Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Obesity-Related Glomerulopathy: From Mechanism to Therapeutic Target
Authors Wei L, Li Y, Yu Y, Xu M, Chen H, Li L, Peng T , Zhao K, Zhuang Y
Received 17 August 2021
Accepted for publication 5 October 2021
Published 28 October 2021 Volume 2021:14 Pages 4371—4380
DOI https://doi.org/10.2147/DMSO.S334199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Lifang Wei,1,* Ye Li,2 Yue Yu,1 Minmin Xu,1 Huilan Chen,1 Lijie Li,1 Ting Peng,1 Kang Zhao,1 Yongze Zhuang3,*
1Department of Nephrology, The Third People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 2The Third People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China; 3Department of Nephrology, 900 Hospital of the Joint Logistics Team, PLA, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lifang Wei; Yongze Zhuang Tel +86 591 62091295
Fax +86 591 22869300
Email [email protected]; [email protected]
Abstract: Obesity-related glomerulopathy (ORG) is a secondary glomerular disease caused by obesity, with clinical manifestations such as proteinuria and glomerulomegaly. Currently, the high incidence of obesity brings a change in the spectrum of kidney diseases across the globe, including China. ORG has become another important secondary nephropathy leading to end-stage renal disease (ESRD), and its incidence has increased significantly. This trend is bound to bring about a serious socioeconomic burden. Therefore, it is urgent to study its pathogenesis and intervention measures. Currently, the occurrence and development mechanisms in ORG are complicated by many factors, which are still unclear. In the past 20 years, with the continuous intensive research on mechanisms such as hypoxia in the metabolic process, immune inflammation, and pyroptosis, there have been new advances in the mechanism of ORG, especially the important role of inflammation in podocyte injury and its impact on the progress of ORG. Here, we briefly review the possible pathogenic role of the inflammasome in the podocyte damage in ORG and summarize the possible therapeutical strategies targeting inflammasome.
Keywords: obesity, NLRP3 inflammasome, ORG, podocyte, Chinese herbal medicines
Introduction
Over the past several decades, the worldwide prevalence of obesity in people has doubled since 1980, including Latin American countries.1–6 As the more years obesity continues, the more damaging coexisting illnesses, such as chronic kidney disease, develop.7 Since the first case of obesity-related glomerulopathy (ORG) was reported in 1974, more and more studies have suggested that obesity has become an independent risk factor for the development of chronic kidney disease (CKD).8,9 ORG usually has an insidious onset, with microalbuminuria or clinically dominant proteinuria as the primary manifestation, with or without impaired renal function, and a small number of patients manifesting with microscopic hematuria or nephrotic syndrome. Pathologically, it is characterized by increased glomerular volume, focal segmental glomerulosclerosis (FSGS) and foot process widening, but the proportion of foot process fusion is low.10 Currently, It is recognized that the diagnosis of ORG should meet the following criterion:10–13 (1) body mass index (BMI, weight in kg/height in meters2) ≥ 30kg/m2(Chinese population should be> 28kg/m2), excluding endocrine obesity, drug-induced obesity and diabetes, accompanied by elevated fasting blood glucose or abnormal glucose tolerance; (2) different clinical levels of proteinuria (> 0.3g/24h), without gross hematuria and obvious microscopic hematuria; (3) renal pathology manifestations of glomerular hypertrophy with or without FSGS, immuno-fluorescence staining being oligo-immune complex deposition, which may be accompanied by IgM, C3 non-specific or segmental deposition; (4) excluding obese patients with primary renal diseases, such as IgA nephropathy, membrane nephropathy, and other secondary factors that could cause increased glomerular volume or FSGS, such as diabetic nephropathy, and lipoprotein glomerulopathy.
In this review, we will mainly describe the pathogenesis and treatment of ORG, especially the application research of Traditional Chinese Medicine (TCM), in order to provide new therapeutic targets for ORG.
BMI and Obesity
In 2016, Okabayashi et al reported 20 patients with moderate obesity (BMI <30 kg/m2) may also develop ORG, probably caused by the renal factor(s), such as low glomerular density, as well as BMI.14 Therefore, does BMI still have its shortcomings in assessing the incidence of ORG?
