Back to Journals » OncoTargets and Therapy » Volume 14
Olaparib Treatment in a Patient with Advanced Gallbladder Cancer Harboring BRCA1 Mutation
Authors Li X , Gao L, Qiu M, Cao D
Received 28 January 2021
Accepted for publication 26 March 2021
Published 22 April 2021 Volume 2021:14 Pages 2815—2819
DOI https://doi.org/10.2147/OTT.S303594
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Xiaofen Li,1 Limin Gao,2 Meng Qiu,1 Dan Cao1
1Department of Abdominal Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Correspondence: Dan Cao; Meng Qiu
Department of Abdominal Oncology, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Tel +86-28-85422589
Fax +86-28-85423609
Email [email protected]; [email protected]
Abstract: Gallbladder cancer is a highly aggressive malignancy with an extremely poor prognosis. Germline BRCA1 mutation in gallbladder cancer is very rare. Herein, we present a case of a 73-year-old Asian patient diagnosed with lymph nodes and peritoneal metastases just two months after surgery for primary gallbladder cancer. The patient had a past history of early-stage breast cancer and received a left radical mastectomy 27 years ago. Next-generation sequencing (NGS) was performed as the patient refused to receive systemic chemotherapy. According to NGS result of germline BRCA1 mutation, the patient was administered first-line Olaparib and obtained a progression-free survival of about 6 months. Furthermore, we briefly summarized the current targeted treatment of gallbladder cancer. To the best of our knowledge, this is the first report of germline BRCA1 p. S451Lfs*20 mutation and first-line Olaparib treatment in a metastatic gallbladder cancer patient. As a highly heterogeneous and aggressive malignancy, we suggest making clinical decisions according to a precision medicine concept based on gene sequencing in advanced gallbladder cancer.
Keywords: gallbladder cancer, BRCA1 mutation, Olaparib
Introduction
Gallbladder cancer, the most common type of biliary tract cancers (BTCs), is a relatively rare but highly aggressive malignancy.1,2 Around the world, the incidence of gallbladder cancer varies by geography and ethnicity, with the highest incidence observed in South America.3,4 It is well known that the prognosis of gallbladder cancer is extremely poor, with a 5-year survival rate of less than 5%.2,4,5
Although with limited effectiveness, the current standard treatment for advanced BTCs is systemic chemotherapy.6 The large Phase 3 study, ABC-02 trial, demonstrated improved survival outcome with the combination of gemcitabine and cisplatin over gemcitabine alone in the first-line setting (PFS, 8.0 vs 5.0 months, p<0.001); (OS, 11.7 vs 8.1 months, p<0.001).7 In recent decades, rapid progress in gene sequencing has identified promising molecular targets in BTCs. Nearly 40% of BTC cases have potentially targetable driver gene alterations, such as FGFR, IDH1/2, PIK3CA, NTRK, ERBB2, BRAF, and so on, with ERBB2 the most frequent gene mutation in gallbladder cancer.8,9 With the rapid development of gene sequencing, molecularly oriented precision medicine has achieved great progress and clinical trials assessing agents targeting FGFR, IDH1 and so on are ongoing.9,10 Recently, pemigatinib, a small-molecule FGFR inhibitor, has been approved as the first targeted agent to treat chemotherapy-refractory advanced iCCA based on a Phase 2 study (FIGHT-202 trial).11 However, so far only data from large-scale Phase 3 trials on first-line treatment of targeted agents has been published.
Herein, we report on a female gallbladder cancer patient harboring germline BRCA1 gene mutation, who received first-line Olaparib and got an optimal prognosis.
Case Presentation
A 73-year-old Chinese female patient was admitted to our hospital in November 2019, complaining of upper abdominal pain for over 2 months. Physical examination presented mild right upper quadrant tenderness. The patient had a history of left radical mastectomy because of early-stage breast cancer 27 years ago, without postoperative treatment. And there was no special family history. Abdominal enhanced computed tomography (CT) showed gallstones and a thickened wall of gallbladder fundus, with enlargement of lymph nodes around the main portal vein. Thoracic CT suggested no obvious abnormality. After routine preoperative examinations, radical cholecystectomy and regional lymphadenectomy were performed on November 15, 2019. Postoperative pathological diagnosis confirmed moderately differentiated adenocarcinoma from the gallbladder (Figure 1), invading the whole layer, involving peripheral fibrofatty tissue and liver parenchyma, with immunohistochemistry (IHC) staining of CK7 (+), CK19 (+), MOC-31 (small partly +), CEA (+), SATB-2 (-), CDX-2 (small partly +) and MIB-1 (+, about 20%). Incisal edges of the distal common bile duct and right hepatic artery were detected for residue cancer cells. The liver incisal edge was negative. Two lymph nodes involving the para-right hepatic artery and posterior pancreatic head lymph nodes were found to be metastatic. The postoperative stage was pT4N1M0, stage IVA. After discussion with her family, the patient refused postoperative chemotherapy.
