Back to Journals » International Journal of General Medicine » Volume 13
Observation of Changes in Helicobacter pylori Antigen and Antibody Positivity According to Non-Invasive Tests Before and After Helicobacter pylori Eradication Therapy in Symptomatic Patients
Authors Zaman A , Shamsuzzaman SM , Bhuiyan F, Hasan MR , Saito T
Received 28 July 2020
Accepted for publication 15 October 2020
Published 12 November 2020 Volume 2020:13 Pages 1093—1103
DOI https://doi.org/10.2147/IJGM.S273368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Anandita Zaman,1 SM Shamsuzzaman,1 Farshid Bhuiyan,2 Md Riasat Hasan,3 Takashi Saito3
1Dhaka Medical College, Department of Microbiology, Dhaka, Bangladesh; 2Holy Family Red Crescent Medical College, Department of Physiology, Dhaka, Bangladesh; 3Division of Clinical Cariology and Endodontology, Department of Oral Rehabilitation, School of Dentistry, Health Sciences University of Hokkaido, Hokkaido, Japan
Correspondence: Md Riasat Hasan
Division of Clinical Cariology and Endodontology, Department of Oral Rehabilitation, School of Dentistry, Health Sciences University of Hokkaido, Hokkaido, Japan
Tel +81-133-23-1129
Fax +81-133-23-1296
Email [email protected]
Background: Non-invasive tests can help with the diagnosis of Helicobacter pylori (H. pylori) infection and in determining patient prognosis following H. pylori eradication therapy. The aim of the study was to detect H. pylori antigens in the stool in symptomatic patients and to observe changes in the antigen test results following H. pylori eradication therapy.
Methods: A prospective study was conducted. Blood, urine and stool samples were collected from 62 dyspeptic patients. Anti-H. pylori IgM and IgG antibodies were detected in the serum by ELISA, anti-H. pylori IgG antibodies were detected in the urine by ICT and H. pylori antigens were detected in the stool by ELISA. Among the 62 patients, 39 (62.90%) were positive with all three methods. These 39 patients were asked to complete a 2-week course of medication and return after 6 weeks following completion of therapy to undergo repeated tests. In total, 3 dropped out of the study.
Results: Among the 62 dyspeptic patients, 41 (66.13%) were positive for serum IgG according to ELISA, 39 (62.90%) were positive for urine IgG according to ICT, 8 (12.90%) were positive for serum IgM according to ELISA, and 42 (67.74%) were positive for HpSA according to ELISA. After eradication therapy, 18 (50.00%) patients were positive for serum IgG, 19 (52.78%) were positive for urine IgG, 4 (11.11%) were positive for serum IgM and 5 (13.88%) were positive for HpSA. The difference in HpSA positivity before and after eradication therapy was statistically significant (P < 0.05).
Conclusion: This study involved non-invasive procedures that can be used as first-line screening tools for the detection of active H. pylori infection to observe the role of HpSA test in diagnosis and assessment of prognosis following eradication therapy for H. pylori.
Keywords: H. pylori, RAPIRUN, ELISA, prognosis, HpSA
Introduction
H. pylori is a spiral-shaped, slow-growing, gram-negative, microaerophilic, multi-flagellated, highly motile bacterium found in the gastric mucosa lining the upper gastrointestinal tract (stomach and duodenum) of humans and animals. It is associated with many different gastric and extra-gastric diseases. H. pylori is a major cause of gastritis and peptic ulcer disease.1–3 It is the first formally recognized bacterial carcinogen. The bacterium was classified by the International Agency for Research on Cancer (IARC) and the World Health Organization (WHO) as a definite class I carcinogen in 1994, putting it in the same class as cigarette smoking.4 Infection by H. pylori usually occurs early in life and, unless eradicated by specific antibiotic treatment, persists throughout an individual’s lifetime.5 H. pylori, which infects more than 50% of the entire world’s population, has become a global health problem associated with numerous benign and malignant gastric diseases, including chronic gastritis, gastric and duodenal ulcers, gastric cancer and gastric MALT (mucosa-associated lymphoid tissue) lymphomas.6–8 Although two main risk factors for peptic ulcer disease (PUD) are H. pylori infection and NSAIDs, but about one-fifth of PUD cases are not related to these two causes.9,10 In a study conducted among chronic cirrhotic patients, it was found that there is no significant association between H. pylori infection and peptic ulcer.11 Various etiological factors for PUD make it even more necessary to evaluate the cause, before blindly prescribing eradication therapy.
