Back to Journals » Clinical Ophthalmology » Volume 14
Ocular Manifestations of Sarcoidosis in a South Florida Population
Authors Laura D, Lee Y , Farhangi M, Salamo O, Mirsaeidi M , Goldhardt R , Galor A
Received 29 August 2020
Accepted for publication 9 October 2020
Published 2 November 2020 Volume 2020:14 Pages 3741—3746
DOI https://doi.org/10.2147/OPTH.S278373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Diana Laura,1,2 Yonghoon Lee,1,2 Monika Farhangi,1,2 Oriana Salamo,1 Mehdi Mirsaeidi,1,3 Raquel Goldhardt,1,2 Anat Galor1,2
1Miami Veterans Administration Medical Center, Miami, FL 33125, USA; 2Bascom Palmer Eye Institute, Department of Ophthalmology, University of Miami, Miami, FL 33136, USA; 3Department of Pulmonology, University of Miami, Miami, FL 33125, USA
Correspondence: Anat Galor
Miami Veterans Administration Medical Center, 1201 NW 16th St, Miami, FL 33125, USA
Email [email protected]
Objective: To describe the ocular manifestations of sarcoidosis in a South Florida population and identify risk factors for the presence of ocular disease.
Design: Retrospective consecutive case series.
Methods: Medical charts of individuals with sarcoidosis seen in the University of Miami pulmonary department were reviewed for ocular disease. Odds ratios were used to identify risk factors for ocular sarcoidosis.
Results: Fourteen of 108 individuals with sarcoidosis had ocular involvement. The mean age of the 14 individuals was 56± 15 years. Seventy-one percent were female, 50% were black, and 21% were Hispanic. Twelve had uveitis of which panuveitis was the most common subtype. Five had ≤ 20/70 vision in at least one eye due to uveitis. Neurosarcoidosis was a risk factor for ocular sarcoidosis (OR 6.14, p=0.03, 95% CI 1.21– 31.09).
Conclusion: Ocular manifestations occurred in a minority of individuals in a pulmonary sarcoidosis clinic in South Florida. Uveitis was the most common ocular manifestation. Neurosarcoidosis was a risk factor for ocular involvement.
Keywords: neurosarcoidosis, sarcoidosis, ocular sarcoidosis, uveitis
Introduction
Sarcoidosis is a chronic granulomatous disease that typically affects the lungs and hilar lymph nodes, though the characteristic non-necrotizing granulomas can affect all organ systems.1,2 Ocular involvement is a well-known manifestation of the disease. The reported frequency of ocular sarcoidosis is highly variable depending on the study population and whether patients are identified in uveitis or pulmonary clinics, ranging from 7% to 50% in the literature.3
A few studies have examined risk factors for the development of ocular involvement in patients with systemic sarcoidosis, with inconsistent findings. For example, one study identified male sex as a risk factor,4 while another found that women were more likely to have eye involvement.2 Race has also been examined as a risk factor with some studies reporting a higher frequency of ocular involvement in blacks compared to whites,5 while others did not find a significant difference among races.6,7 However, even in the latter studies, blacks developed ocular sarcoidosis at a significantly younger age than whites.6,7 With regards to other organ system involvement, prior studies found that posterior segment or fundus involvement was associated with a higher frequency of CNS involvement,8,9 though they did not provide statistical analysis supporting one organ involvement as an actual risk factor for the other.
The epidemiology of sarcoidosis has been less well studied in populations with a large proportion of Hispanics. For example, in studies from Chicago10 and California,6 Hispanic patients comprised only 3% and 4% of patients studied. We bridge this knowledge gap by describing the clinical characteristics of ocular sarcoidosis in a Miami population, an area where Hispanics make up approximately 65% of the population. Furthermore, we evaluate risk factors for the presence of eye disease.
Materials and Methods
Study population: A cohort of patients seen in the University of Miami (UM) Sarcoidosis Program diagnosed with systemic sarcoidosis was identified from the Registry for Advanced Sarcoidosis, an ongoing prospective study at the UM hospital. Inclusion criteria for sarcoidosis diagnosis followed guidelines established by the American Thoracic Society which included physician diagnosis supported by histopathologic evidence of non-caseating granulomas in the absence of mycobacterial infection. However, biopsy was not a requirement for diagnosis and was circumvented in certain clinical situations as deemed appropriate by the treating physician (eg, bilateral hilar lymphadenopathy apparent on computerized tomography (CT), clinical features of Löfgren’s syndrome).11,12 All individuals diagnosed with sarcoidosis at the time of chart review were included in the current study. The Sarcoidosis Program protocol states that any individual with ocular symptoms is referred to an ophthalmologist for screening.