 |
Figure 1 Representative postoperative pathological images of gallbladder mass (hematoxylin and eosin stain). |
About two months after surgery, routine postoperative CT imaging on January 10, 2020 revealed peritoneal nodules and enlarged lymph nodes in the ligamentum hepatogastricum and mesentery regions, which indicated metastases. The patient refused chemotherapy again and accepted the suggestion of a gene test. Next-generation sequencing (NGS) of the operative specimen and plasma were performed and showed germline mutation (c.1352_1364del, p. S451Lfs*20) in BRCA1 and somatic mutation in TP53 (exon7 p. E258G) and MUTYH (exon13 p. E420*) genes (3D Medicines Shanghai, China). Tissue-based tumor mutational burden (TMB) was 6.15. Microsatellite status was stable (MSS) and the IHC result of programmed death-ligand 1 (PD-L1) was negative. No meaningful mutations were found in other genes, including the mismatch repair gene (MMR), ERBB2, IDH1/2 and FGFR1/2. It is worth mentioning that the patient’s family members also received gene sequencing using blood samples. As she had no siblings and her parents were dead, her only daughter and daughter’s son received gene sequencing. The results showed that her daughter has the same germline mutation of BRCA1, while her grandson harbors no germline mutation.
Given that BRCA1 mutation was proven to be a predictor of the Olaparib effect, we prescribed Olaparib 300mg every 12 hours as a first-line treatment on January 20, 2020. Unfortunately, grade 4 anemia occurred after taking Olaparib for about 1 month and was treated with a blood transfusion. The Olaparib dose was then decreased to 150mg every 12 hours and no severe adverse reaction was found. About three months after Olaparib administration, a CT scan revealed significant shrinkage of the peritoneal nodules (Figure 2), with the largest diameter decreasing from 1.58 cm to 0.98 cm, which indicated a partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.12 After taking Olaparib for 6 months, on July 31, 2020 an abdominal CT scan showed a new lesion in the remnant liver and enlarged peritoneal nodules, which meant progressed disease (PD). Considering the dosage of Olaparib was not enough, we suggested to the patient that the Olaparib dosage be reinstated at 300mg every 12 hours. But after careful discussion with her family, our patient decided to stop anti-tumor treatment and obtained a progression-free survival (PFS) of about 6 months. At the most recent follow-up, on March 8, 2021, she was alive and receiving the best supportive care at the local hospital.
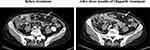 |
Figure 2 CT images of peritoneal metastasis before and after Olaparib treatment (white arrows: peritoneal metastases). |
Discussion
Biliary tract cancers (BTCs), originating from the epithelium of bile ducts and the gallbladder, are characterized by high aggressiveness, advanced disease at presentation and a poor prognosis. Based on anatomical location, BTCs are classified into intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA) and gallbladder cancer. Surgery is the only curative treatment approach for BTCs. However, most patients develop distant metastases after surgical resection, suggesting the importance of adjuvant chemotherapy.6 A meta-analysis including 10 retrospective studies showed that, compared to resection alone, adjuvant chemotherapy may prolong OS significantly (HR=0.42; 95% CI 0.22–0.80) in patients with gallbladder cancer.13 The large randomized Phase 3 trial, the BILCAP study, proved that adjuvant capecitabine was associated with improved recurrence-free survival (25.9 vs 17.4 months, HR=0.70, 95% CI 0.54–0.92, p=0.0093) and OS (53 vs 36 months, HR=0.75, 95% CI 0.58–0.97, p=0.028), compared to observation, in the per-protocol analysis.14 Thus, we suggested that our patient receive adjuvant treatment after surgery, as her postoperative stage was pT4N1M0. But our patient refused. This was one of the main reasons for distant metastases just two months after surgery.
In general, about half of BTCs have driver gene alterations, which vary considerably based on anatomical location.8,15,16 It has been reported that the most common altered genes in gallbladder cancer are TP53 (59%), CDKN2A/B (19%), ARID1A (13%) and ERBB2 (16%).15 BRCA1/2 gene mutations are very rare in gallbladder cancer. A study investigating the molecular profile of 1292 patients with BTCs showed that, in gallbladder cancer cases, BRCA1 mutated in 0.3% and BRCA2 mutated in 3.8%.17
BRCA genes are found to be directly associated with hereditary breast cancer, consisting of BRCA1 and 2.18 The proteins encoded by BRCA1/2 play an important role in the repair of double-strand DNA breaks via homologous recombination. Thus, BRCA1/2 mutated cells have defects in double-strand DNA break repair. Olaparib, an oral poly ADP-ribose polymerase (PARP) inhibitor, can effectively suppress cell repair of single-strand DNA breaks. For tumor cells with BRCA1/2 mutations, use of Olaparib leads to repair defects in both double-strand and single-strand DNA breaks, resulting in cell death. Clinical trials have shown the effectiveness of Olaparib in breast cancer, ovarian cancer and pancreatic cancer with germline BRCA1/2 mutations.19–21 In 2017, a large Phase 3 trial, the OlympiAD study, published its results in the New England Journal of Medicine, which proved a significant benefit of Olaparib monotherapy over standard therapy in patients with HER2-negative metastatic breast cancer and a germline BRCA mutation.19 Later, the SOLO1 study and the POLO study showed the significant effectiveness of Olaparib maintenance therapy among metastatic ovarian cancer and pancreatic cancer patients with a germline BRCA mutation.20,21 However, as for BRCA mutated gallbladder cancer, there is no clinical trial studying the efficacy of Olaparib. To the best of our knowledge, only one case report has been published about BRCA1 mutated gallbladder cancer treated with Olaparib.22 In that case report, by Xie et al, the patient harbored the BRCA1 Q858* mutation and obtained a PFS of 3 months after Olaparib administration. The mutation site of our patient (p. S451Lfs*20) is different from the similar published case. Besides, our patient got a better outcome, with a PFS about 6 months.