The prevalence of H. pylori infection is very high in Bangladesh. In a study conducted in 1995, 92% of the Bangladeshi population was found to be H. pylori positive by ELISA.12 Other studies performed in Bangladesh reported that more than 50% of infants were positive for H. pylori by the age of 2 years.13,14 In another recent study conducted in Bangladesh, 92.7% of subjects were tested positive for H. pylori by stool antigen test, which is very high.15 A reliable diagnosis and administration of H. pylori eradication therapy would result in a reduction of the incidence of gastroduodenal diseases, including gastric cancer associated with H. pylori infection and also decrease the number of new infections in the future. Assessment of prognosis following eradication therapy is necessary to detect treatment failure (which is quite common now due to multidrug resistance of organisms), recurrence and relapse.
A number of diagnostic tools, including invasive and non-invasive methods, are available for the diagnosis of H. pylori infection. Invasive tests include culture, histology, rapid urease test and detection of genetic material by PCR of endoscopic biopsy tissue. Non-invasive methods include the urea breath test (UBT), serology, antibody detection in the urine and antigen detection in the stool and other molecular methods.16 Also, a recent study has shown significant correlation between H. pylori infection and monocytosis in areas with high infection percentage.17 But there is no definite method as a gold standard to detect H. pylori infection.18,19
To date, invasive tests such as RUT, histology and culture have been considered as the standard methods, but all of them require endoscopy, which is expensive and inconvenient, has a risk of complications and is only available in gastroenterology departments of tertiary level hospitals. In context of Bangladesh, using endoscopy for diagnosis of H. pylori infection is not a practical solution because of its expense, associated hazards and unavailability at all levels of health-care facilities. Moreover, it would be inhumane to attempt to convince patients to undergo a second endoscopy for prognostic purpose.
As a result, simple, rapid, reliable and non-invasive diagnostic tests have become essential in clinical practice. Out of the non-invasive tests, till date, the most popular and well-accepted method among clinicians for diagnosis of H. pylori infection is, detection of antibodies in the blood. Serological tests can detect anti-H. pylori IgM, IgG and IgA antibodies in serum, but only the IgG antibody test shows reliable results, but can be found even months after a successful treatment.20–22.So while it can fairly be used to diagnose H. pylori infection, is not a reliable tool for assessing prognosis following eradication therapy.
C-urea breath test is non-invasive, safe, accurate, highly sensitive and specific and a reliable prognosis tool following eradication therapy, but is very expensive, technically difficult.23
A quick and easy non-invasive test based on Immunochromatographic Test (ICT) assay for qualitative detection of H. pylori antibody in urine, RAPIRUN® (Otsuka Pharmaceutical Co., Ltd. Tokyo, Japan) has been evaluated in many countries showing high sensitivity, specificity, and accuracy.24,25 However, this test needs more validation in population before being used in clinical practice. Some other newer non-invasive tests like DNA detection in the stool and saliva are quite expensive, time consuming and require technical expertise. Currently, these tests are still in research and development.26
The detection of antigens in the stool by ELISA has shown in various studies to be an effective diagnostic test owing to its high specificity and sensitivity seen in various studies.27–29 In infected individuals, H. pylori sticks to the gastric epithelial wall and is excreted in the feces, thus is a direct test of initial infection and is in superiority of serologic tests.30 The stool antigen (HpSA) test is simple, rapid, reliable, non-invasive, hazard free and comparatively inexpensive. Hence, it has the potential to become the preferred test among practitioners as a diagnostic and prognostic tool.
Although the Helicobacter pylori Stool Antigen (HpSA) test is a reliable way to establish the presence of H. pylori, still, in this study, the adjunctive use of other non-invasive tests, namely, serology (ie, serum antibody IgM and IgG) and the rapid detection of antibodies in urine (RAPIRUN) were used to ensure the reliability of the diagnosis of H. pylori infection, while still keeping the main focus of the study in determining the value of the HpSA test in diagnosis of H. pylori infection and assessment of prognosis following eradication therapy.
The aim of the study was to detect H. pylori antigens in the stool in symptomatic patients, observe the changes in the antigen test results following H. pylori eradication therapy, detect H. pylori IgM and IgG antibodies in the serum by ELISA, detect H. pylori IgG antibodies in the urine by ICT, detect H. pylori antigens in the stool by ELISA and compare the results before and after H. pylori eradication therapy.
Materials and Methods
Study Design and Ethics
This prospective study was carried out in the Department of Microbiology of Dhaka Medical College, Bangladesh, in collaboration with Bikrampur Bhuiyan Medical College, Bangladesh, and Health Sciences University of Hokkaido, Japan. A pre-designed data collection form/questionnaire was completed prior to sample collection. The protocol was approved by the Research Review Committee (RRC) of the Department of Microbiology of Dhaka Medical College, and ethics approval was obtained from the Ethical Review Committee (ERC) of Dhaka Medical College, approval number DMC/Ethical/2013/11. The study was conducted in accordance with Helsinki Declaration as revised in 2013.
