Back to Journals » Clinical Ophthalmology » Volume 14
Ocular Manifestations and Biometrics in Marfan’s Syndrome from Eastern Nepal
Authors Suwal R , Khadka S , Joshi P
Received 3 July 2020
Accepted for publication 10 August 2020
Published 25 August 2020 Volume 2020:14 Pages 2463—2472
DOI https://doi.org/10.2147/OPTH.S269364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Rinkal Suwal,1 Simanta Khadka,2 Purushottam Joshi3
1Department of Optometry, BP Eye Foundation, Hospital for Children, Eye, ENT and Rehabilitation Service (CHEERS), Bhaktapur, Nepal; 2Department of Vitreo-Retina, Bharatpur Eye Hospital, Bharatpur, Chitwan, Nepal; 3Department of Vitreo-Retina, Mechi Eye Hospital, Birtamod, Jhapa, Nepal
Correspondence: Simanta Khadka
Department of Vitreo-Retina, Bharatpur Eye Hospital, Bharatpur, Chitwan, Nepal
Tel +977-9841572286
Fax +977-056-523333
Email [email protected]
Purpose: To evaluate the ocular characteristics of Marfan’s syndrome (MFS) fulfilling the revised Ghent-2 nosology in Eastern Nepal.
Materials and Methods: A hospital-based observational and cross-sectional study was conducted. Ocular manifestations and biometrics were incorporated. Patients were subdivided into adults (16 years or older) and children (5– 15 years). Ocular biometric parameters consisted of values of refractive error, keratometry readings, anterior chamber depth (ACD), central corneal thickness (CCT), lens thickness (LT) and axial length (AL).
Results: A total of 34 eyes of 17 patients with MFS were included, where 32 eyes were phakic. Mean age of the study participants was 14.5 ± 9.1 years. The mean best corrected visual acuity (BCVA) of phakic eyes was 0.99 ± 0.82 LogMAR. Myopia greater than − 3 diopters (D) was present in 28/34 (82.35%) eyes. The average spherical equivalent was − 12.34 ± 8.85 D. Ectopia lentis (EL) was present in 24/32 (75%) eyes where superonasal was the most common subluxation in 10/24 (41.7%) eyes. AL was longer in adults 26.54 ± 4.42 mm compared to 25.21 ± 1.93 mm in children. Likewise, LT in adults was 4.9 ± 0.70 mm and 4.40 ± 0.59 mm in pediatric participants. Flat corneas were noted in both the groups with an average of 41.53 ± 2.21 D. The mean CCT and ACD were 524.62 ± 21.74 μm and 3.64 ± 0.80 mm, respectively. There was a negative association between the AL and the average corneal curvature (Kmed, correlation coefficient − 0.11, p=0.54).
Conclusion: Myopia is the foremost ocular involvement with significant visual disability in MFS. Though, AL and corneal curvature are not included in the revised Ghent-2 nosology, we strongly recommend these parameters to be considered during ophthalmic evaluation in suspected and diagnosed cases of MFS in the absence of genetic testing.
