Back to Journals » Infection and Drug Resistance » Volume 15
Ocular Lesions in Brucella Infection: A Review of the Literature
Authors Ma C , Li H, Lu S, Li X , Wang S, Wang W
Received 22 October 2022
Accepted for publication 14 December 2022
Published 22 December 2022 Volume 2022:15 Pages 7601—7617
DOI https://doi.org/10.2147/IDR.S394497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chao Ma,1,* Haoyu Li,2,3,* Shuwen Lu,4 Xian Li,5,6 Shuai Wang,1 Wenzhan Wang1
1Department of Ophthalmology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 2Department of Ophthalmology, the Second Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China; 3Hunan Clinical Research Centre of Ophthalmic Disease, Changsha, Hunan, People’s Republic of China; 4Department of Ophthalmology, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, People’s Republic of China; 5Manchester Royal Eye Hospital, Manchester University NHS Foundation Trust, Manchester, England; 6School of Pharmacy and Optometry, Faculty of Biology, Medicine and Health, the University of Manchester, Manchester, England
*These authors contributed equally to this work
Correspondence: Wenzhan Wang, Department of Ophthalmology, the First Affiliated Hospital of Zhengzhou University, 1 Jianshe East Road, Zhengzhou, Henan, 450052, People’s Republic of China, Tel +86 371-66278091, Email [email protected]
Abstract: Ocular lesions due to Brucella infection are uncommon and easily overlooked in clinical management, but must be differentiated from non-infectious eye diseases and treated promptly to protect the patient’s vision. We reviewed the relevant literature and identified 47 patients with ocular complications of Brucella infection. Among them, 28 showed ocular neuropathy, 15 presented with uveitis, and four patients displayed other ocular symptoms. Ocular symptoms accompanying Brucella infection require prompt diagnosis and treatment. The main methods of diagnosis are intraocular fluid tests and blood tests. Early diagnosis and treatment with suitable antibiotics are central to protecting the patient’s vision. Notably, in terms of mechanism of injury, Brucella infection is chronic and cannot be eliminated by phagocytes, and can cause damage to the eye by inducing autoimmune reactions, antigen-antibody complex production, release of endogenous and exogenous toxins, and bacterial production of septic thrombi in the tissues. In this review, we summarize the ocular symptoms, diagnosis, treatment and prognosis of Brucella infection, and discuss the mechanisms of Brucella in ocular lesions, providing a reference for the diagnosis and treatment of Brucella ocular lesions.
Keywords: Brucella, infection mechanism, ocular, ocular neuropathy, uveitis
Introduction
Brucellosis is a zoonotic disease that can be transmitted from animals to humans by direct or indirect contact with infected animals or their products, for example, through ingestion of raw milk or other unpasteurised milk products.1–3 Other common routes of infection in humans include local cuts and abrasions, infection via the conjunctival sac of the eye, or inhalation of aerosols.4 Brucellosis is caused by pathogenic bacteria of the genus Brucella, classically described as six species, four of which are recognised as zoonotic: Brucella melitensis, Brucella abortus, Brucella canis and Brucella suis. Most cases of brucellosis are associated with B. melitensis, which is due to the presence of many virulence-associated factors.5,6 In humans infected with this Gram-negative bacterium, the disease may cause a wide range of symptoms in the nervous system, musculoskeletal system and heart, ranging from mild flu-like symptoms to systemic manifestations with severe complications. The clinical signs and symptoms of brucellosis are not clearly specific and the diagnosis relies on a combination of clinical manifestations such as fever, epidemiological and serological findings.7 The use of anterior chamber water to detect Brucella antibodies was used earlier, but there may be cases where the anterior chamber water dose is not sufficient for detection.8 These can be combined with ocular symptoms, which may include ocular uveitis, optic papilloedema and keratitis.9 In this study, we reviewed patients with Brucella infection presenting with ocular symptoms, to investigate the pathogenesis of Brucella spp. and the possible mechanisms of action relating to ocular infection. This review is also intended to serve as a reference for the management of ocular lesions occurring with Brucella infection.
Literature Review
We searched PubMed for articles related to Brucella infection-caused ocular lesions. The search formula used was: (((((((((((papilledema) OR (keratitis)) OR (neuritis)) OR (uveitis)) OR (optic neuritis)) OR (Endophthalmitis)) OR (eye)) OR (ocular)) OR (ophthal*)) OR (eyeball)) OR (optic nerve)) AND (brucell*). We included only reports from 1950–2022, excluding those not written in English. Patients were included in the review if Brucella infection was found at the time of diagnosis in combination with ocular symptoms. The following data were collected: age, sex, site of ocular Brucella infection, systemic infection, treatment modality and treatment outcome.
Results and Discussion
The initial search returned 286 records, and a total of 87 articles met our inclusion criteria after screening by title, abstract and article content. We thereby identified 47 patients with ocular lesions associated with Brucella infection (Table 1). The average age of the patients at the time of presentation was 27.9 years. There were 28 cases of ocular neuropathy, 15 cases of uveitis, and four cases of other ocular lesions.
 |  |  |  |
Table 1 Summary of 47 Patients with Brucella Infection Causes Eye Disease |
B. melitensis is a Gram-negative bacillus and an intracellular bacterium that accounts for approximately 70% of Brucella infections.57 The most common symptoms of B. melitensis infection in adults are arthralgia, fever, malaise, sweating, lethargy, myalgia and tremor.58,59 The most frequent ocular complications associated with brucellosis are optic neuropathy, uveitis, cataracts, vitreous lesions, ocular atrophy, macular degeneration, glaucoma, retinal neovascularisation and retinal detachment by retraction.60,61 Other, less frequent, complications include dacryocystitis and lacrimal ductitis.62 Ocular uveitis caused by Brucella infection most often manifests as posterior uveitis, followed by total uveitis.44 In this review, by exploring the relevant literature, we will describe the infectious process and consider possible mechanisms associated with ocular pathogenesis in brucellosis.
From the risk factors of the patients included in this study, the majority of patients had a clear history of exposure, such as consumption of unpasteurized milk and cheese products, a history of working in animal husbandry and a history of travel to areas with developed animal husbandry, and only a few patients had no clear history of exposure, but the patients themselves lived in infected areas. Brucella infection is associated with exposure to diseased animals and consumption of unpasteurized milk and cheese products, and veterinarians, laboratory workers, slaughterhouse workers and farmers are at higher risk of developing the disease compared to the normal population.27,63 Therefore, the patient’s past history is also an important reference for the diagnosis of Brucella infection.