In clinical practice, BMI is usually used to assess the degree of obesity,15 but CKD patients often experience changes in body composition, such as muscle and adipose tissue, visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). BMI cannot distinguish the distribution of these tissues, especially the distribution of adipose tissue because there is considerable number of evidence that, compared with SAT, excessive VAT will bring more insulin resistance, and increase the risk of kidney disease.16 More recent data highlight that waist circumference (WC) as a measure of abdominal obesity which is related to VAT, provides an indicator of body composition and adds critical information along with BMI in clinical evaluations.17–20
Recently, studies have put forward the concept of Metabolic Healthy Obesity (MHO), which is a healthy way of storing fat with small fat cells (hyperplasia), and Metabolic Unhealthy Obesity (MUO), which is large hypoxic fat cells.21 Necrosis may occur in the large hypoxic fat cells due to hypoxia in the metabolic process, leading to immune cell infiltration, inflammation, and insulin resistance in adipose tissue, and ultimately leading to increased circulating lipids and glucose, as well as ectopic lipid deposition which causes inflammation in liver, muscle, heart, kidney, pancreas and other tissues.
It is declared that obesity is a chronic, relapsing progressive disease.22 The metabolic state of the human body can change from a “metabolic health” state to an unhealthy phenotype.23 This transition may be related to continued obesity, aging, insufficient vascularization, hypoxia, decreased adiponectin secretion.24 And it gradually declines SAT ability to proliferate and effectively store lipids.25
Obesity-Related Genes
According to a new study by researchers at the University of Chicago, a series of genetic variations affect the expression of obesity-related genes in the brain and adipose tissue. The research team found that changes in the expression of obesity-related genes are associated with changes in metabolism and behavior, indicating that these variants have a combined effect that increases the risk of obesity. The strongest genetic association with human obesity corresponds to a set of genetic variations in a gene called FTO. More than 40% of people have one copy of these variants, and 16% have two copies, which increases their risk of becoming obese by 70%. Their study shows that multiple variants on a common haplotype modify the regulatory properties of several enhancers targeting IRX3 and IRX5 from mega-base distances. They demonstrate that these enhancers affect gene expression in multiple tissues, including adipose and brain, and impart regulatory effects during a restricted temporal window.26 These findings may also provide us with orientation of gene therapy targets for obesity. And whether these genes are related to ORG remains to be further studied.
Risk Factors of ORG
ORG is a kidney disease secondary to obesity, but obesity is not the only factor that causes ORG. The degree of renal damage in ORG is not necessarily related to the severity of obesity. Current research indicates that there are the following additional or susceptibility factors to explain the significant differences in susceptibility to kidney injury caused by obesity between individuals:27 (1) visceral fat obesity; (2) low nephron number, may due to low birth weight, intra-uterine growth retardation and preterm birth; (3) obesity-associated conditions or complications (eg, sleep apnea syndrome, pulmonary hypertension and right ventricular overload, nonalcoholic fatty liver disease); (4) congenital or acquired nephron mass reduction (eg, unilateral renal agenesis, nephrectomy); (5) progressive loss of functioning nephron (eg, chronic kidney disease of any cause, aging).
Pathogenesis of ORG
However, the mechanism by which obesity causes kidney disease is unclear. The pathogenesis of ORG is mainly summarized in three aspects: hemodynamic changes and renin-angiotensin-aldosterone system (RAAS) activation, adipose tissue-related factors and inflammation. The progression mechanism of ORG is also diverse and very complex, among wtih podocyte damage caused by chronic low-grade lipid accumulation, compensatory hyperplasia, fibrosis, oxidative stress and apoptosis is particularly important (Figure 1).
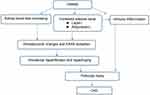 |
Figure 1 Pathogenesis of ORG. |
Hemodynamic Changes and RAAS Activation
Hemodynamic changes can appear in the early stages of obesity. Histopathologically, FSGS found in ORG are very likely caused by single-nephron hyperfiltration following abnormally increased renal plasma flow and filtration fraction. As the body weight increases, the whole body volume and kidney load also increase, which leading to increased kidney blood flow, high glomerular filtration, and high pressure within the glomerular. Those changes cause the glomerular basal membrane expansion, glomerular hypertrophy, and ultimately lead to renal impairment, such as decreased podocyte density and detachment of podocytes.28,29 Denic et al described a human model of the handling of multiple proteins by the proximal renal tubule. This model suggests that hyperfiltration itself may cause proteinuria since renal protein excretion appears to be very sensitive to single-nephron glomerular filtration rate (SNGFR). Increased SNGFR decreases the time that glomerular filtrate proteins are available for proximal tubular endocytosis, so protein reabsorption decreases and proteinuria develops.30,31 Changes in the structure of the glomerulus lead to narrowing of the glomerular filtration cavity, reduced filtration area, decreased urinary sodium excretion, and water and sodium retention. Long-term unbalanced sodium-salt balance regulation and ultrafiltration will further aggravate glomerular damage and form a vicious circle.