Keywords: corneal astigmatism, ectopia lentis, flattened cornea, Marfan’s syndrome, ocular biometry
Introduction
Marfan’s syndrome (MFS) is a heritable connective tissue disorder with clinical manifestations that involves skeletal, cardiovascular and ocular systems.1 Mutation in the fibrillin-1 gene located at chromosome 15q21.1 is the established primary defect which leads to familial cases in the majority with autosomal dominance pattern of inheritance.2,3 This condition was first reported by Antoine-Bernard Marfan,4 which carries his name and the estimated incidence of 1 to 3 among 10,000 live births.1,5
Beighton et al first proposed the clinical diagnostic criteria for MFS as “Berlin criteria”.6 This criteria was revised in Ghent, Belgium after validation of mutation in fibrillin-1 gene and named Ghent-1 nosology.7 Subsequently, this nosology was updated in 2010 to Ghent-2 nosology and it exclusively mentioned ectopia lentis (EL) and aortic root aneurysm as major criteria for diagnosing MFS.8
EL was first reported in MFS by Börger.9 About 50–80% of eyes with MFS may have EL.10 EL is mostly superotemporal. However, the direction of subluxations may be variable. Defective lenticular zonules and abnormal ciliary process are responsible for EL. Myopia is the second most common criterion in MFS, accounting for approximately 40% of the cases.11 Patients with MFS develop lenticular myopia due to spherophakia and axial myopia caused by increased axial length.12 The revised nosology has included myopia >−3 diopters (D) as a diagnostic criteria whenever EL is absent. EL is a key factor for astigmatism. Other ocular manifestations in MFS may include flattened cornea, increased globe size giving an appearance of pseudoproptosis, miotic pupil and glaucoma.8 Furthermore, degenerative pathologies like retinal detachment (RD) and early cataracts are common, however, these are not a part of the updated Ghent criteria. Significant ocular involvements are found in approximately 54% of patients,13 which helps to substantiate the diagnosis of MFS. Other typical manifestations and signs include tall stature, long fingers and toes (arachnodactyly), long limbs, flat feet, increased arm span to body height, joint flexibility, high arched palate, dental deformities, hernias, scoliosis, pectus carinatum, pectus excavatum and spontaneous pneumothorax.8,11,14 Ophthalmic clues may help in the early diagnosis of MFS, which is vital not only to restore sight, but also to save lives.
There are various series of ocular findings and biometric parameters in MFS from different parts of the globe. However, reports from Nepal are mostly limited to case reports. Hence we have conducted this study to evaluate the ocular biometric parameters in MFS visiting a tertiary eye care centre located in Eastern Nepal.
Materials and Methods
A hospital-based observational, cross-sectional study was conducted in Mechi Eye Hospital (MEH). Patients were examined from July 2016 to December 2018 where they were sent for ophthalmic evaluation as a part of interdisciplinary referral system from the nearby institutions and hospitals. Diagnosed cases of MFS fulfilling the revised Ghent-2 nosology,8 were included. Whereas other connective tissue disorders and unverified MFS were excluded. Diagnosis of MFS was made on the basis of presence of family history and EL, which was considered sufficient for positive MFS. In the absence of family history, a systemic score of more than seven points and/or echocardiographic demonstration of aortic dilatation (whenever available) and/or EL was considered a reliable indicator of MFS. Written informed consent was obtained from each participant or from a legal guardian/parent when the participant was younger than 18 years of age. The study was approved by the ethical clearance committee of Mechi Eye Hospital. All procedures and data collection were conducted in accordance to the tenets of the Declaration of Helsinki.
The examination of all the patients was carried out by a registered ophthalmologist and board certified optometrist trained in handling all the devices necessary for ocular evaluation. All examinations and measurements were conducted in the same examination session. The collected variables from the participants included, age, gender, visual acuity (VA), presenting complaint and duration of ocular symptoms. A complete ocular, medical and family history was obtained. VA was assessed by Snellen’s visual acuity chart as per the norms of International Council of Ophthalmology.15 Tumbling “E” chart was used for patients with no formal education.
All participants were subjected to slit lamp biomicroscope (Haag Streit, BQ 900, Switzerland) for comprehensive ophthalmic evaluation by an ophthalmologist. Retinal periphery was evaluated after dilatation of the pupil by indirect ophthalmoscopy and scleral depression. Tropicamide 0.8% with phenylephrine 5% (Tropicamet plus®, Sun Pharmaceuticals Ltd, Mumbai, India) was instilled for pupillary dilatation in all the patients whereas cyclopentolate 1% (Cyclopent®, Sun Pharmaceuticals Ltd, Mumbai, India) was used for pediatric participants. Uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA) was documented. For BCVA, refraction from both phakic and aphakic portion was performed and determined after subjective acceptance with tolerance for estimation of net refractive value. BCVA was determined for phakic eyes only at the time of presentation and aphakic/pseudophakic eyes were excluded. Orthoptics evaluation was carried out to determine ocular deviation. Prism cover test was utilized to quantify the deviation if present. Intraocular pressure (IOP) was measured by noncontact tonometer (Reichert 7, Reichert Technologies, NY, USA). The maximum and minimum corneal curvature values were calculated using Zeiss keratometer (Carl Zeiss Meditec, Germany). Kmed value was calculated in diopters (D) by averaging the maximum (Kmax) and minimum (Kmin) values of corneal curvature. The corneal astigmatism (Cast) was calculated as difference between Kmax and Kmin values. Estimation of central corneal thickness (CCT), axial length (AL), anterior chamber depth (ACD), lens thickness (LT) values were obtained by contact A-scan biometry (4 sight Accutome, 24–8000, PA, USA).