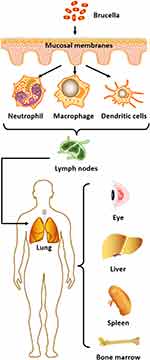
Brucella infection generally occurs through either direct contact with infected animals, or contact with the secretions of infected animals. Although some studies have shown that Brucella can invade the respiratory tract and the oral, conjunctival, lacrimal, vaginal and foreskin mucosa, the exact mechanism of epithelial cell invasion in these tissues is currently unknown.64 Brucella infection can be divided into three stages. First, the pathogen invades the host within two days of infection. In the second stage, known as the acute phase of the infection, the pathogen replicates in the reticuloendothelium and in different organs of the reproductive system, usually between two days to three weeks post-infection. In the third stage, known as the chronic phase, the pathology varies in different tissues and can last from six months to one year or more.65,66
The main pathogenic mechanism of B. melitensis is invasion of host cells, which relies on a variety of virulence factors released by the bacterium, enabling the pathogen to evade clearance by the host’s immune defences. Thus, B. melitensis can survive, replicate and proliferate inside host cells, then enter organs and tissues through macrophages to form foci of infection, or migratory foci.67 For example, B. abortus is naturally resistant to killing by neutrophils, macrophages and dendritic cells.68,69 When brucellae enter the body, these are the major phagocytic cell types that engulf and internalise the invading bacteria, and together, they constitute the primary host cells for Brucella replication.70 Internalisation results in intracellular Brucella-containing vesicles that interact with components of the endocytic and secretory pathways, such as early endosomes and vacuoles, which intersect with bacterial replication inside the endoplasmic reticulum. In this process, the bacteria avoid fusion with lysosomes, not only prolonging the survival of macrophages but also promoting dendritic cell maturation.71,72 Brucella ends its intracellular cycle by recruiting components of the autophagic apparatus to the vacuole, to complete the replication process via bacterial egress and enable infection of neutrophils and other cells.73,74
Once Brucella enters neutrophils, it survives in the phagosome and persists for a period of time, resisting the bactericidal activity of these leukocytes.75 After infecting immune cells, the pathogen will next move to the regional lymph nodes, but the infected host will not yet show signs of disease.76 Finally, Brucella spreads through the systemic lymphatic circulation to different organs of the reticuloendothelial system, including the lungs, spleen, liver, bone marrow, and even the eyes.77
In summary, pathogenic brucellae enter host cells, exert self-protective mechanisms to avoid being broken down by lysosomes, and then enter the next phase to infect the target organs after replication is completed. The host’s immune system cannot effectively eliminate the pathogen and block the infection process (Figure 1). Brucella infections exhibit a range of non-characteristic symptoms such as fever, arthralgia, myalgia, headache and neurological deficits, making them difficult to diagnose. However, if diagnosis or therapeutic intervention is not properly managed, the bacteria may invade and replicate inside vital organs—such as the bone marrow, heart and brain—leading to severe systemic reactions, and perhaps even the death of the patient.78 Therefore, early and definitive diagnosis of brucellosis, along with targeted pharmacological treatment, is of considerable clinical significance and can reduce the occurrence of serious complications in affected patients.
Effect of Neurobrucellosis on the Ocular Nerves
The incidence of neurobrucellosis in Brucella infection is 0.5–25%, and its clinical manifestations include meningitis, meningeal vascular involvement, parenchymal brain dysfunction, peripheral neuropathy, and behavioural abnormalities of varying severity.79,80 Previous studies on Brucella brain infections have shown that pathogenic bacteria reaching the reticuloendothelial system can enter the circulation and spread to the meninges, causing meningitis or meningoencephalitis.81 The toxins released cause endothelial damage leading to arteritis and cerebral ischemia, and which can also result in vascular occlusion causing cerebral infarction.27
Pathogenesis and Clinical Manifestations of Neurobrucellosis
Brucella can provoke an inflammatory response at the site of the lesion by direct action, but also by secreting cytokines and endotoxins that affect the structure and function of the peripheral nerves, spinal cord, meninges or blood vessels.82,83 Thus, the pathogenesis of Brucella infection leading to neuropathy is thought to be mediated by the action of cytokines or bacterial endotoxins on neuronal tissue, cytotoxic T lymphocytes, and immune mechanisms that cause demyelinating lesions in the brain and spinal cord white matter.84,85 The above mechanisms of injury may lead to perivascular infiltration of monocytes, lymphocytes and macrophages. In turn, cellular infiltration can contribute to intimal oedema and proliferative neuropathy, resulting in segmental demyelination, remyelination, axonal degeneration and proliferative neuropathy.35 Brucella infection causes an immune-mediated response in the central nervous system, resulting in vasculopathy not only associated with cranial neuropathy, but also closely related to optic nerve-related diseases.14
Thus, Brucella can cause damage not only to the brain and peripheral nervous system, but also to the optic nerve, usually manifesting as optic nerve oedema.13 Optic neuritis, abducens nerve palsy and other cranial nerve involvement may therefore be part of the neuropathy of brucellosis. Cranial nerve involvement in brucellosis mainly includes the trigeminal, facial, abducens, actinic or optic nerves. Neurobrucellosis can involve any one of these nerves, either alone or in combination.29,86 Optic disc oedema may occur due to axoplasmic congestion of unmyelinated axons of the optic nerve papillae, secondary to inflammation associated with demyelination behind the optic nerve bulb. In terms of ocular neuropathies, the most susceptible ocular nerves are the optic and abducens nerves.87 Thus, brucellosis complications can include optic neuritis and abducens nerve palsy/paralysis, but the mechanisms are not understood.
In the event of optic nerve oedema due to Brucella infection, one of the possible pathogeneses is vasculitis, a characteristic manifestation of brucellosis.23 The prognosis for patients with optic nerve papilloedema secondary to vasculitis is relatively poor, and most will eventually develop optic nerve atrophy.25 In addition to optic nerve injury due to vasculitis, increased intracranial pressure due to Brucella infection can also lead to optic nerve papillary oedema and elevated cerebrospinal fluid (CSF) pressure. This generally has less effect on visual acuity and pupillary reflexes, unlike optic neuritis due to vasculitis, which exhibits relatively more afferent pupillary disturbance and significant vision loss.88,89 Thus, unlike optic nerve oedema due to vasculitis, patients with optic papilloedema due to increased intracranial pressure can be more effectively treated.
One previous report concerned a 14-year-old female patient presenting with increased intracranial pressure and strabismus, with bilateral abducens nerve involvement and bilateral papilloedema, who had normalised vision and reduced papilloedema when followed up after six months of treatment.90 Akhondian showed that increased intracranial pressure in an 11-year-old child, accompanied by optic papilloedema and blurred vision, was due to brucellosis and that visual acuity recovered and optic papilloedema subsided after one month of treatment.20 Therefore, the treatment outcomes for optic neuritis and optic nerve oedema caused by Brucella infection differ considerably, and the presence of combined vascular inflammation may be an important indicator when evaluating visual prognosis.
As well as vision loss due to optic nerve oedema, another important neurological symptom of Brucella infection of the eye is ocular abducens nerve palsy. The abducens nerve is one of the cranial nerves, and cranial nerve palsy may be due to endotoxin-induced spasm involving the central auditory pathway, or ischemia of the nerve tissue. The abducens nerve (also known as the sixth cranial nerve, or cranial nerve VI) has the longest intracranial course and is susceptible to direct or indirect injury, for example, through microvascular infarction or direct compression.11,91 Furthermore, abducens nerve palsy may have a similar pathogenesis to optic neuritis—including intracranial meningeal inflammation leading to meningeal infection—given the fact that Brucella can attack Schwann cells after reaching the neuraxis via the circulatory system or lymphatic system, leading to demyelination.92 Since the abducens and optic nerves are among the cranial nerves, the mechanism of occurrence may be similar to that of optic neuritis, and we believe that vascular inflammation may also be involved in the pathology of abducens nerve palsy.