Inclusion Criteria
Adult patients with specific symptoms indicating possibility of gastritis and\or peptic ulcer disease and\or gastric carcinoma and\or gastric lymphoma. Specific symptoms include
- Upper abdominal or lower chest pain with or without relationship to food
- Regurgitation, heartburn and water brash
- Anorexia, nausea and vomiting
- Bloating, belching and flatulence
- Hematemesis, hematochezia and melena.
Exclusion Criteria
- Patients who were unwilling to be included in this study and those who refused to come back for retesting.
- Patients who had partial or complete gastrectomy or gastro-jejunostomy.
- Patients who had received H. pylori eradication therapy in past two months.
- Patients who had taken any antibiotic, colloidal bismuth compound, proton-pump inhibitor in the last month and/or taking anti-acids for the last two days.
- Patients who have known hypersensitivity towards penicillin group of drugs.
In this study, 62 dyspeptic patients were tested for the presence of anti-H. pylori serum IgM and IgG antibodies by ELISA, IgG antibodies in the urine by RAPIRUN® and H. pylori HpSA by ELISA. In this study, patients who were positive for HpSA, serum IgM and/or IgG and urine IgG were selected as candidates for H. pylori eradication therapy. All the patients received the same medication, Pylotrip® (Square Pharmaceuticals Ltd., Bangladesh) (Table 1) for a period of two weeks. Six weeks after the completion of therapy, the same tests were repeated to detect changes in their status and to assess how reliable the detection of HpSA by ELISA is for assessment of prognosis.
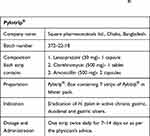 |
Table 1 Eradication Therapy Used in the Study, Pylotrip® (Square Pharmaceuticals Ltd., Dhaka, Bangladesh) |
Sample Collection
A total of 62 samples each of blood, urine and stool were collected from patients of sexes in different age groups suffering from symptoms indicating gastritis, peptic ulcer disease, gastric carcinoma and/or gastric lymphoma who visited Bikrampur Bhuiyan Medical College in the first phase (before the prescription of H. pylori eradication therapy) and were tested for monoclonal H. pylori antigens in the stool, serum IgM and IgG and urine IgG. Positive gold standard test was defined as positive serum H. pylori IgG antibody.
None of the patients had gone through any investigation for detection of H. pylori infection prior to this study. Out of 62 patients, 24 have received medications such as antacids, PPIs and H2 receptor antagonists from time to time for symptoms related to H. pylori infection, but never received the eradication therapy. Samples were collected by following procedures:
Serum
With all aseptic precaution, 3 mL of venous blood was collected from each patient. After collection, the blood was kept at room temperature for one hour, followed by centrifugation at 3000 rpm for 10 minutes. The serum was separated and was taken in a cryotube and kept at ≤ −20º C until serological tests were performed with a maximum preservation time limit of 30 days.31 The diluted sample was stored at 2–8ºC for up to 7 days.
Urine
Around 10 mL of random voided urine was collected from each patient in a 50 mL sterile, airtight container at room temperature. Test was performed with minimum delay. Then, the sample was discarded, as it would be of no further use.
Stool
A clean, dry, waterproof, airtight container containing no detergent, preservatives or transport media was supplied to each patient and asked to come with freshly passed stool in the morning next day. A spoonful of stool from the sample was taken. Proper dilution technique was performed and a small amount of diluted stool sample was pipetted into a cryotube. It was stored at 2–8ºC and held up to maximum time of 72 hours. When the test could not be performed within this time frame, it was frozen immediately upon receipt at ≤−20ºC until tested. Specimen was allowed to freeze and thaw twice at most.
Patients who were positive for all three tests, namely monoclonal HpSA, serum IgG and urine IgG, were considered infected with H. pylori and were selected to receive H. pylori eradication therapy of specific brand and combination (Table 1) for a period of two weeks. In the second phase, six weeks after the completion of the eradication therapy, blood, urine and stool samples were taken from the 36 patients who returned for repeated tests. In total, 3 dropped out of the study.
Tests Performed
Detection of Anti-H. pylori IgM in the Serum by ELISA
ELISA for the anti-H. pylori IgM antibody was performed using a commercial test kit (SERION ELISA® Classic Helicobacter pylori IgM, Serion, Germany). The manufacturer’s instructions were followed during the procedure.
The presence of IgM antibodies against H. pylori was confirmed when the serum level exceeded 30 U/mL.