Refractive errors were estimated with regard to both spherical and cylindrical power and expressed in diopters (D). Spherical equivalent was calculated to determine the average refractive error. History of previous RD surgery was reviewed. History of implantation of band buckle or silicon oil instillation which could influence the AL was considered and excluded. All measurements were conducted on both eyes of the participants, except for LT in aphakic and pseudophakic patients. K value was not calculated for patients who underwent previous lens extraction surgery. Patients were divided into adults (age 16 years or older) and children (aged 5–15 years). Additionally, anthropometric measurements; height and arm span as well as other typical signs of MFS were evaluated for academic purposes.
The collected data were analyzed with statistical package for the social sciences software (SPSS v.20, IBM Corporation, Armonk, NY, USA). Categorical variables were presented in frequency and percentage. Analysis of noncategorical data was performed by inferential statistics. Pearson's correlation was used to determine the association between Kmed and AL. VA recorded with Snellen chart was expressed as logarithm of minimum angle of resolution (LogMAR) equivalent for analysis.
Results
Demographic Data
A total of 34 eyes of 17 patients (11 males and six females) of diagnosed MFS were included in this study. Our study comprised of 12 patients younger than 15 years and five patients aged 16 years and above. The mean age was 14.5 ± 9.1 years (range: 5–43 years) in this series. (Figure 1) shows the distribution of patients. Positive family history was present in 5/17 (29.4%) participants, where one member in the first generation was affected.
 |
Figure 1 Age-wise distribution of patients. |
Ocular Findings
One eye (2.9%) was aphakic at presentation following surgery elsewhere, 1/34 (2.9%) eye was pseudophakic who underwent lens extraction with anterior chamber intraocular lens (ACIOL) in our eye care center in the past. Similarly, 32/34 (91.2%) eyes were phakic at the time of examination. Seven out of 34 eyes (20.6%) of four patients had divergent strabismus where alternate exotropia was present in two patients, exophoria in one patient and left exotropia in one patient respectively. Comparison of the biometric parameters between children and adult participants were depicted in (Table 1). However, there was no statistically significant difference between the groups in the ocular biometric parameters.
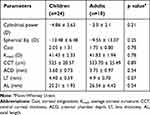 |
Table 1 Ocular Biometric Parameters of Children and Adult Participants with MFS |
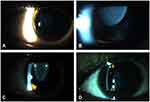
The Cast and Kmed value was not obtained in two eyes which has undergone previous lens extraction surgery, similarly AL estimation was excluded in one eye which had previous history of implantation of encircling band buckle. EL was present in 24/32 (75%) of the phakic eyes. Superonasal was the commonest subluxation which was present in 10/24 (41.7%) eyes followed by superotemporal in 5/24 (20.8%) eyes (Table 2) (Figure 2). Two eyes which were aphakic and pseudophakic at presentation were excluded, as the history of previous EL could not be established. EL was not present in both eyes of a single patient, however high myopia of ≥ −11 D was present in both eyes which fulfilled the major criteria of Ghent-2 nosology. A single eye had previous history of RD surgery done in our center. The incidence of RD at presentation in our series of MFS accounted for 3/34 (8.82%) eyes. In addition, 4/34 (11.8%) had significant lenticular opacification. These patients were referred to department of vitreo-retina for further management.
 |
Table 2 Direction of Lens Subluxation in Eyes of MFS Patients (n=24 Eyes) |
The mean UCVA of the 34 eyes of the study participant was 1.46 ± 0.72. Similarly, the mean BCVA of 32 eyes 0.99 ± 0.82. The distribution of UCVA and BCVA is presented in (Figure 3).