Although any meningitis can cause abducens nerve palsy through direct inflammation, increased intracranial pressure, or both, one study found that brucellosis patients can develop abducens nerve palsy before the onset of meningitis symptoms. This suggests that cranial nerve involvement and symptom onset may precede meningeal infection.17 Furthermore, abducens nerve palsy can also occur during neurobrucellosis drug treatment.93 Sahin et al additionally showed that Brucella infection may negatively affect multiple cranial nerves, including the optic, vestibulocochlear and abducens nerves.25 Therefore, patients with acute abducens nerve palsy should undergo a thorough neurological and medical evaluation. Myasthenia gravis, paraneoplastic diseases, trauma, and tumours should be prioritised; and syphilis, nodal disease, and Lyme disease may be rare etiologies.94 Neuroimaging and CSF analysis are recommended when other neurological symptoms are present. When the diagnosis is unclear, the possibility of Brucella infection must be considered in patients from endemic areas and with a history of possible exposure.
In summary, the possible pathogeneses of ocular neuropathy caused by Brucella may be briefly explained as follows.32,35,60
- Optic nerve and abducens nerve infection may be an extension of meningeal infection secondary to inflammation of the meninges.
- Brucella can reach the nervous system early in sepsis through the bloodstream, or during the chronic phase through the bloodstream or lymphatic system. The pathogen attacks and destroys Schwann cells, leading to demyelination. The damaged Schwann cells release antigens which in turn trigger an autoimmune response, causing the body’s immune system to attack Schwann cells directly.
- Nerve infection is a vascular inflammatory process in which bacterial antigens, antibodies and complement complexes accumulate in the vascular nerves. This induces vascular and perivascular infiltration of monocytes, lymphocytes and macrophages.
- Brucella infection directly releases large amounts of endogenous and exogenous toxins. Brucella endotoxin acts mainly on the cell membrane of Schwann cells, or on the capillary endothelium of vascular nerves, provoking vascular and perivascular inflammatory responses. Furthermore, antibodies produced against endogenous and exogenous toxins can reach Schwann cells through the blood–nerve barrier, and may act in concert with endotoxins to deposit immune complexes and cause direct damage to the neurovasculature.
Detection Method of Neurobrucellosis
Brucella detection relies mainly on biochemical tests, including microbiological culture, serological tests (Coombs test, immunocapture agglutination, Brucellacapt, enzyme-linked immunosorbent assay and indirect fluorescent antibody test) and molecular tests (real-time PCR).95 Neurobrucellosis is difficult to diagnose definitively in clinical care, because it is based on positive CSF bacterial cultures or elevated titres of any Brucella antibodies, and abnormal CSF measurements: CSF cell count >106/L, decreased glucose and increased protein.96,97 In addition, In the detection of Brucella abortus, the accounting amplification test assay is also one of the commonly used methods. These include conventional PCR methods, in-house PCR, nested PCR, PCR enzyme immunoassay in microplate format, real-time PCR, quantitative RT-PCR and multiplex real-time PCR.98–101 Recently, a new method based on loop-mediated isothermal amplification was used for Brucella detection.102 Besides, although in vitro bacterial culture is the gold standard for diagnosis of neurobrucellosis, the culture positivity rate is quite low (<15%) and can easily result in false-negative results in clinical management.103 Moreover, brucellae take a long time to culture, and this may delay patient treatment while waiting for the results. Particularly in countries where Brucella infection is endemic, diagnosis therefore needs to be based on screening for a history of ingestion of unpasteurised cow’s or goat’s milk, or other dairy products, or exposure to raw lamb, excluding other clinically important etiologies.
In neurobrucellosis, detection of Brucella requires screening for the bacterium in blood, bone marrow or CSF. Serum and CSF titres above 1:160 and 1:80, respectively, and CSF abnormalities manifesting as increased protein and lymphocytosis, strengthen the sensitivity of laboratory diagnosis.26,104,105 At present, neurobrucellosis has neither a typical clinical presentation nor a specific CSF presentation by which to make a clear clinical diagnosis,106 and treatment with doxycycline prior to lumbar puncture may also result in a negative CSF culture.10 Therefore, the timing of the test is crucial for diagnosis. Since it is difficult to isolate and culture Brucella from blood and CSF, serological tests for IgG and IgM are also important.107
In areas of brucellosis outbreaks, greater caution is needed in the treatment of optic neuritis, requiring associated imaging and exclusion of those with clinical suspicion of neuromyelitis optica. It is important to bear in mind that neurobrucellosis may cause similar signs and symptoms of increased intracranial pressure, and thus, optic neuritis.22,108 Therefore, determination of CSF pressure by lumbar puncture is necessary for a definitive diagnosis in patients suspected of having increased intracranial pressure leading to optic nerve oedema. In addition, PCR diagnostic methods are considered to be as sensitive and accurate as the blood culture technique.109 In recent years, metagenomic next-generation sequencing (mNGS), a new detection technology, has also been applied. It requires only a small amount of intraocular fluid for high-throughput analysis, and detection is fast and sensitive with the results obtained within three days.13 Thus, the test may serve as a new and effective method for the diagnosis of neurobrucellosis, and ongoing advances in testing methods may offer improved assistance for the diagnosis and treatment of patients with Brucella infection.
Treatment of Neurobrucellosis
In neurobrucellosis, intracranial hypertension secondary to optic nerve atrophy rarely leads to persistent vision loss, although it has been reported in more than 50% of cases. With appropriate antibiotic treatment, however, there is usually complete regression without loss of vision.110 If patients are not diagnosed and treated promptly, optic nerve atrophy secondary to optic nerve papilloedema can lead to severe visual impairment that cannot be recovered, and permanent vision loss.28 This may be related to blockage of the fast and slow phase of axial blood flow associated with optic nerve papilloedema, which in turn leads to impairment of normal physiological activity of the nerve, inducing optic atrophy. Therefore, prompt initiation of medication is required for neurological symptoms of brucellosis.
Furthermore, Brucella infection can cause multisystemic lesions and has a prolonged disease course, so treatment requires long-term drug therapy. Although there are no clinical trials to guide optimal treatment, the combination of doxycycline and rifampin is effective.111,112 Use of ciprofloxacin in addition to doxycycline and rifampicin was found to be more successful in clinical treatment of neurobrucellosis.113 However, there is a special consideration in the treatment of central nervous system complications of brucellosis; namely, how to achieve high drug concentrations in the CSF following administration. It was recently reported that treatment success is significantly higher with intravenous ceftriaxone-based regimens than with oral regimens. It has been suggested that ceftriaxone is more efficacious in brucellosis, possibly due to the high rate of free diffusion of the drug in the humoral circulation.114 Therefore, whether a regimen of ceftriaxone combined with ciprofloxacin and rifampicin might be more effective for patients with combined neuroleptic brucellosis is an open question, and a direction that needs further investigation. In addition, future studies should address how ceftriaxone, doxycycline, ciprofloxacin and rifampicin can be best used in combination for optimal treatment of Brucella infection.