Data extracted: All medical records were reviewed for the presence of ocular sarcoidosis, as defined in the International Criteria for the Diagnosis of Ocular Sarcoidosis established at the International Workshop on Ocular Sarcoidosis, and for other relevant information.13 The following information was extracted: age, sex, race, ethnicity, type of ocular involvement, laterality, visual acuity at final visit, duration of follow-up, base levels of Angiotensin Converting Enzyme (ACE), lysozyme, C-reactive protein (CRP), and CD4 count, history of cancer, and organs affected by sarcoidosis.
Data were summarized with descriptive statistics. Using SPSS statistics software (SPSS, Inc., Chicago, IL), odds ratios were calculated from binary logistic regression analyses and used to identify risk factors for ocular sarcoidosis.
Results
A total of one hundred eight individuals with a diagnosis of sarcoidosis were enrolled into the Registry for Advanced Sarcoidosis at the time of chart review. The mean age of this cohort was 55 years (range 24–81) and 66 (61%) were female. Forty-one (38%) individuals were black and 23 (21%) were Hispanic. Of these, 14 individuals (13%) were found to have ocular sarcoidosis. The mean age of the 14 individuals with ocular sarcoidosis was 56 years. Seventy one percent were female, 50% were black, and 21% were Hispanic. Nine of these individuals had bilateral ocular involvement, while five individuals had unilateral findings (Table 1).
 |
Table 1 Demographics and Uveitis Characteristics of the Individuals with Orbital Involvement |
Twelve patients had a history of uveitis. Nine patients had uveitis characterized as chronic, two as acute, and one that could not be further subtyped. Panuveitis was seen in four individuals and was thus the most common subtype of uveitis in our population.
Three patients had isolated anterior uveitis and three had isolated posterior uveitis. One patient had anterior-intermediate uveitis. One patient had uveitis that could not be further specified. All patients with posterior and panuveitis had choroidal involvement in the form of multifocal peripheral atrophic scars (Figure 1). Of the 10 individuals who underwent fluorescein angiography, 4 had evidence of retinal vasculitis. Anterior uveitis was significantly more frequent in Hispanics (100%) than non-Hispanics (9.1%), p=0.005 but did not vary by gender and race.
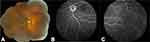 |
Figure 1 Fundus photograph (A) and fluorescein angiography (B and C) displaying punched out chorioretinal lesions in two patients with sarcoid panuveitis. |
Six individuals had structural complications associated with uveitis. Three patients had macular edema, one choroidal neovascularization (CNV), two glaucoma, and one retinal detachment. Of note, one patient had both macular edema and glaucoma. Five individuals had moderate or worse (≤20/70) vision in at least one eye due to uveitis. Two individuals had a history of biopsy-proven lacrimal gland granulomas consistent with sarcoidosis, and both maintained good vision.
Of all the factors examined in Table 2, neurosarcoidosis was the only factor found to portend a risk for ocular sarcoidosis (OR 6.14, p=0.03, 95% CI 1.21–31.09). Ocular involvement was also more frequent with ear/nose/throat (ENT) involvement, but this factor did not reach statistical significance (OR 7.67, p=0.05, 95% CI 0.99–58.57).
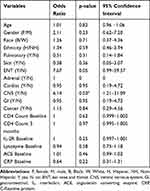 |
Table 2 Uni-Variable Logistic Regression Analysis Evaluating Risk Factors for Ocular Disease in a Cohort of Individuals with Sarcoidosis |
Discussion
This study provides data on characteristics of ocular sarcoidosis in a cohort of individuals with systemic sarcoidosis seen in a pulmonary clinic in South Florida. Ocular manifestations occurred in a minority of individuals (13%), which is consistent with prior studies. Two of the largest prospective studies of patients with biopsy-confirmed sarcoidosis in the United States reported frequencies of ocular involvement of 11.8%2 and 23%.14 However, there is a wide range of reported frequencies, likely due to the population studied, the study design, and the definition of ocular involvement. For example, the frequency of ocular involvement was lower in individuals admitted to a hospital (19%, 101 of 532)8 compared to those seen in an eye clinic (80%, 65 of 81).6 However, in the latter study, keratoconjunctivitis sicca was included in the definition of ocular involvement and was found in 25 (31%) individuals, slightly less frequently than uveitis, which was found in 33 (41%) of individuals.6 Other studies, such as a retrospective study utilizing the national Veteran Affairs (VA) database, defined eye involvement as orbital granulomas, scleritis and uveitis, and found eye disease in 8.3% of 15,130 patients with a sarcoid diagnosis (via International Classification of Disease codes), a frequency more similar to the one seen in our study.15
Uveitis was the most common ocular manifestation of sarcoidosis in our study, consistent with prior studies.3,5,6 In our series, panuveitis was the most common subtype of uveitis. Our findings are in line with a retrospective study from France of 83 individuals (predominantly white) with biopsy-proven sarcoidosis in which panuveitis was the most common uveitis sub-type (55%, 34/62 in whites and 43%, 9/21 in non-whites).7 Similarly, a study of a predominantly black cohort of 63 individuals with biopsy-proven sarcoidosis in Chicago also reported a higher frequency of panuveitis among patients with sarcoid uveitis (42%, 10/24 in whites and 54%, 21/59 in blacks).10 Other studies, however, such as a prospective study in Minnesota of a predominantly white population,3 as well as the VA study (50% black),15 found anterior uveitis to be the most common uveitis sub-type.