 |
Figure 3 Distribution of UCVA and BCVA in the total participants. Percentage is mentioned in the parentheses. |
The mean cylindrical power of the total eyes was −4.18 ± 3.23 D (−1 to −12 D) where cylindrical power was estimated in 22/34 (64.7%) eyes. The cylindrical power could not be estimated in remaining 12 eyes due to abnormal scissors reflex. The average spherical equivalent was estimated in 31/34 (91.2%) eyes which was −12.34 ± 8.85 D (+6 to −26.50 D) and estimation of refractive error in the remaining three eyes could not be performed due to absence of appreciable glow in retinoscopy. The average value of Cast was 1.97 ± 1.20 (0.50–5.75). The calculated mean value of CCT, ACD, and LT was 524.62 ± 21.74 μm (486–557 μm), 3.64 ± 0.80 mm (1.96–5.04 mm) and 4.53 ± 0.65 mm (3.45–6.06 mm) respectively. The overall Kmed value of the study participants was found to be 41.53 ± 2.21 D (34.39–44.17 D) and the mean AL of the study group was 25.61 ± 2.90 mm (21.61–31.88 mm). There was a negative association between the AL and the Kmed (correlation coefficient −0.11, p=0.54) (Figure 4). Other typical features in our patients with MFS are shown in (Figure 5).
 |
Figure 4 Linear correlation plot for Kmed and AL. |
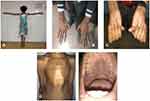 |
Figure 5 Typical features of MFS. (A) Tall stature with long arm span length. (B) Arachnodactyly. (C) Positive Steinberg’s sign. (D) Pectus carinatum. (E) High arched palate. |
Discussion
In this observational study, we have included ocular manifestations and biometric parameters of 34 eyes of 17 patients. To the best of our knowledge, this could be the foremost study to be reported from Nepal including a series of patient with MFS according to the Ghent-2 criteria.
The participants included in our study belonged to younger age group with an average age of 14.5 ± 9.1 years (5–43 years). The family history was traced positive in 5/17 (29.4%) patients where a family member in the first generation had similar symptoms. Sporadic cases of MFS were reported to occur in more than (25%) cases.16 Lack of genetic registry and inaccessible genetic screening test could have led to under reporting of this heritable condition in low resource setup like ours.
Strabismus was present in 7/34 (20.6%) eyes of the patients in our series. Similarly, Konradsen and Zetterstrom had reported 9% strabismus as a presenting ocular finding in MFS.17 The average BCVA was 0.10 ± 0.32 LogMAR as reported by Drolsum et al,18 0.13 ± 0.25 LogMAR by Gehle et al,19 along with 0.3 LogMAR stated by Konradsen and Zetterstrom.17 The BCVA in our cohort of MFS was relatively less with an average of 0.99 ± 0.82 LogMAR. The VA was severely deteriorated in four eyes due to RD which have contributed to low average BCVA. In our case, there were 2/17 (11.76%) cases with mild visual impairment, 4/17 (23.52%) patients with moderate visual impairment, four patients (23.52%) with severe visual impairment and one patient (5.88%) with blindness which were classified according to ICD-11 classification of visual impairment.20 In our case, the plausible explanation for mild to severe visual impairment could be attributed to subluxation of the lens and the primary reason for blindness was RD. The other explanation for decreased VA is amblyopia. Many studies have also reported similar findings of decreased VA in MFS.17,18,21 Amblyopia and reduced visuomotor coordination are the identified risk factors for visual maldevelopment in MFS.22,23 Hence proper refraction, glass prescription, surgery and vision therapy are recommended for patients with MFS for visual rehabilitation.