Not only renal hemodynamic changes caused by obesity, but also RASS activation is an important factor in the renal damage pathway.32 Previous studies have shown that increased loop reabsorption sodium, high leptin, high insulin, sympathetic nervous system (SNS) activation and activated RASS cause an increasing in angiotensin (Ang II), while renal sodium retention and urinary sodium excretion are being increased, further aggravating the kidney damage. Ang II is closely related to the occurrence and development of ORG. Therefore, blocking the activation of RASS has a better effect on reducing obesity-related glomerular proteinuria and delaying the progression of renal function.
Role of Adipose Tissues and Inflammation
In obesity, Adipose tissue not only has the function of storing and providing energy, but also as an endocrine organ to widely influence and regulate the body’s energy metabolism and various functions. Adipose tissue in and around the kidneys secretes adipokines, such as leptin, adiponectin, cytokines (TNF‑α, IL‑6, and IL‑1β) in local inflammation, and the chemotaxis of a variety of cell populations such as macrophages, endothelial cells, fibroblasts, and leukocytes. The adipokines can activate c‑Jun N‑terminal kinase (JNK) and nuclear factor κ‑light‑chain‑enhancer of activated B cells (NF‑κB) signaling pathways. These inflammatory signaling pathways are involved in the phosphorylation of different proteins and transcriptional factors, causing an increased secretion of proinflammatory molecules (TNF‑α, IL‑6), chemokines [monocyte chemoattractant protein 1 (MCP‑1), and proatherogenic mediators [plasminogen activator inhibitor‑1 (PAI‑1)].33 Furthermore, many studies have provided evidence that the activation of complement system can increase adipose tissue inflammation which can conversely promote the production of complement components in adipose tissue and enhance adipocyte hypertrophy.34–37 Inflammation is often associated with leukocyte infiltration, via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and reactive oxygen species (ROS) generation. These mechanisms all lead to glomerulosclerosis, renal fibrosis, and finally proteinuria.38 At present, we mainly focus on leptin, adiponectin and inflammation.
Leptin
Leptin is mainly a small peptide hormone produced by adipose tissue, especially visceral fat, which can be bound to receptors in the hypothalamus to regulate the balance of energy intake. It can affect kidney function through direct and indirect effects. At the same time, when the level of renal function decreases, the level of leptin in the body will also increase significantly, further impairing renal function. On the one hand, leptin can be bound to specific receptors in the glomerulus to cause up-regulation of the pro-fibrotic transforming growth factor-β (TGF-β) response and increase the production of extracellular matrix components, directly affecting the kidneys. On the other hand, leptin also causes high blood pressure by activating SNS, which indirectly affects the kidneys. Current studies have shown that leptin activates SNS through the JAK/STAT pathway and binding to the melanocortin 4 (MC4) receptor. These mechanisms indicate that obesity plays an important role in kidney disease and its related hypertension.39
Adiponectin
Adiponectin has anti-atherosclerosis, anti-inflammatory and anti-diabetic properties, by which activate a number of important regulatory cell signaling mechanisms, including adenosine monophosphate‐activated protein kinase (AMPK), mitogen‐activated protein kinase (p38-MAPK) signaling pathways and lipid metabolism pathways.40 There are two receptors that adiponectin binds to, Adiponectin receptor 1 (AdipoR1) and Adiponectin receptor 2 (AdipoR2). At the renal level, AdipoR1 is found in the podocytes and proximal tubule. The protective effect of adiponectin on the kidney is also closely related to podocytes. Xu et al used palmitic acid (PA) to induce podocyte injury in vitro. After adiponectin was added, the expression of nucleotide binding and oligomerization domain like receptor protein 3 (NLRP3) inflammasome-related proteins and inflammatory cytokines (IL-18 and IL-1β) decreased, indicating that adiponectin down-regulates ROS/NF-κ B/NLRP3 pathway to protect podocytes.41 These mechanisms demonstrate a link between hypo-adiponectinemia and kidney dysfunction.