The second aim of this study was to evaluate risk factors for ocular sarcoidosis. Several studies have evaluated whether racial differences affect the risk of ocular sarcoidosis without a clear consensus. Some studies reported that ophthalmic disease was more common in blacks,5 while other studies, including our current one, did not find a relationship between race and ocular involvement.6 One study did find that whites had a higher frequency of posterior segment involvement compared to blacks. However, they defined posterior segment involvement broadly and included vitritis, punched out lesions, snowballs, cystoid macular edema (CME), periphlebitis, vitreous opacities, posterior vitreous detachment, and/or epiretinal membrane.16
We did not find Hispanic ethnicity to be a risk factor for ocular sarcoidosis, perhaps due to our small sample of Hispanics, but we did find a higher frequency of anterior uveitis in Hispanics versus non-Hispanics. It is important to note, however, that only three subjects with ocular sarcoidosis were Hispanic, and one of those patients had a uveitis that could not be further subtyped based on chart review, and was therefore excluded from this statistical analysis. Thus, a larger study is needed to confirm our findings.
Biological sex has also been identified as a potential risk factor for eye involvement,2,4 though reports are conflicting. A large multicenter case control study of 736 patients with biopsy-proven sarcoidosis found that women were more likely to have eye involvement,2 while a retrospective study in Louisiana of 109 patients with biopsy-proven sarcoidosis found that male sex was a risk factor for ocular sarcoidosis.4 Of note, the former study had a higher proportion of black patients than the latter. In our study, sex did not affect risk of ocular disease.
In our cohort, neurosarcoidosis was the only significant risk factor for ocular sarcoidosis. An association between central nervous system (CNS) and ocular sarcoidosis has been previously suggested in a study where 14 out of 46 (30%) individuals with neurosarcoidosis had concomitant uveitis.17 Within uveitis, posterior segment involvement is most closely related to CNS disease, as highlighted by a review of 44 cases where 14 individuals with posterior segment inflammation had CNS involvement (35%).9 Of note, the overall reported frequency of CNS disease in sarcoidosis is 2%.18 In support of this connection, of 202 individuals with biopsy-proven sarcoidosis and uveitis, CNS involvement was found in 10 of 51 individuals (20%) with posterior segment disease compared to 16 of 151 (11%) individuals without posterior segment disease.8 Several theories may explain the noted association between ocular and neurosarcoidosis. First, a similar pathogenesis may occur in both compartments with disruptions in the blood-retina and blood-brain barriers leading to autoimmune manifestations in both locations.17 Second, granulomatous infiltration may start in the optic nerve and cause secondary intraocular inflammation.19 Conversely, granulomas in the eye (eg, choroid) can cause a local inflammatory response that then spreads to the central nervous system via the optic nerve sheath.
Identification of risk factors for ocular sarcoidosis is crucial, as untreated chronic inflammation can lead to permanent vision loss. Given the aforementioned conflicting data regarding which patient population is most likely to develop ocular sarcoidosis, we recommend that all patients with systemic sarcoidosis undergo ophthalmology screening with slit lamp exam and dilated fundus exam annually, a protocol that is now being established at our center. This is especially important given that ocular involvement in sarcoidosis can be asymptomatic.20 Treatment is titrated based on the severity of ocular disease, and additional ophthalmic medications given, as necessary.