The estimation of LT was possible in all the eyes except for pseudophakic and aphakic eyes which were excluded. The minimum age of the enrolled patient was five years, relative cooperation from the patient and the guardian/parent during biometry made the reading possible. The Average LT in children and adults group were 4.40 ± 0.59 mm and 4.9 ± 0.70 mm respectively. LT was comparatively higher in adults which might be due to increased lenticular opacification in this group as well as spherical shape of the lens. The onset of nuclear sclerosis was found 10 to 20 years earlier compared to other healthy individuals of the same age group.24 Studies have also revealed an excellent visual outcome without any complications with scleral fixated intraocular lens implantation in eyes with MFS.25 AL was also found longer in adults than children. However, difference in biometrics values (LT and AL) of children and adults with MFS were not statistically significant. Available literature had shown statistically significant difference between these two groups where longer AL and thicker LT was present in adults compared to children.26
The major criteria of the nosology includes EL other than cardiovascular anomaly.8 Nontraumatic etiology of EL,27 either unilateral or bilateral is the major ocular finding of MFS.7,11,28 In our study, 24/32 (75%) of phakic patients had EL. The most common direction of subluxation of lens was superonasal 10/24 (41.7%). Maumenee had reported (77%) superotemporal subluxation to be the commonest in her study.29 In contrast, superior subluxation was also found as a common occurrence (79.6%).19
Spherical equivalent depends upon both corneal curvature and AL. Hyperopic shift is the result of flatter cornea,30 whereas myopic shift is caused by long AL.31 Position of the lens in terms of tilting as well as subluxation could also induce both myopia and astigmatism.17 In our study, 28/34 (82.35%) eyes had myopia greater than −3.00 D (Ghent-2 nosology in the absence of EL). Lens related astigmatism, with a minimum difference of one D in subjective refraction and Cast were found in 15/34 (44.11%) eyes. Furthermore, Cast was slightly higher in our series than the study by Konradsen et al, where they have reported higher Cast than controls with mean of 1.6 D.32 The same defect that affects the zonules is hypothesized to affect the connective tissues within the cornea resulting in increased Cast.32,33 With the rule astigmatism (WTR) was the commonest astigmatic type in 13/34 (38.23%) eyes which is due to vertical steepening of the cornea. In accordance with our study, Konradsen et al also reported WTR to be most common type of astigmatism.32
Despite longer AL, ACD was decreased which is evident in other studies as well.19,34 Mean AL in our series was 25.61 ± 2.90 mm which slightly differs from previously reported AL in MFS by Setala et al (25 mm with EL),35 Drolsum et al (24.80 mm),18 Konradsen and Zetterstrom (24.73 mm)17 and Maumenee (24.65 mm).29 Similarly, Kmed value was comparable with the study by Drolsum et al (41.8 D),18 Maumenee (41.38 D),29 Sultan et al (41.4 D),36 and Gehle et al (41.78).19 These findings validate a flatter cornea in MFS where the mean Kmed estimated was 41.53 ± 2.21 D compared to normal reference Kmed of 43.5 D.37,38 Since, EL may not present in all cases of MFS, these corneal signs of flatter corneal curvature and increased Cast could be considered positive ocular signs for MFS. In our study, CCT was within normal range with an average of 524.62 ± 21.74 μm, which was analogous to recent studies performed by Gehle et al,19 and Kinori et al.26 In contrast, thinner corneas have also been reported in MFS.21,32,36 However CCT is not favored as an important ocular clue for diagnosis.26 A negative association was established between the AL and the Kmed with correlation coefficient of −0.11 in our study. In agreement similar negative association was reported between AL and Kmed, with correlation coefficient of −0.33.26 Thus the patient with MFS with longer AL might have flat corneal curvature and these parameters can also be correlated with EL.26
Megalocornea was not noticed in our participants which is uncommon in MFS. However, some literature have reported this rare entity but without additional evidence suggestive of glaucoma.39,40 Other possible anterior segment findings in MFS includes miotic and poorly dilating pupil, iris abnormalities like transillumination defects, and hypoplastic stroma.29,41 Studies have also revealed secondary glaucoma and phacolytic glaucoma.18,29,42,43 Posterior staphyloma as a posterior segment manifestation in MFS have also been reported,44 however, it is another rarity.29,41 RD at presentation in our study was found in 3/34 (8.82%) eyes. This is proportionate to the study where the reported incidence of RD varies from (8%)29 to as high as (12%).28 The risk factor for RD might be due to traction on the vitreous base induced by subluxated lens, which leads to tears and holes on the peripheral retina.23 We had a younger average age group in our series, nonetheless, the reported average age for RD is 33 years with earlier onset in females and more frequent after lens surgery.45 With recent advancements in techniques of RD surgery, successful surgical and visual outcomes have been reported.46–48
This heritable connective tissue disorder originated by malformation of a single gene on chromosome 15q. Mortality in MFS was found to be 70% in 1972 and 48% in 1995 accounted mostly due to aortic complication.22 Studies have reported increase in life expectancy with mean age of 32 years (1972) to 45 years (1998) with advancement in medical and surgical techniques.22 Cardiovascular features are the significant life-compromising issues in MFS as aortic aneurysm/dissection, mitral valve prolapse are common.49 Cardiovascular features are often later signs than that of ocular features.50 Hence ophthalmologists may be the first to suspect this entity. Visual disability might further compromise the quality of life in this vulnerable group. Hence, interdisciplinary approach is mandatory for diagnosis and management of MFS, which can not only prolong the survival, but also enhance the standard of living. Although, we had limited numbers of participants, our study is compliant with other similar reported literature in ocular characteristics in MFS.