NLRP3 Inflammasome
In addition to lipid metabolism disorders, activation of RAAS and SNS in the pathogenesis and progression of ORG, the activation of glomerular immune inflammation also plays a unique role.42
As is known to all, immune inflammation is the body’s innate response to various injury factors in vivo and in vitro. When body’s metabolism is abnormal, a large number of internal danger signals will be released by damaged and dead cells, which are collectively referred to a danger-associated molecular patterns (DAMPs).43 DAMPs can interact with a wide range of germline-encoded pattern recognition receptors (PRRs) to initiate a pro-inflammatory response related to metabolic disorders.44 Since the inflammasome was reported in 2002, the mechanism of inflammation caused by the inflammasome has been paid more and more attention. NLRP3 is the particular important member in the nucleotide-binding oligomerization domain-like receptor (NLR) family. NLRP3 inflammasome is an intracellular multi-protein complex (NLRP3/ASC/caspase-1 complex), which contains NLRP3, apoptosis-related spot-like protein (ASC) and caspase-1. When PRRs are activated by the DAMPs, it can promote K+ efflux, Ca2+ signaling, mitochondrial dysfunction, and ROS production, lysosomal disruption, etc., leading to NLRP3 inflammasome activation and finally resulting in cells releasing factors such as interleukins (IL-18 and IL-1β).45,46
As an important secretory organ, adipose tissue can secrete a variety of adipokines. When human body consumes too much energy, adipokines will activate adipose tissue macrophages (ATM) to shift from anti-inflammatory M2 to pro-inflammatory M1 and trigger inflammation. This was also confirmed by the research of He et al.47
Podocyte Injury in ORG
As is known to all, Podocytes are the main component of the glomerular filtration barrier. Podocytes cover the surface of glomerular capillaries through their crossed foot processes. The stability of this structure is essential for maintaining glomerular function.48,49 When podocytes are damaged, the stability of this structure will be destroyed, and the dysfunction, injury, and apoptosis of podocytes which are critical for the pathogenesis of proteinuria and development of ORG are inevitable.50 Through the study of ORG kidney pathology, it was found that the podocyte foot process was widened, some of the foot process disappeared, the density and number of podocytes was decreased, and the podocytes were even detached from the basement membrane. These structural changes eventually lead to the destruction of the function of the podocytes. Because the regenerative capacity of podocytes is extremely small, once it is damaged, it is difficult to repair.51,52 Therefore, it is vital to protect podocytes from damage and apoptotic cell death.
However, the damage mechanism of podocytes in ORG is still not very clear. At present, it is agreed that podocyte injury contains a variety of pathophysiological processes. In recent years, with the in-depth study of inflammasomes, more and more researchers have paid attention to the pathogenic role of inflammation in ORG podocytes injury.53–55 In our review, we sort out and analyze the research on the signal pathways of podocyte injury in ORG in the past 20 years, providing a new perspective to alleviate podocyte damage and delay the progression of ORG. These studies are mainly based on animal models or cell cultures, and there is a lack of research data on human kidney tissue (Table 1).
 |
Table 1 The Pathway in the Podocyte Injury |
Therapeutic Target
At present, there is still a lack of specifically effective drug for the treatment of ORG. However, with the continuous in-depth research on the pathogenesis of ORG, the treatment of ORG has gradually changed from traditional body mass reduction, antihypertensive, metabolism regulation to targeted therapy that can improve kidney metabolism and inhibit inflammatory pathway.
Traditional Treatment
In actual clinical practice, it is generally believed that actively reducing proteinuria is an important means to delay the progression of CKD. Therefore, the application of proteinuria control drugs is a common treatment for ORG, such as angiotensin-converting enzyme inhibitor (ACEI), angiotensin II type 1 receptor blocker (ARB), ACEI/ARB combination. At the same time, controlling the intake of protein, NaCl, and fat, and if necessary, combining treatment with lipid-lowering drugs and aldosterone antagonists are adopted.
However, the most basic treatment is to lose weight. Currently, there are already many clinical guidelines to guide the treatment of obesity.70–73 The main measures include lifestyle changes, exercise (including walking and cycling in our daily commuting mode74), weight loss with drugs, and bariatric surgery. In drug weight loss, recent studies have confirmed that sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 agonists protect kidney while losing weight.75–78 However, the efficacy and safety of these two drugs still need to be further clarified by a large randomized prospective study with long-term follow-up. And among all of those therapies, personalized precision medicine may contribute to the medical management and care of obesity patients.79
Traditional Chinese Medicine in ORG
Although people currently agree that in the early stages of the disease, active weight control is the most effective treatment for proteinuria, the current methods of weight loss still cannot effectively control obesity. And as the disease progresses, there is still a lack of effective long-term weight loss methods.