As with all studies, our findings must be considered in light of our study limitations, which include a small sample size of individuals with ocular sarcoidosis, a cross-sectional methodology to evaluate risk factors, and variable systemic treatments that may have affected the presence of ocular disease. Furthermore, only individuals with ocular symptoms were screened by an ophthalmologist so our frequency estimate may have missed individuals with asymptomatic eye involvement.
Conclusions
Despite these limitations, our study adds information on the epidemiology and risk factors for ocular sarcoidosis in a novel population. We found that a minority of patients had ocular disease and that uveitis, specifically panuveitis, was the most common manifestation. Neurosarcoidosis was the only identified risk factor for ocular sarcoidosis, supporting the association between CNS and ocular sarcoidosis established in prior studies. This risk factor should necessitate a thorough neurologic exam in patients with ocular sarcoidosis.
Ethics Statement
All protocols have obtained ethical approval from the University of Miami Institutional Review Board, IRB # 20,150,612. Informed consent was received from the study participants and the guidelines outlined in the Declaration of Helsinki were followed.
Funding
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
Disclosure
Anat Galor reports support by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH2010820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
The authors report no other potential conflicts of interest for this work.
This paper and/the abstract of this paper were presented at the American Thoracic Society Conference as a poster presentation/conference talk with interim findings. The poster’s abstract was published in “Poster Abstracts” in American Journal of Respiratory and Critical Care Medicine 2018, 197, A4805.J.
References
1. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234. doi:10.1056/NEJM199704243361706
2. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi:10.1164/ajrccm.164.10.2104046
3. Ungprasert P, Tooley AA, Crowson CS, Matteson EL, Smith WM. Clinical characteristics of ocular sarcoidosis: a population-based study 1976–2013. Ocul Immunol Inflamm. 2017;1–7.
4. Janot AC, Huscher D, Walker M, Grewal HK, Yu ML, Saketkoo LA. Cigarette smoking and male sex are independent and age concomitant risk factors for the development of ocular sarcoidosis in a new orleans sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2016;32:138–143.
5. Jabs DA, Johns CJ. Ocular involvement in chronic sarcoidosis. Am J Ophthalmol. 1986;102:297–301. doi:10.1016/0002-9394(86)90001-2
6. Evans M, Sharma O, LaBree L, Smith RE, Rao NA. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. 2007;114:325–333. doi:10.1016/j.ophtha.2006.05.074
7. Febvay C, Kodjikian L, Maucort-Boulch D, et al. Clinical features and diagnostic evaluation of 83 biopsy-proven sarcoid uveitis cases. Br J Ophthalmol. 2015;99:1372–1376. doi:10.1136/bjophthalmol-2014-306353
8. Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–655. doi:10.1016/0002-9394(78)90184-8
9. Gould H, Kaufman HE. Sarcoid of the fundus. Arch Ophthalmol. 1961;65:453–456. doi:10.1001/archopht.1961.01840020455023
10. Birnbaum AD, Oh FS, Chakrabarti A, Tessler HH, Goldstein DA. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol. 2011;129:409–413. doi:10.1001/archophthalmol.2011.52
11. Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other granulomatous disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173.
12. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183:573–581. doi:10.1164/rccm.201006-0865CI
13. Herbort CP, Rao NA, Mochizuki M; members of Scientific Committee of First International Workshop on Ocular S. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm. 2009;17:160–169. doi:10.1080/09273940902818861
14. Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–127.
15. Birnbaum AD, French DD, Mirsaeidi M, Wehrli S. Sarcoidosis in the national veteran population: association of ocular inflammation and mortality. Ophthalmology. 2015;122:934–938. doi:10.1016/j.ophtha.2015.01.003
16. Khalatbari D, Stinnett S, McCallum RM, Jaffe GJ. Demographic-related variations in posterior segment ocular sarcoidosis. Ophthalmology. 2004;111:357–362. doi:10.1016/S0161-6420(03)00793-0
17. Menezo V, Lobo A, Yeo TK, Du Bois RM, Lightman S. Ocular features in neurosarcoidosis. Ocul Immunol Inflamm. 2009;17:170–178. doi:10.1080/09273940802687812
18. Ricker W, Clark M. Sarcoidosis; a clinicopathologic review of 300 cases, including 22 autopsies. Am J Clin Pathol. 1949;19:725–749. doi:10.1093/ajcp/19.8.725
19. Matsou A, Tsaousis KT. Management of chronic ocular sarcoidosis: challenges and solutions. Clin Ophthalmol. 2018;12:519–532. doi:10.2147/OPTH.S128949
20. Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Doc Ophthalmol. 1989;72:287–296. doi:10.1007/BF00153496
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