Detection of Anti-H. pylori IgG in the Serum by ELISA
ELISA for the anti-H. pylori IgG antibody was performed using a commercial test kit (SERION ELISA® Classic Helicobacter pylori IgG, Serion, Germany). The manufacturer’s instructions were followed during the procedure.
The presence of IgG antibodies against H. pylori was confirmed when the serum level exceeded 50 U/mL.
Detection of Anti-H. pylori IgG in Urine by the Rapid Detection Test (RAPIRUN®)
ICT for the detection of anti-H. pylori IgG antibody was performed using a rapid detection test kit (RAPIRUN®, Otsuka Pharmaceuticals, Japan). The manufacturer’s instructions were followed during the procedure.
•Single band on control (C) panel: Test is valid.
•No band on control (C) panel: Test is invalid.
•Band on both control (C) and test (T) panel: Test is valid and positive.
•Band on test (T) panel, no band on control (C) panel: Test is false positive and invalid.
Detection of Helicobacter pylori Antigen in Stool Specimens by ELISA
ELISA for the H. pylori antigen (monoclonal) in stool specimens was performed using a commercial test kit (Premier Platinum HpSA Plus®, Meridian Bioscience Europe, Italy). The manufacturer’s instructions were followed during the procedure.
By spectrometric dual wavelength (450/630 nm):
Negative: <0.100
Positive: ≥0.100
Negative Control: <0.100
Positive Control: ≥0.600
Statistical Analysis
The results of the study were recorded systematically, and the statistical analysis of the data was performed using SPSS 20.0 for Windows 7. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of serum ELISA were calculated manually using a standard formula. The level of significance was set at P<0.05.
Results
It was observed that 52% of patients had heartburn, and 39% of patients reported that they experienced episodes of vomiting. Pain (35%) and flatulent dyspepsia (47%) were the other 2 common complaints (Figure 1)
 |
Figure 1 Bar diagram showing the clinical presentations in patients (n=62). |
Among the 62 patients, 15 (25%) patients were current or past smokers, and 47 (75%) were non-smokers. In addition, 21 (33.87%) patients had a habit of using tobacco/jarda, and 41 (66.13%) did not have a habit of taking tobacco/jarda. Additionally, 2 (3.23%) patients had a history of alcohol consumption, and 60 (96.77%) patients had never consumed alcohol (Figure 2).
 |
Figure 2 Bar diagram showing personal habits regarding smoking, tobacco/jarda consumption and alcohol intake in the study population (n=62). |
The study population was between 18 and 70 years old, with a mean age of 35.50 years. Among the 62 patients, 35.48% (n=22) were in the 31–40 year age group; 22 (35.48%) were male, and 40 (64.52%) were female, with a female–male ratio of 1.8:1 (Table 2).
 |
Table 2 Age and Sex Distributions of the Study Population (n=62). The Age and Sex Distributions of the Selected Patients. Female: Male Ratio = 1.8:1 |
Among these 62 patients, 7 (11.29%) were positive according to all 4 tests, and 39 (62.90%) were positive according to 3 tests (HpSA, serum IgG and urine IgG). Forty-two (67.74%) were positive only for HpSA, 41 (66.13%) were positive only for serum IgG, 39 (62.90%) were positive for urine IgG and 8 (12.90%) were positive for serum IgM (Table 3).
 |
Table 3 Number of H. pylori-Positive Patients According to the 4 Different Methods Before Eradication Therapy (n=62) |
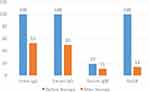
Thirty-six patients returned for retesting after therapy. Among them, before therapy, 100.00% (n=36) were positive for urine IgG, 100.00% (n=36) were positive for serum IgG, 100.00% (n=36) were positive for HpSA and 19.44% (n=7) were positive for serum IgM. After therapy, 52.78% (n=19) were positive for urine IgG, 50% (n=18) were positive for serum IgG, 13.88% (n=5) were positive for HpSA, and 11.11% (n=4) were positive for serum IgM. The difference in HpSA positivity before and after eradication therapy was statistically significant (p<0.05) (Figure 3).
Among the 62 patients, 42 (67.74%) were positive for HpSA, and 20 (32.26%) were negative for HpSA. Of the 42 (67.74%) HpSA-positive patients, 39 (95.12%) were positive for serum IgG, 3 (14.29%) were negative for serum IgG, 38 (97.44%) were positive for urine IgG, 4 (17.39%) were negative for urine IgG, 7 (87.50%) were positive for serum IgM and 35 (64.81%) were negative for serum IgM. Among the 20 (32.26%) HpSA-negative patients, 2 (4.88%) were positive for serum IgG, 18 (85.71%) were negative for serum IgG, 1(2.56%) was positive for urine IgG, 19 (92.61%) were negative for urine IgG, 1 (12.50%) was positive for serum IgM, and 19 (35.19%) were negative for serum IgM (Table 4).