Brucella Infection in Uveitis
Uveitis is one of the most prevalent inflammatory eye diseases and ocular infections also play an important role in the development of uveitis.115 Accounting for approximately 10% of all global cases of blindness—and another common ocular symptom after Brucella infection, in addition to nerve damage in the eye. Depending on the inflammatory process and anatomical location, uveitis can be classified into four main subtypes: anterior uveitis, intermediate uveitis, posterior uveitis and total uveitis.116 According to one source, posterior uveitis is the most common manifestation in Brucella infection, followed by total uveitis.39 However, it has also been suggested that anterior uveitis is the predominant form of ocular uveitis in brucellosis, followed by chorioretinitis (a type of posterior uveitis) and total uveitis.117 Brucella-associated uveitis usually occurs after the acute phase of a systemic infection, and is currently regarded as either a noninfectious immune response, or a form of infectious chorioretinitis with low-virulence bacteria found in the choroid.48 Uveitis in brucellosis can present as granulomatous or non-granulomatous inflammation, and it can develop in one or both eyes.118 Early treatment with corticosteroids may provide relief, but uveitis will frequently recur when these agents are used alone.119
Pathogenesis and Clinical Manifestations of Uveitis
Previous studies related to ocular pathology have shown that host–pathogen interactions between humans and Brucella species can cause damage by two mechanisms: first, direct invasion of bacteria into ocular tissue and production of septic emboli on uveal tissue; and second, production of immunoglobulins and circulating immune complexes following infection.62,120 This supports the idea that systemic steroid use improves vision in brucellosis patients because corticosteroid hormone administration modulates the immune component of the body.45 Brucella can cause chorioretinitis in the eye, which usually presents as multifocal lesions or nodular or topographical changes with retinal oedema and haemorrhage, and these symptoms are often observed in conjunction with the manifestations of uveitis.121 Development of Brucella uveitis can seriously affect a patient’s vision, so further exploration of the pathogenesis and possible treatment are necessary to try to save the patient’s sight.
Thus, host–pathogen interactions in brucellosis that lead to the development of ocular uveitis can currently be explained by the following two postulated mechanisms.122,123
- Brucellae directly invade ocular tissue, via blood or lymphatic tissue circulation, and produce septic emboli in the uveal tissue, resulting in focal chorioretinitis.
- Immune responses to Brucella infection lead to deposition of immunoglobulins and circulating immune complexes in the uveal tissue, producing an exaggerated autoimmune response.
In severe infections, these immune complexes—combined with direct microbial invasion of the uveal tissue—lead to increased transmural pressure in the choroidal vessels, causing plasma accumulation and plasmacytic choroidal detachment. This could explain how Brucella infection results in uveitis combined with choroidal detachment.
In patients with Brucella-associated uveitis, the eye may show sclerosis, fine keratoconjunctival deposits in the corneal endothelium (KP), yellowish-white corneal stromal deposits, anterior chamber flash, cells floating in the anterior chamber fluid, normal iris without nodules, cloudy vitreous, or normal optic disc and posterior pole but with chorioretinal plaques around the retina. In severe cases, exudative retinal detachment and choroidal detachment may occur.47 Combined chorioretinitis usually presents as multifocal, nodular or geographic nodular chorioretinitis with retinal oedema and haemorrhage.124
Detection Method of Uveitis
In terms of diagnosis, ocular symptoms can provide support for the diagnosis of uveitis, but laboratory analysis is also required to diagnose uveitis due to Brucella infection.125 As discussed above, routine clinical blood cultures, CSF culture methods and molecular detection techniques are widely used for microbiological testing of systemic and localised infections.126 However, traditional culture methods have a low positive detection rate—especially for B. abortus, a slow-growing and uncommon microorganism—which limit their use in guiding clinical diagnosis and treatment.127 Moreover, outside brucellosis endemic areas, physicians do not routinely consider this organism and many health care facilities are not equipped to culture it. As a result, patients may be delayed in diagnosis, or even misdiagnosed.
In contrast, despite their improved detection efficiency, molecular diagnostic techniques such as quantitative polymerase chain reaction(qPCR) and gene chips are not suitable for ocular sample analysis because they require specific primer sets, can only target a limited number of pathogens, and have a high demand for sample volume.128,129 Compared to the above methods, metagenomic next-generation sequencing(mNGS) is an unbiased high-throughput sequencing technique that can, in principle, detect all pathogens in clinical samples in a short period of time.130 It can also provide information on antibiotic resistance by comparing sequenced genes in microorganisms against antibiotic resistance databases.131 In clinical management of patients who do not require vitrectomy, a slit lamp puncture of approximately 0.2 mL of atrial fluid is sufficient for mNGS. This is a more convenient procedure, with lower risk and fewer complications, than extracting vitreous fluid.132 However, if a patient has developed an exudative retinal detachment requiring surgical treatment, vitreous fluid testing is also recommended. The reason for this is that in patients with ocular Brucella infections, the vitreous, as a non-circulating fluid, contains higher antibody titres against brucellae compared to serum and atrial fluid.47 Therefore, vitreous specimens, where available, are important in the diagnosis of ocular brucellosis. Clinically, ocular symptoms can suggest that patients have uveitis, but systemic serologic testing, along with atrial fluid and vitreous cavity fluid testing, can all help clarify the underlying cause. The combined use of multiple tests can increase the accuracy of diagnosis, and direct the selection of therapeutic agents.
Treatment of Uveitis
Current drug regimens for the treatment of brucellosis combined with uveitis employ two or more antibiotics, including doxycycline, rifampicin, streptomycin, or gentamicin.133 In patients with a clear diagnosis and receiving antibiotics, systemic and topical glucocorticoid therapy may alleviate ocular symptoms. As for the duration of treatment, Brucella infections are difficult to clear and generally require at least two or three months of regular medication to achieve a therapeutic effect.42 Early discontinuation of treatment may lead to disease recurrence and the development of drug-resistant bacteria. It is therefore crucial to appreciate the importance of initiating medication after a clear diagnosis. Furthermore, as with all infectious uveitis, direct treatment with immunosuppressive agents due to initial misdiagnosis may lead to a worsening of the patient’s condition, in which case an extended period of treatment may be necessary.41 Therefore, immunosuppressive agents should be used with caution when the aetiology of uveitis is not clear.
Other Ocular Lesions in Brucella Infection
As well as causing optic nerve-related diseases and uveitis, Brucella infections can also affect the ocular appendages. Lacrimal ductitis can occur, perhaps through direct invasion of the ocular structures, suggesting that Brucella species are capable of colonising the epidermis directly.54 Nevertheless, the condition manifests as damage to the lacrimal duct and lacrimal gland tissue. Patients present with excessive protrusion of the eye, increased lacrimal secretions and unilateral lacrimal gland inflammation. Association with brucellosis is confirmed by serology, lacrimal gland secretion culture and histopathology.56 Systemic infections can cause mastitis and pancreatitis, and infection of these secretory glands may have the same pathogenesis as lacrimal gland infections, lending support to the idea that Brucella affects the exocrine glands.49,134 Subcutaneous abscesses caused by brucellae are relatively rare, but can be associated with the development of conjunctivitis and lacrimal gland infections, which are soft tissue infections usually associated with penetrating injuries.135,136 The Brucella vaccine bottle was accidentally dropped and some of its contents entered the veterinarian’s conjunctival sac, resulting in the development of bilateral keratoconjunctivitis. After two months of infection, the Brucella titer reached 1/320. The treatment was not effective and eventually the patient’s uncontrolled infection caused total ophthalmia resulting in eye removal.137 Therefore, localized infection caused by Brucella can also lead to uncontrolled infection and blindness, which needs to be taken into account by physicians and patients in clinical practice.
In our opinion, it is possible that lacrimal gland infections and conjunctivitis caused by Brucella are direct infections caused by patient exposure to these bacteria in the eye. Another plausible explanation is that Brucella accesses the lesion site from the lymph nodes, bone and blood circulation. Because there is no history of penetrating injury or other clinically relevant manifestations in the vast majority of patients, and because abscesses at different sites may be the result of bacterial infections, serologic data from patients may also suggest chronic limited brucellosis.36 Further pathological and histological studies are required in the future to reveal the underlying mechanisms of abscess occurrence. Brucella invasion of the lacrimal gland is extremely rare, but the mechanism of glandular injury causing abscess may be similar to endophthalmitis, as involvement of brucellosis-associated lacrimal gland inflammation appears to be a new extraocular infectious reaction.