Limitations and Recommendations
Limitations for this study are relatively small numbers of patients, noncomparison with similar age and gender matched control groups, lack of incorporation of genetic testing for fibrillin-1 gene which is primarily due to unavailability of the services, cross-sectional nature of the study. The outcomes of lens extraction and retinal surgery under the vitreo-retina department is not included, which we intend to report separately in future. Prospective study with long-term follow-up of these patients would add more insight into their ocular as well as general physical status.
Conclusion
Myopia is the foremost ocular involvement with significant visual disability in MFS. Early detection of MFS through multidisciplinary approach is necessary. Longer AL with flat cornea can be an additional ocular findings for establishing the diagnosis of MFS. Though, AL and corneal curvature are not included in the revised Ghent-2 nosology; we strongly recommend these parameters to be considered during ophthalmic evaluations in suspected and diagnosed cases of MFS whenever genetic study is not available.
Abbreviations
MFS, Marfan’s syndrome; EL, ectopia lentis; D, diopters; RD, retinal detachment; VA, visual acuity; UCVA, uncorrected visual acuity; BCVA, best corrected visual acuity; IOP, intraocular pressure; Kmed, average corneal curvature; Kmax, maximum values of corneal curvature; Kmin, minimum values of corneal curvature; Cast, corneal astigmatism; CCT, central corneal thickness; AL, axial length; ACD, anterior chamber depth; LT, lens thickness; LogMAR, logarithm of minimum angle of resolution; ACIOL, anterior chamber intraocular lens; WTR, with the rule astigmatism.
Ethics
The confidentiality of the study participants was ensured. Consent was obtained from the parent/legal guardian to publish the photograph depicted in Figure 5.
Acknowledgment
The authors wish to thank all the patients and their respective guardians/parents for taking part in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366(9501):1965–1976. doi:10.1016/S0140-6736(05)67789-6
2. Dietz HC, Cutting CR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337–339. doi:10.1038/352337a0
3. Dietz HC, Pyeritz RE, Hall BD, et al. The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21. 3. Genomics. 1991;9(2):355–361. doi:10.1016/0888-7543(91)90264-F
4. Marfan AB. Un cas de deformation congenitale des quatres membres plus prononcee aux extremities characterisee par lallongement des os avec un certain degre d amincissement. Bull Mem Soc Med Hop Paris. 1896;13:220.
5. Gray J, Bridges A, Faed M, et al. Ascertainment and severity of Marfan syndrome in a Scottish population. J Med Genet. 1994;31(1):51–54. doi:10.1136/jmg.31.1.51
6. Beighton P, De Paepe A, Danks D, et al. International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet. 1988;29(3):581–594. doi:10.1002/ajmg.1320290316
7. De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62(4):417–426. doi:10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R
8. Loeys BL, Dietz HC, Braverman AC, et al. The revised ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. doi:10.1136/jmg.2009.072785
9. Börger F. Über zwei Fälle von Arachnodaktylie. Z Kinderheilk. 1915;12(2–3):161. doi:10.1007/BF02222690
10. Nelson SER. Ocular Manifestations of Autosomal Dominant Systemic Conditions. Duane’s Clinical Ophthalmology on CD-Rom. Vol. 3. Philadelphia: Lippincott Williams & Wilkins; 2006.