Among the 62 patients, 41 (66.13%) were positive for serum IgG, and 21 (33.87%) were negative for serum IgG. Among the 41 (66.13%) serum IgG-positive patients, 39 (92.86%) were positive for HpSA, and 2(10.00%) were negative for HpSA. Among the 21 (33.87%) serum IgG-negative patients, 3(7.14%) were positive for HpSA, and 18 (90.00%) were negative for HpSA. The HpSA test showed sensitivity, specificity, PPV, NPV and accuracy of 95.12%, 85.71%, 92.86%, 90.00% and 91.94%, respectively, when measured against serum IgG (Table 5).
 |
Table 5 Shows the Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and Accuracy of the Serum IgG, Urine IgG and Serum IgM Test (Against HpSA Test) |
Before therapy, for serum IgG test, sensitivity was 92.86%, specificity was 90.00%, PPV was 95.12%, NPV was 95.71%, and accuracy was 91.94%. For urine IgG test, sensitivity was 90.48%, specificity was 95.00%, PPV was 97.44%, NPV was 92.61%, and accuracy was 91.94%. For serum IgM test, sensitivity was 16.67%, specificity was 95.00%, PPV was 87.50%, NPV was 35.19%, and accuracy was 41.94% when measured against the HpSA test (Table 6).
After the completion of therapy (n=36), only 5 (13.89%) patients were positive for HpSA, and 31 (86.11%) were negative. Among the 5 (13.86%) HpSA-positive patients, 4 (22.22%) were positive for serum IgG, 1 (05.56%) was negative for serum IgG, 3 (15.79%) were positive for urine IgG, 2 (11.76%) were negative for urine IgG, 4 (100%) were positive for serum IgM, and 1 (03.12%) was negative for serum IgM. Of the 31 (86.11%) HpSA-negative patients, 14 (77.73%) were positive for serum IgG, 17 (94.44%) were negative for serum IgG, 16 (84.21%) were positive for urine IgG, 15 (88.24%) were negative for urine IgG, 0 (00.00%) were positive for serum IgM, and 32 (100%) were negative for serum IgM (Table 7).
 |
Table 7 Relationship of the Serum IgG, Urine IgG and Serum IgM Test and the HpSA Test for the Detection of H. pylori Infection After Therapy (n=36) |
For the HpSA test, after therapy, the sensitivity was 22.22%, the specificity was 94.44%, the PPV was 80% and the NPV was 54.84%, when measured against serum IgG test (Table 7).
Table 8 shows the relationship between HpSA test results before and after eradication therapy. Of the 62 dyspeptic patients, before eradication therapy, 42 (67.74%) were positive for HpSA, and 20 (32.26%) were negative. After the completion of therapy (n=36), only 5 (13.89%) were positive for HpSA, and 31 (86.11%) were negative.
 |
Table 8 Relationship Between HpSA Test Results Before and After Eradication Therapy. P-value is <0.001 |
Discussion
A number of diagnostic tools, including invasive and non-invasive methods, are available for the diagnosis of H. pylori infection. Invasive tests include culture, histology, the rapid urease test and the detection of genetic material by PCR in specimens obtained by endoscopic biopsy. The non-invasive methods include the urea breath test, serology, antibody detection in urine and antigen detection in stool. However, no single test has yet been shown to be reliable enough to be used alone as a gold standard. All of the tests have their own limitations, disadvantages and shortcomings. Thus, this study was conducted to observe the changes in H. pylori stool antigen (HpSA) test both before and after eradication therapy to assess its reliability as a diagnostic and prognostic tool.
In the present study, Figure 1, shows the different clinical presentations in patients, and the two most common symptoms were heartburn (52%) and flatulent dyspepsia (47%). H. pylori infection has been associated with dyspepsia and non-ulcer dyspepsia, although this association is still unconfirmed.32
Figure 2, shows the personal habits of the subjects in this study. Smokers and/or tobacco (jarda) chewers were found to have a lower risk of H. pylori infection (p<0.001) than non-smokers and those who do not chew tobacco (jarda). This finding is in agreement with that of a study from the USA that reported that negated any significant relationship between smoking and H. pylori infection.33 However, most studies have shown a relationship between smoking and the occurrence of H. pylori infection. In one study, 73 out of 147 patients with gastric and duodenal ulcers were (49.6%) smokers.34 The lower number of smokers in this study might be because the majority of the study population was female, and females in Bangladesh generally do not smoke. On the other hand, tobacco/jarda consumption was observed in a greater proportion of patients than in previous studies, possibly because women in Bangladesh, especially elderly individuals, have a habit of chewing tobacco. Only a small percentage (3%) of patients reported alcohol consumption, so no conclusion about the association between alcohol consumption and H. pylori infection could be drawn.