Conclusions
In conclusion, we have reviewed the general and ocular pathogenesis of virulent Brucella species (Figure 2). Brucella infection is uncommon in non-endemic regions, and misdiagnosis or underdiagnosis may occur; therefore, ophthalmic clinicians should consider ocular pathology due to brucellosis as part of the diagnostic process. In patients with a known history of epidemiological risk or contact, brucellosis should certainly be included as a possible cause of optic neuritis, abducens nerve palsy, uveitis and lacrimal sacculitis. In such cases, systemic and intraocular fluid testing will be an important adjunct to other diagnostic procedures. Prevention strategies include, among others, avoidance of unpasteurised dairy products (milk or cheese) and animal contact (farm animals, or raw beef and lamb) in endemic areas. Although it is difficult to draw firm conclusions regarding optimal treatment, combination therapy with antibiotics is appropriate, when tolerated, in systemic brucellosis. For ocular lesions associated with Brucella infection, early pharmacological intervention may be an important factor in restoring and protecting the patient’s vision.
 |
Figure 2 Ocular pathogenesis of Brucella. |
Data Sharing Statement
The literature used and cited in this study is available in peer-reviewed journals and is publicly accessible.
Funding
This work was supported by the Joint construction project of Henan Medical Science and technology (LHGJ20220370).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3(2):213–221. doi:10.3201/eid0302.970219
2. Thakur SD, Kumar R, Thapliyal DC. Human brucellosis: review of an under-diagnosed animal transmitted disease. J Commun Dis. 2002;34(4):287–301.
3. Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6(10):e1865. doi:10.1371/journal.pntd.0001865
4. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–2336. doi:10.1056/NEJMra050570
5. Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–541. doi:10.1146/annurev-micro-090110-102905
6. Young EJ. An overview of human brucellosis. Clin Infect Dis. 1995;21(2):283–289; quiz 290. doi:10.1093/clinids/21.2.283
7. Avijgan M, Rostamnezhad M, Jahanbani-Ardakani H. Clinical and serological approach to patients with brucellosis: a common diagnostic dilemma and a worldwide perspective. Microb Pathog. 2019;129:125–130. doi:10.1016/j.micpath.2019.02.011
8. Poletti J, Poletti A, Larmande A. Diagnostic et traitement de l'uvéite brucellose. Med Trop. 1981;41(5):523–525. French.
9. Galińska EM, Zagórski J. Brucellosis in humans--etiology, diagnostics, clinical forms. Ann Agric Environ Med. 2013;20(2):233–238.
10. Sarmiento Clemente A, Amerson-Brown MH, Foster CE. An adolescent with neurobrucellosis caused by Brucella abortus cattle vaccine strain RB51. Pediatr Infect Dis J. 2021;40(9):e353–e355. doi:10.1097/inf.0000000000003200
11. Havalı C, Çağan E. A rare pediatric case of neurobrucellosis with bilateral optic neuritis. Turk J Pediatr. 2020;62(6):1094–1098. doi:10.24953/turkjped.2020.06.023
12. Turel O, Abdillah FK, Yozgat CY, et al. A rare presentation of neurobrucellosis in a 6-year-old pediatric patient with sagittal sinus thrombosis. Neuropediatrics. 2021;52(1):48–51. doi:10.1055/s-0040-1715482
13. Geng L, Feng Y, Li D, et al. Meningoencephalitis, coronary artery and keratitis as an onset of brucellosis: a case report. BMC Infect Dis. 2020;20(1):654. doi:10.1186/s12879-020-05358-z
14. Mergen B, Sarici AM, Baltu F, Ozaras R, Adaletli I. An unusual presentation of sixth nerve palsy: neurobrucellosis. GMS Ophthalmol Cases. 2019;9:Doc13. doi:10.3205/oc000102
15. Bains R, Dahhan T, Belzowski A, Heinze ER, Wong AL, Clements PJ. An interesting case of neurobrucellosis mimicking neuropsychiatric lupus. Case Rep Rheumatol. 2018;2018:9793535. doi:10.1155/2018/9793535
16. Sharma PP, Murali MV, Hamdi T. Neurobrucellosis presenting as pseudotumor cerebri: first report from Oman. Oman Med J. 2017;32(6):507–509. doi:10.5001/omj.2017.96
17. Dar W, Latief M, Dar I, Sofi N. Meningitis, polyradiculopathy, and optic nerve involvement in neurobrucellosis: a rare clinical presentation. Neurol India. 2017;65(5):1142–1144. doi:10.4103/neuroindia.NI_127_16
18. Ibrahimagić O, Smajlović D, Dostović Z, Iljazović A, Kojić B, Zonić L. Neurobrucellosis and cerebral venous sinus thrombosis: a case report. Acta Clin Belg. 2017;72(5):343–345. doi:10.1080/17843286.2016.1251385
19. Tajdini M, Akbarloo S, Hosseini SM, et al. From a simple chronic headache to neurobrucellosis: a case report. Med J Islam Repub Iran. 2014;28:12.
20. Akhondian J, Ashrafzadeh F, Beiraghi Toosi M. A Rare presentation of neurobrucellosis in a child with Recurrent transient ischemic attacks and pseudotumor cerebri (A case report and review of literature). Iran J Child Neurol. 2014;8(2):65–69.
21. Tugcu B, Nacaroglu SA, Coskun C, Kuscu DY, Onder F. Chronic meningitis complicating intracranial hypertension in neurobrucellosis: a case report. Semin Ophthalmol. 2015;30(5–6):429–431. doi:10.3109/08820538.2013.874472
22. Sinopidis X, Kaleyias J, Mitropoulou K, Triga M, Kothare SV, Mantagos S. An uncommon case of pediatric neurobrucellosis associated with intracranial hypertension. Case Rep Infect Dis. 2012;2012:492467. doi:10.1155/2012/492467
23. Marques R, Martins C, Machado I, Monteiro JP, Campos N, Calhau P. Unilateral optic neuritis as a presentation of neurobrucellosis. Pediatr Rep. 2011;3(2):e11. doi:10.4081/pr.2011.e11
24. Sahin OG, Pelit A, Turunc T, Akova YA. Ophthalmoparesis, papillitis and premacular hemorrhage in a case with endocarditis: a rare presentation of Brucellosis. Indian J Ophthalmol. 2010;58(2):164–166. doi:10.4103/0301-4738.60098
25. Sahin E, Yilmaz A, Ersöz G, Uğuz M, Kaya A. Multiple cranial nerve involvement caused by Brucella melitensis. South Med J. 2009;102(8):855–857. doi:10.1097/SMJ.0b013e3181ac0628
26. Tonekaboni SH, Karimi A, Armin S, Khase LA, Sabertehrani AS. Neurobrucellosis: a partially treatable cause of vision loss. Pediatr Neurol. 2009;40(5):401–403. doi:10.1016/j.pediatrneurol.2008.12.011
27. Vinod P, Singh MK, Garg RK, Agarwal A. Extensive meningoencephalitis, retrobulbar neuritis and pulmonary involvement in a patient of neurobrucellosis. Neurol India. 2007;55(2):157–159. doi:10.4103/0028-3886.32790
28. Miyares FR, Deleu D, ElShafie SS, et al. Irreversible papillitis and ophthalmoparesis as a presenting manifestation of neurobrucellosis. Clin Neurol Neurosurg. 2007;109(5):439–441. doi:10.1016/j.clineuro.2007.01.010
29. Karakurum Goksel B, Yerdelen D, Karatas M, et al. Abducens nerve palsy and optic neuritis as initial manifestation in brucellosis. Scand J Infect Dis. 2006;38(8):721–725. doi:10.1080/00365540500466614
30. Levy J, Shneck M, Marcus M, Lifshitz T. Brucella meningitis and papilledema in a child. Eur J Ophthalmol. 2005;15(6):818–820. doi:10.1177/112067210501500628
31. Karapinar B, Yilmaz D, Vardar F, Demircioglu O, Aydinok Y. Unusual presentation of brucellosis in a child: acute blindness. Acta Paediatr. 2005;94(3):378–380. doi:10.1111/j.1651-2227.2005.tb03085.x
32. Tunç M, Durukan H. Bilateral severe visual loss in brucellosis. Ocul Immunol Inflamm. 2004;12(3):233–236. doi:10.1080/092739490500183
33. Ciftçi E, Erden I, Akyar S. Brucellosis of the pituitary region: MRI. Neuroradiology. 1998;40(6):383–384. doi:10.1007/s002340050605
34. Estevão MH, Barosa LM, Matos LM, Barroso AA, da Mota HC. Neurobrucellosis in children. Eur J Pediatr. 1995;154(2):120–122. doi:10.1007/bf01991914