11. Nemet AY, Assia EI, Apple DJ, Barequet IS. Current concepts of ocular manifestations in Marfan syndrome. Surv Ophthalmol. 2006;51(6):561–575. doi:10.1016/j.survophthal.2006.08.008
12. Maumenee IH. The Marfan syndrome is caused by a point mutation in the fibrillin gene. Arch Ophthalmol. 1992;110(4):472–473. doi:10.1001/archopht.1992.01080160050027
13. Faivre L, Collod-Beroud G, Loeys B, et al. Effect of mutation type and location on clinical outcome in 1013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet. 2007;81(3):454–466. doi:10.1086/520125
14. Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979;300(14):772–777. doi:10.1056/NEJM197904053001406
15. Universale CO. Visual acuity measurement standard. Ital J. 1984;1–39.
16. Collod-Béroud G, Boileau C. Marfan syndrome in the third millennium. Eur J Med Genet. 2002;10(11):673–681. doi:10.1038/sj.ejhg.5200876
17. Konradsen TR, Zetterström C. A descriptive study of ocular characteristics in Marfan syndrome. Acta Ophthalmol. 2013;91(8):751–755. doi:10.1111/aos.12068
18. Drolsum L, Rand‐Hendriksen S, Paus B, Geiran OR, Semb SO. Ocular findings in 87 adults with ghent‐1 verified Marfan syndrome. Acta Ophthalmol. 2015;93(1):46–53. doi:10.1111/aos.12448
19. Gehle P, Goergen B, Pilger D, Ruokonen P, Robinson PN, Salchow DJ. Biometric and structural ocular manifestations of Marfan syndrome. PLoS One. 2017;12(9):e0183370. doi:10.1371/journal.pone.0183370
20. World Health Organization. Blindness and vision impairment. World Health Organization: Geneva, Switzerland. Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment.
21. Kara N, Bozkurt E, Baz O, et al. Corneal biomechanical properties and intraocular pressure measurement in Marfan patients. J Cataract Refract Surg. 2012;38(2):309–314. doi:10.1016/j.jcrs.2011.08.036
22. Dean JC. Marfan syndrome: clinical diagnosis and management. Eur J Med Genet. 2007;15(7):724–733. doi:10.1038/sj.ejhg.5201851
23. Remulla JF, Tolentino FI. Retinal detachment in Marfan’s syndrome. Int Ophthalmol Clin. 2001;41(4):235–240. doi:10.1097/00004397-200110000-00021
24. Traboulsi E, Maumenee I. Subluxation of the crystalline lens and associated systemic disease. Oxf Monogr Med Genet. 1998;36(1):605–628.
25. Konradsen T, Kugelberg M, Zetterström C. Visual outcomes and complications in surgery for ectopia lentis in children. J Cataract Refract Surg. 2007;33(5):819–824. doi:10.1016/j.jcrs.2007.01.032
26. Kinori M, Wehrli S, Kassem IS, Azar NF, Maumenee IH, Mets MB. Biometry characteristics in adults and children with Marfan syndrome: from the Marfan eye consortium of Chicago. Am J Ophthalmol. 2017;177:144–149. doi:10.1016/j.ajo.2017.02.022
27. Jarett W. Dislocation of the lens. Arch Ophthalmol. 1967;78(3):289–296. doi:10.1001/archopht.1967.00980030291006
28. Cross HE, Jensen AD. Ocular manifestations in the Marfan syndrome and homocystinuria. Am J Ophthalmol. 1973;75(3):405–420. doi:10.1016/0002-9394(73)91149-5