In this study, among symptomatic patients (n=62), the percentage of serum IgG-positive was 66.13% (n=41). In two studies conducted in Bangladesh, it was found that seroprevalence of H. pylori infection in adult population was approximately 90%-92%.12,13 Detection of anti-H. pylori antibodies in serum has been universally a convenient instrument for the well-accepted “test and treat” strategy employed by physicians owing to its fairly good specificity and sensitivity.
In the present study, selected patients with serum IgG positivity before therapy was 100% (n=36), which reduced to 50.00% (n=18) when re-evaluation after eradication therapy (Figure 3) which is similar to another study where reduction in seropositivity by 50% following eradication therapy could be seen.35 These circulating antibodies decrease relatively slowly and can persist at constant levels for months to years in infected individuals with unchanged antibody titer even after eradication of the infection. Because of this slow reduction in the antibody titer it is difficult to differentiate between past and present infections using this test, and it does not provide very useful information regarding prognosis after eradication therapy.
In this study, out of 62 symptomatic patients, 8(12.90%) were positive for serum IgM against H. pylori (Table 3) despite having a high percentage of HpSA positivity (67.74%), which indicates acute infection (n=8) with sensitivity, specificity and accuracy of 16.67%, 95.00% and 41.94%, respectively (Table 5). Therefore, it does not seem to be a very reliable test for diagnosing acute H. pylori infection.
In the present study, a rapid ICT known as RAPIRUN® was used to detect IgG against H. pylori in urine. In this study, out of 62 symptomatic patients, 39 (62.90%) were positive for urine IgG according to the rapid detection test (RAPIRUN®) (Table 3). It showed some promising results with regard to sensitivity, specificity, PPV, NPV and accuracy of 90.48%, 95.00%, 97.44%, 92.61% and 91.94%, respectively (Table 5). In two different studies conducted in Indonesia and Vietnam, the sensitivity, specificity and accuracy were seen to be 83-85%, 90-94% and 87-93%, respectively.24,25 However, after evaluation in all the studies, the conclusion was made that the rapid urine test can be used for a rough diagnosis, but the diagnosis needs to be confirmed by other methods, and that the test was not suitable for evaluating the results of eradication therapy.36
In the present study, out of 62 untreated symptomatic patients, 42 (67.74%) had positive results on the H. pylori stool antigen (HpSA) test (Table 3). In contrast, 41 (66.13%) out of 62 patients had positive results for the serum IgG test. The detection of serum anti-H. pylori IgG is a well-accepted method for the diagnosis of H. pylori infection that is used by physicians worldwide. When the results of both methods were compared before therapy (Table 6), the p-value of <0.001 indicates that there is a significant difference, indicating the potential of the HpSA test as a first-line tool for identifying H. pylori infection in symptomatic patients. Also the sensitivity, specificity and accuracy for the HpSA test was 95.12%, 85.71% and 91.94%, respectively (Table 4). This result is very similar to that of another study performed in Bangladesh, which showed a sensitivity of 95.9%, a specificity of 92.3%, a PPV of 93% and an NPV of 95% for the stool antigen test and is in consistency with some newer studies.27–29 So the current study shows that HpSA test can be a reliable tool for diagnosis of H. pylori infection.
Through this study, it can be seen that the HpSA detection test can be used as a reliable method for screening for H. pylori infection in symptomatic patients prior to endoscopy or in places where the facility is not available. It was also found to be a good method for monitoring prognosis following H. pylori eradication therapy. As a consequence, with regard to the detection of H. pylori infection and the assessment of post-therapy prognosis, the HpSA test seems to be a good alternative to tests such as biopsies and urea breath tests. This technique has the following advantages over other techniques,
- It is simple, fast, safe, inexpensive, needs no preparation prior to sample collection, requires no expertise for collection and preparation of specimen, can be obtained easily, even from new-born children.
- The sensitivity and specificity are high (according to this study 95.12% and 85.71%, respectively).
- Most importantly, the HpSA test can become negative as soon as two weeks following H. pylori eradication therapy.