What is noteworthy is that our patient had grade 4 anemia after taking Olaparib for one month and then received a reduced dose. In the large randomized trials on Olaparib in breast cancer and pancreatic cancer, anemia was the most common grade 3 or higher adverse event, occurring in 11–16% of patients, and often leading to dose reduction.19,21 Therefore, we suggest closely monitoring complete blood count whilst taking Olaparib and giving timely treatment for anemia.
In conclusion, the patient in our case suffered highly aggressive gallbladder cancer with a germline BRCA1 mutation. She responded well to first-line Olaparib and obtained an optimal quality of living. To our knowledge, this is the first report of germline BRCA1 p. S451Lfs*20 mutation and first-line Olaparib usage in a metastatic gallbladder cancer patient. As a highly heterogeneous malignancy, we recommend gene sequencing in advanced gallbladder cancer and making treatment strategy decisions based on the precision medicine concept.
Abbreviations
NGS, next-generation sequencing; BTC, biliary tract cancer; CT, computed tomography; IHC, immunohistochemistry; TMB, tumor mutational burden; MSS, microsatellite stable; PD-L1, programmed death-ligand 1; MMR, mismatch repair gene; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressed disease; PFS, progression-free survival; iCCA, intrahepatic cholangiocarcinoma; eCCA, extrahepatic cholangiocarcinoma; OS, overall survival; HR, hazard ratio; CI, confidence interval; PARP, poly ADP-ribose polymerase.
Ethical Approval
Informed written consent was obtained from the patient for publication of this report and any accompanying images. This case report was approved by the West China Hospital, Sichuan University Ethic Committee for Clinical Investigation.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi:10.3322/canjclin.51.6.349
2. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi:10.2147/CLEP.S37357
3. Narayan RR, Creasy JM, Goldman DA, et al. Regional differences in gallbladder cancer pathogenesis: insights from a multi-institutional comparison of tumor mutations. Cancer. 2019;125(4):575–585. doi:10.1002/cncr.31850
4. Hori M, Saito E. Gallbladder cancer incidence rates in the world from the Cancer Incidence in Five Continents XI. Jpn J Clin Oncol. 2018;48(9):866–867. doi:10.1093/jjco/hyy119
5. Shukla SK, Singh G, Shahi KS, et al. Staging, treatment, and future approaches of gallbladder carcinoma. J Gastrointest Cancer. 2018;49(1):9–15. doi:10.1007/s12029-017-0036-5
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. version 5; 2020. Accessed from: www.nccn.org.
7. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi:10.1056/NEJMoa0908721
8. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi:10.1038/ng.3375
9. Chakrabarti S, Kamgar M, Mahipal A. Targeted therapies in advanced biliary tract cancer: an evolving paradigm. Cancers (Basel). 2020;12(8):2039. doi:10.3390/cancers12082039
10. Tella SH, Kommalapati A, Borad MJ, et al. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020;21(1):e29–e41. doi:10.1016/S1470-2045(19)30733-8
11. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi:10.1016/S1470-2045(20)30109-1
12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
13. Ma N, Cheng H, Qin B, Zhong R, Wang B. Adjuvant therapy in the treatment of gallbladder cancer: a meta-analysis. BMC Cancer. 2015;15:615. doi:10.1186/s12885-015-1617-y
14. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi:10.1016/S1470-2045(18)30915-X
15. Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122(24):3838–3847. doi:10.1002/cncr.30254
16. Okamura R, Kurzrock R, Mallory RJ, et al. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int J Cancer. 2020;148(3):702–712.
17. Spizzo G, Puccini A, Xiu J, et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5(3):e000682. doi:10.1136/esmoopen-2020-000682
18. Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137(2):343–350. doi:10.1016/j.ygyno.2015.02.017
19. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi:10.1056/NEJMoa1706450
20. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi:10.1056/NEJMoa1810858
21. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA -mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi:10.1056/NEJMoa1903387
22. Xie Y, Jiang Y, Yang X-B, et al. Response of BRCA1 -mutated gallbladder cancer to olaparib: a case report. World J Gastroenterol. 2016;22(46):10254–10259. doi:10.3748/wjg.v22.i46.10254
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