29. Maumenee IH. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684.
30. Sheridan M, Douthwaite WA. Corneal asphericity and refractive error. Ophthalmic Physiol Opt. 1989;9(3):235–238. doi:10.1111/j.1475-1313.1989.tb00899.x
31. Grosvenor T, Scott R. Role of the axial length/corneal radius ratio in determining the refractive state of the eye. Optom Vis Sci. 1994;71(9):573–579. doi:10.1097/00006324-199409000-00005
32. Konradsen TR, Koivula A, Kugelberg M, Zetterström C. Corneal curvature, pachymetry, and endothelial cell density in Marfan syndrome. Acta Ophthalmol. 2012;90(4):375–379. doi:10.1111/j.1755-3768.2010.01996.x
33. Heur M, Costin B, Crowe S, et al. The value of keratometry and central corneal thickness measurements in the clinical diagnosis of Marfan syndrome. Am J Ophthalmol. 2008;145(6):997–1001. doi:10.1016/j.ajo.2008.01.028
34. Konradsen TR, Koivula A, Kugelberg M, Zetterström C. Accommodation measured with optical coherence tomography in patients with Marfan’s syndrome. Ophthalmology. 2009;116(7):1343–1348. doi:10.1016/j.ophtha.2009.01.023
35. Setälä K, Ruusuvaara P, Karjalainen K. Corneal endothelium in Marfan syndrome A clinical and specular microscopic study. Acta Ophthalmol. 1988;66(3):334–340. doi:10.1111/j.1755-3768.1988.tb04606.x
36. Sultan G, Baudouin C, Auzerie O, De Saint Jean M, Goldschild M, Pisella P-J. Cornea in Marfan disease: orbscan and in vivo confocal microscopy analysis. Invest Ophthalmol Vis Sci. 2002;43(6):1757–1764.
37. Fledelius HC, Stubgaard M. Changes in refraction and corneal curvature during growth and adult life: a cross‐sectional study. Acta Ophthalmol. 1986;64(5):487–491. doi:10.1111/j.1755-3768.1986.tb06959.x
38. Olsen T, Arnarsson A, Sasaki H, Sasaki K, Jonasson F. On the ocular refractive components: the Reykjavik eye study. Acta Ophthalmol Scand. 2007;85(4):361–366. doi:10.1111/j.1600-0420.2006.00847.x
39. Bastola P. Marfan syndrome: case series from a single family. Nepal J Med Sci. 2012;1(2):132–134. doi:10.3126/njms.v1i2.6614
40. Consul B, Mehrotra A, Mathur G. Marfan’s syndrome with bilateral megalocornea and subluxated cataractous lenses. Indian J Ophthalmol. 1966;14(6):262.
41. Allen R, Straatsma B, Apt L, Hall M. Ocular manifestations of the Marfan syndrome. Trans Am Acad Ophthalmol Otolaryngol. 1967;71(1):18–38.
42. Latasiewicz M, Fontecilla C, Millá E, Sánchez A. Marfan syndrome: ocular findings and novel mutations—in pursuit of genotype–phenotype associations. Can J Ophthalmol. 2016;51(2):113–118. doi:10.1016/j.jcjo.2015.12.019
43. Rand-Hendriksen S, Lundby R, Tjeldhorn L, et al. Prevalence data on all ghent features in a cross-sectional study of 87 adults with proven Marfan syndrome. Eur J Med Genet. 2009;17(10):1222–1230. doi:10.1038/ejhg.2009.30
44. Tolentino F, Schepens C, Freeman H. Systemic conditions with vitreoretinal degeneration. In: Vitreoretinal Disorders: Diagnosis and Management. Philadelphia: Saunders; 1976:269–289.
45. Chandra A, Ekwalla V, Child A, Charteris D. Prevalence of ectopia lentis and retinal detachment in Marfan syndrome. Acta Ophthalmol. 2014;92(1):e82–e83. doi:10.1111/aos.12175
46. Lee S-Y, Ang C-L. Results of retinal detachment surgery in Marfan syndrome in Asians. Retina. 2003;23(1):24–29. doi:10.1097/00006982-200302000-00004
47. Sharma T, Gopal L, Shanmugam MP, et al. Retinal detachment in Marfan syndrome: clinical characteristics and surgical outcome. Retina. 2002;22(4):423–428. doi:10.1097/00006982-200208000-00005
48. Shrestha C, Shrestha V. Marfan syndrome with bilateral retinal detachment. Med J Shree Birendra Hosp. 2014;13(2):52–54. doi:10.3126/mjsbh.v13i2.13118
49. Robinson PN, Arteaga-Solis E, Baldock C, et al. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43(10):769–787. doi:10.1136/jmg.2005.039669
50. Stanković-Babić G, Vujanović M, Đorđević-Jocić J, Cekić S. Ocular features of Marfan syndrome. FU Med Biol. 2008;15(1):37–40.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.