Our study has a limitation of choice for gold standard. The choice of gold-standard test greatly affects the results of non-invasive testing for H. pylori and use of a single test as the gold standard increases the error rate. Tests such as RUT, histology, culture and UBT would have been more reliable for the purpose of comparison while evaluating sensitivity and specificity for the HpSA test, both before and after therapy. In absence of these tests serum IgG against H. pylori was determined as gold standard. It is a fair tool for comparison before therapy, but after therapy, its validity does not have high enough reliability itself to be compared with the HpSA test for determination of specificity and sensitivity. However, in the present study, out of 36 HpSA-positive patients before therapy, only 5(13.88%) were positive following eradication therapy (Figure 3). The p-value for the difference in the number of patients who were positive for HpSA before and after therapy was <0.05, which is significant (Table 8).
Conclusion
As a completely non-invasive and comparatively inexpensive procedure, the stool HpSA detection test can be used as a first-line screening tool for the detection of active H. pylori infection in symptomatic patients in locations where highly technical and specialized investigations such as endoscopy are not available. The use of this test can also eliminate the need for endoscopy and the unnecessary discomfort, expense, and complications that can result. The HpSA can be used in mass surveys to identify and eradicate H. pylori infection to reduce the load of gastric malignancies. Most importantly, it can be used as a prognostic tool to assess the success of eradication therapy and the rate of recurrence, provided that patient compliance is good. However, reliable culture or other highly invasive biopsy-based tests might be, it is challenging or even impossible to repeat them after eradication therapy, considering all of their drawbacks. Therefore, the stool antigen test shows a promise as a prognostic tool and represents progress in the next generation of investigations.
Ethics Approval and Informed Consent
Ethical Approval
The protocol was approved by the Research Review Committee (RRC) of the Department of Microbiology of Dhaka Medical College, and ethics approval was obtained from the Ethical Review Committee (ERC) of Dhaka Medical College (approval number DMC/Ethical/2013/11). The study was conducted in accordance with Helsinki Declaration as revised in 2013.
Informed Consent
All participants have signed informed consent.
Funding
This work was supported by the Inoue Foundation for Science, International Exchange Promotion Aid.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161(4):626–633. doi:10.1093/infdis/161.4.626.
2. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. doi:10.1016/S0140-6736(83)92719-8
3. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–1416. doi:10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3
4. Goodwin CS, Worsley BW. Microbiology of Helicobacter pylori. Gastroenterol Clin North Am. 1993;22:5–19.
5. Imrie C, Rowland M, Bourke B, Drumm B. Is Helicobacter pylori infection in childhood a risk factor for gastric cancer? Pediatrics. 2001;107(2):373–380. doi:10.1542/peds.107.2.373
6. Smith SM, O’Morain C, McNamara D. Helicobacter pylori resistance to current therapies. Curr Opin Gastroenterol. 2019;35(1):6–13. doi:10.1097/MOG.0000000000000497
7. Quaglia NC, Dambrosio A. Helicobacter pylori: A foodborne pathogen? World J Gastroenterol. 2018;24(31):3472–3487. doi:10.3748/wjg.v24.i31.3472
8. Sjomina O, Pavlova J, Niv Y, Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23(Suppl 1):e12514. doi:10.1111/hel.12514
9. Kanno T, Iijima K, Koike T, et al. Accommodation in a refugee shelter as a risk factor for peptic ulcer bleeding after the Great East Japan Earthquake: a case–control study of 329 patients. J Gastroenterol. 2015;50(1):31–40. doi:10.1007/s00535-014-0940-4
10. Kiss S, Zsikla V, Frank A, Willi N, Cathomas G. Helicobacter negative gastritis: polymerase chain reaction for Helicobacter DNA is a valuable tool to elucidate the diagnosis. Aliment Pharmacol Ther. 2016;43(8):924–993. doi:10.1111/apt.13564
11. Rahimkhani M, Ghofrani H. HELICOBACTER PYLORI AND PEPTIC ULCER IN CIRRHOTIC PATIENTS. Pak J Med Sci. 2008;24(6):849–852.
12. Ahmad MM, Rahman M, Rumi AK, et al. Prevalence of Helicobacter pylori in Asymptomatic Population a Pilot Serological Study in Bangladesh. J Epidemiol. 1997;7(4):251–254. doi:10.2188/jea.7.251
13. Bardhan PK. Epidemiological Features of Helicobacter pylori Infection in Developing Countries. Clin Infect Dis. 1997;25(5):973–978. doi:10.1086/516067
14. Bhuiyan TR, Qadri F, Saha A, Svennerholm A-M. Infection by Helicobacter pylori in Bangladeshi children from birth to two years: relation to blood group, nutritional status, and seasonality. Pediatr Infect Dis. 2009;28(2):79–85. doi:10.1097/INF.0b013e31818a5d9d
15. Nahar S, M. Kaderi Kibria K, Hossain M, et al. Epidemiology of H. pylori and its Relation with Gastrointestinal Disorders, A Community-based Study in Dhaka, Bangladesh. J Gastroenterol Hepatol Res. 2018;7(5):2709–2716. doi:10.17554/j.issn.2224-3992.2018.07.795
16. Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38(1):55–66. doi:10.1007/s10096-018-3414-4
17. Rahimkhani M, Mordadi A, Kazemian K, Khalili H. Comparison of Helicobacter Pylori Detection: it’s Association with Leukocytosis and Monocytosis. Infectious Disorders - Drug Targets. 2020;20. doi:10.2174/1871526520666200707113955.