35. Abd Elrazak M. Brucella optic neuritis. Arch Intern Med. 1991;151(4):776–778.
36. Guvenc H, Kocabay K, Okten A, Bektas S. Brucellosis in a child complicated with multiple brain abscesses. Scand J Infect Dis. 1989;21(3):333–336. doi:10.3109/00365548909035705
37. Diaz Espejo CE, Villalobos Chaves F, Sureda Ramis B. Chronic intracranial hypertension secondary to neurobrucellosis. J Neurol. 1987;234(1):59–61. doi:10.1007/bf00314012
38. Xi H, Zhang L, Xu B, Liu H, Metagenomic Next-Generation LS. Sequencing to investigate infectious endophthalmitis of Brucella: a case report. Front Med. 2022;9:847143. doi:10.3389/fmed.2022.847143
39. AlMutairi N, AlRubaie K, Asharari KS, Albalawi HB. Unusual bilateral panuveitis uveitis with brucellosis. Saudi J Ophthalmol. 2021;35(2):143–145. doi:10.4103/1319-4534.337852
40. Adusumilli H, Krupa L, Shetty NS, Rao S. Bilateral serous choroidal detachment in brucellosis and its management and outcome: literature review and case report. Indian J Ophthalmol. 2020;68(6):1204–1206. doi:10.4103/ijo.IJO_1418_19
41. Oray M, Cebeci Z, Kir N, Turgut Ozturk B, Oksuz L, Tugal-Tutkun I. Endogenous Brucella endophthalmitis: a case report. Saudi J Ophthalmol. 2017;31(2):106–108. doi:10.1016/j.sjopt.2017.03.002
42. Al-Kharashi AS. Endogenous endophthalmitis caused by Brucella melitensis. Retin Cases Brief Rep. 2016;10(2):165–167. doi:10.1097/icb.0000000000000217
43. Akyol L, Aslan K, Özgen M, Sayarlioglu M. Bilateral sacroiliitis and uveitis comorbidity: brucellosis? Ankylosing spondylitis? BMJ Case Rep. 2015;2015. doi:10.1136/bcr-2015-211461
44. Mohammadi Z, Dehghani A, Ghanbari HO, Akhlaghi MR, Nasrollahi K, Salam H. Ocular manifestations in a child with systemic brucellosis. J Res Med Sci. 2014;19(7):677–679.
45. Rabinowitz R, Schneck M, Levy J, Lifshitz T. Bilateral multifocal choroiditis with serous retinal detachment in a patient with Brucella infection: case report and review of the literature. Arch Ophthalmol. 2005;123(1):116–118. doi:10.1001/archopht.123.1.116
46. Hatipoglu CA, Yetkin A, Ertem GT, Tulek N. Unusual clinical presentations of brucellosis. Scand J Infect Dis. 2004;36(9):694–697. doi:10.1080/00365540410017554
47. Akduman L, Or M, Hasanreisoğlu B, Kurtar K. A case of ocular brucellosis: importance of vitreous specimen. Acta Ophthalmol. 1993;71(1):130–132. doi:10.1111/j.1755-3768.1993.tb04975.x
48. Walker J, Sharma OP, Rao NA. Brucellosis and uveitis. Am J Ophthalmol. 1992;114(3):374–375. doi:10.1016/s0002-9394(14)71813-6
49. Gasser I, Almirante B, Fernández-Pérez F, Mendoza C. Bilateral mammary abscess and uveitis caused by Brucella melitensis--report of a case. Infection. 1991;19(1):44–45. doi:10.1007/bf01643759
50. Tabbara KF, al-Kassimi H. Ocular brucellosis. Br J Ophthalmol. 1990;74(4):249–250. doi:10.1136/bjo.74.4.249
51. Rolando I, Carbone A, Haro D, Gotuzzo E, Carrillo C. Retinal detachment in chronic brucellosis. Am J Ophthalmol. 1985;99(6):733–734. doi:10.1016/s0002-9394(14)76058-1
52. Lidgett K, Wilson MM. Ocular lesions associated with brucellosis: reports of two cases. Med J Aust. 1955;42(22):900–901. doi:10.5694/j.1326-5377.1955.tb49129.x
53. Bhasin A, Singal RK, Chaudhary D, et al. Thrombotic thrombocytopenic purpura in a patient with Brucella infection. J Assoc Physicians India. 2021;69(10):11–12.
54. Vempuluru VS, Mitra S, Tripathy D, Mohapatra S, Rath S. Isolation of unusual bacteria in canaliculitis: a series of four cases. Saudi J Ophthalmol. 2021;35(1):66–70. doi:10.4103/1319-4534.325778
55. Imamura H, Sakai N, Alexander MJ. Flow-diverter stenting of intracavernous internal carotid artery mycotic aneurysm. J Stroke Cerebrovasc Dis. 2019;28(7):e81–e82. doi:10.1016/j.jstrokecerebrovasdis.2019.04.026
56. Bekir NA, Güngör K, Namiduru M. Brucella melitensis dacryoadenitis: a case report. Eur J Ophthalmol. 2000;10(3):259–261. doi:10.1177/112067210001000312
57. Martins Rda C, Irache JM, Gamazo C. Acellular vaccines for ovine brucellosis: a safer alternative against a worldwide disease. Expert Rev Vaccines. 2012;11(1):87–95. doi:10.1586/erv.11.172
58. Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14(6):e469–e478. doi:10.1016/j.ijid.2009.06.031
59. Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. Human brucellosis in Kuwait: a prospective study of 400 cases. Q J Med. 1988;66(249):39–54.
60. Puig Solanes M, Heatley J, Arenas F, Guerrero Ibarra G. Ocular complications in brucellosis. Am J Ophthalmol. 1953;36(5):675–689. doi:10.1016/0002-9394(53)90310-9
61. Woods AC. Ophthalmology’s stake in brucellosis. Trans Am Acad Ophthalmol Otolaryngol. 1951;55:616–624.
62. Rolando I, Olarte L, Vilchez G, et al. Ocular manifestations associated with brucellosis: a 26-year experience in Peru. Clin Infect Dis. 2008;46(9):1338–1345. doi:10.1086/529442
63. Mousa AR, Koshy TS, Araj GF, et al. Brucella meningitis: presentation, diagnosis and treatment--A prospective study of ten cases. Q J Med. 1986;60(233):873–885.
