. 2019. doi:10.23736/S0026-4806.19.06209-8
32. Liu S, Wang H, Mu J, et al. MiRNA-211 triggers an autophagy-dependent apoptosis in cervical cancer cells: regulation of Bcl-2. Naunyn Schmiedebergs Arch Pharmacol. 2019;393:359–370.
33. Chen XY, Chen Y, Qu CJ, et al. Vitamin C induces human melanoma A375 cell apoptosis via Bax- and Bcl-2-mediated mitochondrial pathways. Oncol Lett. 2019;18(4):3880–3886. doi:10.3892/ol.2019.10686
34. Qin XY, Wang YN, Liu HF, et al. Anti-cancer activities of metal-based complexes by regulating the VEGF/VEGFR2 signaling pathway and apoptosis-related factors Bcl-2, Bax, and caspase-9 to inhibit angiogenesis and induce apoptosis. Metallomics. 2019;12:92–103.
35. Zhang C, Yu H, Shen Y, et al. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch Med Sci. 2015;11(5):1081–1094. doi:10.5114/aoms.2015.54865
36. Wei Z, Lyu B, Hou D, et al. Mir-5100 mediates proliferation, migration and invasion of oral squamous cell carcinoma cells via targeting SCAI. J Invest Surg. 2019; 1–8. doi:10.1080/08941939.2019.1637484
37. Zhang JJ, Xu WR, Chen B, et al. The up-regulated lncRNA DLX6-AS1 in colorectal cancer promotes cell proliferation, invasion and migration via modulating PI3K/AKT/mTOR pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8321–8331. doi:10.26355/eurrev_201910_19143
38. Wen S, Sun L, An R, et al. A combination of Citrus reticulata peel and black tea inhibits migration and invasion of liver cancer via PI3K/AKT and MMPs signaling pathway. Mol Biol Rep. 2019;47:507–519.
39. Chen T, Yu Q, Xin L, et al. Circular RNA circC3P1 restrains kidney cancer cell activity by regulating miR-21/PTEN axis and inactivating PI3K/AKT and NF- kB pathways. J Cell Physiol. 2019;235:4001–4010.
40. Wei X, Jia Y, Lou H, et al. Targeting YAP suppresses ovarian cancer progression through regulation of the PI3K/Akt/mTOR pathway. Oncol Rep. 2019;42(6):2768–2776. doi:10.3892/or.2019.7370
41. Jia X, Wen Z, Sun Q, et al. Apatinib suppresses the proliferation and apoptosis of gastric cancer cells via the PI3K/Akt signaling pathway. J BUON. 2019;24(5):1985–1991.
42. Wu YJ, Lin SH, Din ZH, et al. Sinulariolide inhibits gastric cancer cell migration and invasion through downregulation of the EMT process and suppression of FAK/PI3K/AKT/mTOR and MAPKs signaling pathways. Mar Drugs. 2019;17(12):668. doi:10.3390/md17120668
43. Zhang XQ, Yao C, Bian WH, et al. Effects of Astragaloside IV on treatment of breast cancer cells execute possibly through regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci Nutr. 2019;7(11):3403–3413. doi:10.1002/fsn3.1154
44. Yang JH, Yu K, Si XK, et al. Liensinine inhibited gastric cancer cell growth through ROS generation and the PI3K/AKT pathway. J Cancer. 2019;10(25):6431–6438. doi:10.7150/jca.32691
45. Reddy D, Kumavath R, Ghosh P, et al. Lanatoside C induces G2/M cell cycle arrest and suppresses cancer cell growth by attenuating MAPK, Wnt, JAK-STAT, and PI3K/AKT/mTOR signaling pathways. Biomolecules. 2019;9(12):792. doi:10.3390/biom9120792
46. Zhang L, Jiang B, Zhu N, et al. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCalpha/ERK1/2 and PI3K/Akt pathway. Med Oncol. 2019;37(1):5. doi:10.1007/s12032-019-1320-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.