18. Patel SK. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20(36):12847–12859. doi:10.3748/wjg.v20.i36.12847
19. Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection - recent developments in diagnosis. World J Gastroenterol. 2014;20(28):9299–9313.
20. Jemilohun AC, Otegbayo JA. Helicobacter pylori infection: past, present and future. Pan Afr Med J. 2016;23:1. doi:10.11604/pamj.2016.23.216.8852
21. Thaker Y. Helicobacter pylori: a review of epidemiology, treatment, and management. J Clin Gastroenterol Treat. 2016;2(2):1–5. doi:10.23937/2469-584X/1510019
22. Malfertheiner P, Megraud F, O’morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646–664. doi:10.1136/gutjnl-2012-302084
23. Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-Urea Breath Test for the Diagnosis of Helicobacter pylori Infection in Children: A Systematic Review and Meta-Analysis. Helicobacter. 2011;16(4):327–337. doi:10.1111/j.1523-5378.2011.00863.x
24. Syam AF, Miftahussurur M, Uwan WB, et al. Validation of Urine Test for Detection of Helicobacter pylori Infection in Indonesian Population. Biomed Res Int. 2015;2015:1–6. doi:10.1155/2015/152823
25. Quach DT, et al. Value of a new stick-type rapid urine test for the diagnosis of Helicobacter pylori infection in the Vietnamese population. World J Gastroenterol. 2014;20(17):5087–5091. doi:10.3748/wjg.v20.i17.5087
26. Kabir S. Review article: clinic-based testing for Helicobacter pylori infection by enzyme immunoassay of faeces, urine and saliva. Aliment Pharmacol Ther. 2003;17(11):1345–1354. doi:10.1046/j.1365-2036.2003.01577.x
27. Safarnezhad Tameshkel F, Karbalaie Niya MH, Kheyri Z, Azizi D, Roozafzai F, Khorrami S. The Evaluation of Diagnostic and Predictive Values of Helicobacter pylori Stool Antigen Test in Iranian Patients with Dyspepsia. Iran J Pathol. 2018;13(1):38–44.
28. Lario S, Ramirez-Lazaro MJ, Montserrat A, et al. Diagnostic accuracy of three monoclonal stool tests in a large series of untreated Helicobacter pylori infected patients. Clin Biochem. 2016;49(9):682–687. doi:10.1016/j.clinbiochem.2016.01.015
29. Islam M, Nahar S, Sarker M, et al. Efficacy of different laboratory tests to diagnose Helicobacter pylori infection. Faridpur Medical College Journal. 2013;8(1):11–14. doi:10.3329/fmcj.v8i1.16890
30. Koletzko S, et al. Evaluation of a novel monoclonal enzyme immunoassay for detection of Helicobacter pylori antigen in stool from children. Gut. 2003;52(6):804–806. doi:10.1136/gut.52.6.804
31. Kato M, Asaka M, Saito M, et al. Clinical usefulness of urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori: a collaborative study in nine medical institutions in Japan. Helicobacter. 2000;5(2):109–19T. doi:10.1046/j.1523-5378.2000.00017.x
32. Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterol. 2005;129(5):1756–1780. doi:10.1053/j.gastro.2005.09.020
33. Khalifa MAAA, Khodiar SEL-F, Almaksoud AA. Cigarette smoking status and Helicobacter pylori infection in non-ulcer dyspepsia patients. Egyptian Journal of Chest Diseases and Tuberculosis. 2014;63(3):695–699. doi:10.1016/j.ejcdt.2014.03.007
34. Butt J, Varga MG, Wang T, et al. Smoking, Helicobacter Pylori Serology, and Gastric Cancer Risk in Prospective Studies from China, Japan, and Korea. Cancer Prev Res. 2019;12(10):667–674. doi:10.1158/1940-6207.CAPR-19-0238
35. Rahimkhani M, Mordadi A. An Overview of Helicobacter Pylori and Diagnostic Methods. Int J App Biol Pharma Techno. 2019;10:022–034.
36. Ullah SS, Shamsuzzaman SM, Ara MN. Seropositivity of Helicobacter pylori among the fish handlers. Mymensingh Med J. 2010;19:219–224.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.