64. Rhyan JC. Pathogenesis and pathobiology of brucellosis in wildlife. Rev Sci Tech. 2013;32(1):127–136. doi:10.20506/rst.32.1.2191
65. Martirosyan A, Moreno E, Gorvel JP. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev. 2011;240(1):211–234. doi:10.1111/j.1600-065X.2010.00982.x
66. Grilló MJ, Blasco JM, Gorvel JP, Moriyón I, Moreno E. What have we learned from brucellosis in the mouse model? Vet Res. 2012;43(1):29. doi:10.1186/1297-9716-43-29
67. Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35(3):323–335. doi:10.1016/j.immuni.2011.09.007
68. Kreutzer DL, Dreyfus LA, Robertson DC. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun. 1979;23(3):737–742. doi:10.1128/iai.23.3.737-742.1979
69. Riley LK, Robertson DC. Brucellacidal activity of human and bovine polymorphonuclear leukocyte granule extracts against smooth and rough strains of Brucella abortus. Infect Immun. 1984;46(1):231–236. doi:10.1128/iai.46.1.231-236.1984
70. Ahmed K, Al-Matrouk KA, Martinez G, Oishi K, Rotimi VO, Nagatake T. Increased serum levels of interferon-gamma and interleukin-12 during human brucellosis. Am J Trop Med Hyg. 1999;61(3):425–427. doi:10.4269/ajtmh.1999.61.425
71. Salcedo SP, Marchesini MI, Lelouard H, et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 2008;4(2):e21. doi:10.1371/journal.ppat.0040021
72. Barquero-Calvo E, Chaves-Olarte E, Weiss DS, et al. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One. 2007;2(7):e631. doi:10.1371/journal.pone.0000631
73. Starr T, Child R, Wehrly TD, et al. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11(1):33–45. doi:10.1016/j.chom.2011.12.002
74. Gorvel JP, Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol. 2002;90(1–4):281–297. doi:10.1016/s0378-1135(02)00214-6
75. Riley LK, Robertson DC. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984;46(1):224–230. doi:10.1128/iai.46.1.224-230.1984
76. Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. 2007;25(3):188–202. doi:10.4103/0255-0857.34758
77. Moreno E, Moriyon I. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc Natl Acad Sci U S A. 2002;99(1):1–3. doi:10.1073/pnas.022622699
78. Moreno E, Barquero-Calvo E. The role of neutrophils in Brucellosis. Microbiol Mol Biol Rev. 2020;84:4. doi:10.1128/mmbr.00048-20
79. Zange S, Schneider K, Georgi E, et al. A headache with surprising outcome: first case of brucellosis caused by Brucella suis biovar 1 in Germany. Infection. 2019;47(5):863–868. doi:10.1007/s15010-019-01312-7
80. Karsen H, Tekin Koruk S, Duygu F, Yapici K, Kati M. Review of 17 cases of neurobrucellosis: clinical manifestations, diagnosis, and management. Arch Iran Med. 2012;15(8):491–494.
81. Shakir RA, Al-Din AS, Araj GF, Lulu AR, Mousa AR, Saadah MA. Clinical categories of neurobrucellosis. A report on 19 cases. Brain. 1987;110(Pt 1):213–223. doi:10.1093/brain/110.1.213
82. Turkoglu SA, Halicioglu S, Sirmatel F, Yildiz M, Yildiz N, Yildiz S. Vasculitis and neurobrucellosis: evaluation of nine cases using radiologic findings. Brain Behav. 2018;8(4):e00947. doi:10.1002/brb3.947
83. Alqwaifly M, Al-Ajlan FS, Al-Hindi H, Al Semari A. Central nervous system brucellosis granuloma and white matter disease in immunocompromised patient. Emerg Infect Dis. 2017;23(6):978–981. doi:10.3201/eid2306.161173
84. Ceran N, Turkoglu R, Erdem I, et al. Neurobrucellosis: clinical, diagnostic, therapeutic features and outcome. Unusual clinical presentations in an endemic region. Braz J Infect Dis. 2011;15(1):52–59.
85. Erdem H, Senbayrak S, Meriç K, et al. Cranial imaging findings in neurobrucellosis: results of Istanbul-3 study. Infection. 2016;44(5):623–631. doi:10.1007/s15010-016-0901-3
86. Tali ET, Keskin T, Oznur II, Simonson T, Yuh WT. MRI of Brucella polyneuritis in a child. Neuroradiology. 1996;38(Suppl 1):S190–192. doi:10.1007/bf02278157
87. Trifiletti RR, Restivo DA, Pavone P, Giuffrida S, Parano E. Diabetes insipidus in neurobrucellosis. Clin Neurol Neurosurg. 2000;102(3):163–165. doi:10.1016/s0303-8467(00)00089-5
88. Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83–99. doi:10.1016/s1474-4422(13)70259-x
89. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593–617. doi:10.1016/j.ncl.2010.03.003
90. Emadoleslami M, Mahmoudian T. A case of pseudotumor cerebri and brucellosis. J Pediatr Infect Dis. 2007;2(4):251–253.
91. Romero M, Sánchez F, Fernández-Bolaños R, Jiménez MD. Optic neuritis as a clinical manifestation of neurobrucellosis. Rev Neurol. 1999;28(4):438.
92. Ono K, Arai H, Endo T, et al. Detailed MR imaging anatomy of the abducent nerve: evagination of CSF into Dorello canal. AJNR Am J Neuroradiol. 2004;25(4):623–626.
93. Yilmaz M, Ozaras R, Mert A, Ozturk R, Tabak F. Abducent nerve palsy during treatment of brucellosis. Clin Neurol Neurosurg. 2003;105(3):218–220. doi:10.1016/s0303-8467(03)00008-8
94. Richards BW, Jones FR, Younge BR. Causes and prognosis in 4278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113(5):489–496. doi:10.1016/s0002-9394(14)74718-x
95. Araj GF. Update on laboratory diagnosis of human brucellosis. Int J Antimicrob Agents. 2010;36(Suppl 1):S12–17. doi:10.1016/j.ijantimicag.2010.06.014
96. Haji-Abdolbagi M, Rasooli-Nejad M, Jafari S, Hasibi M, Soudbakhsh A. Clinical and laboratory findings in neurobrucellosis: review of 31 cases. Arch Iran Med. 2008;11(1):21–25.
97. Sanchez-Sousa A, Torres C, Campello MG, et al. Serological diagnosis of neurobrucellosis. J Clin Pathol. 1990;43(1):79–81. doi:10.1136/jcp.43.1.79
98. Sanjuan-Jimenez R, Colmenero JD, Bermúdez P, Alonso A, Morata P. Amplicon DNA melting analysis for the simultaneous detection of Brucella spp and Mycobacterium tuberculosis complex. Potential use in rapid differential diagnosis between extrapulmonary tuberculosis and focal complications of brucellosis. PLoS One. 2013;8(3):e58353. doi:10.1371/journal.pone.0058353
99. Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22(1):130–138. doi:10.2144/97221bi01
100. Al-Nakkas A, Mustafa AS, Wright SG. Large-scale evaluation of a single-tube nested PCR for the laboratory diagnosis of human brucellosis in Kuwait. J Med Microbiol. 2005;54(Pt 8):727–730. doi:10.1099/jmm.0.45772-0
101. Navarro E, Serrano-Heras G, Castaño MJ, Solera J. Real-time PCR detection chemistry. Clin Chim Acta. 2015;439:231–250. doi:10.1016/j.cca.2014.10.017
102. Moeini-Zanjani A, Pournajaf A, Ferdosi-Shahandashti E, et al. Comparison of loop-mediated isothermal amplification and conventional PCR tests for diagnosis of common Brucella species. BMC Res Notes. 2020;13(1):533. doi:10.1186/s13104-020-05377-8
103. Ge Y, Guan HZ, Fan SY, Zhu R, Ma XJ, Li TS. A clinical analysis of 20 patients with neurobrucellosis. Zhonghua Nei Ke Za Zhi. 2017;56(10):729–733. doi:10.3760/cma.j.issn.0578-1426.2017.10.004
104. Yetkin MA, Bulut C, Erdinc FS, Oral B, Tulek N. Evaluation of the clinical presentations in neurobrucellosis. Int J Infect Dis. 2006;10(6):446–452. doi:10.1016/j.ijid.2006.05.007
105. McLean DR, Russell N, Khan MY. Neurobrucellosis: clinical and therapeutic features. Clin Infect Dis. 1992;15(4):582–590. doi:10.1093/clind/15.4.582
106. Guven T, Ugurlu K, Ergonul O, et al. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis. 2013;56(10):1407–1412. doi:10.1093/cid/cit072
107. Omar FZ, Zuberi S, Minns RA. Neurobrucellosis in childhood: six new cases and a review of the literature. Dev Med Child Neurol. 1997;39(11):762–765. doi:10.1111/j.1469-8749.1997.tb07379.x
108. Gise RA, Heidary G. Update on pediatric optic neuritis. Curr Neurol Neurosci Rep. 2020;20(3):4. doi:10.1007/s11910-020-1024-x
109. Hekmatimoghaddam S, Sadeh M, Khalili MB, Mollaabedin M, Sazmand A. Comparison of PCR, Wright agglutination test and blood culture for diagnosis of brucellosis in suspected patients. Pak J Biol Sci. 2013;16(22):1589–1592. doi:10.3923/pjbs.2013.1589.1592
110. Zheng N, Wang W, Zhang JT, et al. Neurobrucellosis. Int J Neurosci. 2018;128(1):55–62. doi:10.1080/00207454.2017.1363747
111. Al-Sous MW, Bohlega S, Al-Kawi MZ, Alwatban J, McLean DR. Neurobrucellosis: clinical and neuroimaging correlation. AJNR Am J Neuroradiol. 2004;25(3):395–401.
112. Maley MW, Kociuba K, Chan RC. Prevention of laboratory-acquired brucellosis: significant side effects of prophylaxis. Clin Infect Dis. 2006;42(3):433–434. doi:10.1086/499112
113. Bektaş O, Ozdemir H, Yılmaz A, et al. An unusual case of neurobrucellosis presenting as demyelination disorder. Turk J Pediatr. 2013;55(2):210–213.
114. Erdem H, Ulu-Kilic A, Kilic S, et al. Efficacy and tolerability of antibiotic combinations in neurobrucellosis: results of the Istanbul study. Antimicrob Agents Chemother. 2012;56(3):1523–1528. doi:10.1128/aac.05974-11
115. O’Connor GR. Current concepts in ophthalmology. Uveitis and the immunologically compromised host. N Engl J Med. 1978;299(3):130–132. doi:10.1056/NEJM197807202990305
116. Selmi C. Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13(4–5):591–594. doi:10.1016/j.autrev.2014.01.006
117. Sungur GK, Hazirolan D, Gurbuz Y, Unlu N, Duran S, Duman S. Ocular involvement in brucellosis. Can J Ophthalmol. 2009;44(5):598–601. doi:10.3129/i09-019
118. Al-Kaff AS. Ocular Brucellosis. Int Ophthalmol Clin. 1995;35(3):139–145. doi:10.1097/00004397-199503530-00011
119. Calhoun FP. Diseases of the uveal tract. AMA Arch Ophthalmol. 1955;53(3):437–455. doi:10.1001/archopht.1955.00930010439021
120. Dernouchamps JP, Vaerman JP, Michiels J, Masson PL. Immune complexes in the aqueous humor and serum. Am J Ophthalmol. 1977;84(1):24–31. doi:10.1016/0002-9394(77)90319-1
121. Güngür K, Bekir NA, Namiduru M. Ocular complications associated with brucellosis in an endemic area. Eur J Ophthalmol. 2002;12(3):232–237. doi:10.1177/112067210201200311
122. Wood RM, Becker B, Woods AC. The effect of adrenal cortex hormonal therapy on experimental Brucella uveitis. Am J Ophthalmol. 1953;36(8):1025–1043. doi:10.1016/0002-9394(53)91883-2
123. Megid J, Mathias LA, Robles CA. Clinical manifestations of brucellosis in domestic animals and humans. Bentham Open. 2010. doi:10.2174/1874318801004010119
124. Rolando I, Vilchez G, Olarte L, et al. Brucellar uveitis: intraocular fluids and biopsy studies. Int J Infect Dis. 2009;13(5):e206–e211. doi:10.1016/j.ijid.2008.12.004
125. Oahalou A, Schellekens PA, de Groot-Mijnes JD, Rothova A. Diagnostic pars plana vitrectomy and aqueous analyses in patients with uveitis of unknown cause. Retina. 2014;34(1):108–114. doi:10.1097/IAE.0b013e31828e6985
126. Taravati P, Lam D, Van Gelder RN. Role of molecular diagnostics in ocular microbiology. Curr Ophthalmol Rep. 2013;1(4). doi:10.1007/s40135-013-0025-1
127. Memish Z, Mah MW, Al Mahmoud S, Al Shaalan M, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40(1):59–63. doi:10.1053/jinf.1999.0586
128. Anand AR, Madhavan HN, Therese KL. Use of polymerase chain reaction (PCR) and DNA probe hybridization to determine the Gram reaction of the infecting bacterium in the intraocular fluids of patients with endophthalmitis. J Infect. 2000;41(3):221–226. doi:10.1053/jinf.2000.0731
129. Brunetto GS, Massoud R, Leibovitch EC, et al. Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations. J Neurovirol. 2014;20(4):341–351. doi:10.1007/s13365-014-0249-3
130. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
131. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
132. Gonzales J, Doan T, Shantha JG, et al. Metagenomic deep sequencing of aqueous fluid detects intraocular lymphomas. Br J Ophthalmol. 2018;102(1):6–8. doi:10.1136/bjophthalmol-2017-311151
133. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–786. doi:10.1016/s1473-3099(07)70286-4
134. Al-Awadhi NZ, Ashkenani F, Khalaf ES. Acute pancreatitis associated with brucellosis. Am J Gastroenterol. 1989;84(12):1570–1574.
135. Busch LA, Parker RL. Brucellosis in the United States. J Infect Dis. 1972;125(3):289–294. doi:10.1093/infdis/125.3.289
136. Ayala-Gaytán JJ, Ortegón-Baqueiro H, de la Maza M. Brucella melitensis cerebellar abscess. J Infect Dis. 1989;160(4):730–732. doi:10.1093/infdis/160.4.730
137. van Rooyen MM. Brucella keratoconjunctivitis. S Afr Med J. 1981;60(5):206–207.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.