The study of the regulation of NLRP3 inflammasomes through diet and fatty acid-induced obesity will open up new ways to treat or alleviate the complications of metabolic inflammatory diseases. The improving knowledge of the pathophysiologic mechanisms of podocyte injury in ORG could bring the development of new antiproteinuric therapy to slow down the progression of ORG. Therefore, based on these visions, people hope to find more effective weight-loss drugs such as Chinese herbal medicines (CHMs).
TCM is based on the principles of the “holism” and “treatment based on syndrome differentiation.” Clinical trials and experimental studies have shown that CHMs have a great beneficial effect on the reduction of proteinuria and improvement of renal function. Several studies on the treatment of ORG with CHMs have been taken out to clarify the possible mechanisms of CHMs. (Table 2) However, most of these studies are animal experiments or in vitro cell studies, and there are no reports on clinical applications. Therefore, its actual clinical efficacy and safety need to be verified by large-scale randomized controlled trial (RCT).
 |
Table 2 The Possible Mechanisms of CHMs in ORG |
Conclusion
ORG is a manifestation of early metabolic disorders, as the course progresses, patients are likely to suffer from the metabolic syndrome. Treatment should be intervened as early as possible in obesity to avoid further damage to renal function which eventually lead to irreversible lesions. With in-depth research on obesity behavior, genetic background, and pathophysiological factors, as well as the complex pathological mechanisms of metabolism and podocyte damage in ORG, it is hoped that there will be more effective treatments for obesity and its related complications. CHMs are a potential treatment for ORG. There is an urgent need to further study the mechanism and conduct good randomized controlled trials to evaluate the effectiveness and safety of TCM.
Acknowledgments
Lifang Wei and Yongze Zhuang are co-corresponding authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi:10.1056/NEJMoa1614362
2. Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin North Am. 2016;45(4):571–579. doi:10.1016/j.gtc.2016.07.012
3. Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642.
4. Kovesdy CP, Furth SL, Zoccali C. World Kidney Day Steering C. Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4:1–10. doi:10.1177/2054358117698669
5. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. doi:10.1056/NEJMsa1909301
6. Jimenez-Mora MA, Nieves-Barreto LD, Montano-Rodriguez A, Betancourt-Villamizar EC, Mendivil CO. Association of overweight, obesity and abdominal obesity with socioeconomic status and educational level in Colombia. Diabetes Metab Syndr Obes. 2020;13:1887–1898. doi:10.2147/DMSO.S244761
7. Gregg EW, Shaw JE. Global health effects of overweight and obesity. N Engl J Med. 2017;377:80–81. doi:10.1056/NEJMe1706095
8. de Vries APJ, Ruggenenti P, Ruan XZ, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417–426. doi:10.1016/S2213-8587(14)70065-8
9. Hall JE, Mouton AJ, da Silva AA, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi:10.1093/cvr/cvaa336
10. Tsuboi N, Koike K, Hirano K, Utsunomiya Y, Kawamura T, Hosoya T. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol. 2013;17(3):379–385. doi:10.1007/s10157-012-0719-y
11. Kambham N, Markowitz GS, Valeri,AM, et al. Obesity-related glomerulopathy: anemerging epidemic. Kidney Int. 2001;59:1498–1509. doi:10.1046/j.1523-1755.2001.0590041498.x
12. Praga M, Hernández E, Morales E, et al. Clinical features and long‐term outcome of obesity‐associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi:10.1093/ndt/16.9.1790
13. Chen HM, Liu ZH, Zeng CH, et al. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis. 2006;48(5):772–779. doi:10.1053/j.ajkd.2006.07.025
14. Okabayashi Y, Tsuboi N, Sasaki T, et al. Glomerulopathy associated with moderate obesity. Kidney Int Rep. 2016;1(4):250–255. doi:10.1016/j.ekir.2016.08.006
15. Bovet P, Chiolero A, Gedeon J. Health effects of overweight and obesity in 195 countries. N Engl J Med. 2017;377:1496–1497.
16. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–1500. doi:10.1161/CIRCRESAHA.120.316101
17. Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61(2):103–113. doi:10.1016/j.pcad.2018.06.004
18. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39(2):79–132.
19. Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines formedical care of patients with obesity. Endocr Pract. 2016;22:1–203.
20. Koulouridis E, Georgalidis K, Kostimpa I, Koulouridis I, Krokida A, Houliara D. Metabolic syndrome risk factors and estimated glomerular filtration rate among children and adolescents. Pediatr Nephrol. 2010;25(3):491–498. doi:10.1007/s00467-009-1364-x
21. Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):405–420. doi:10.1210/endrev/bnaa004
22. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: the obesity society 2018 position statement. Obesity. 2019;27(1):7–9. doi:10.1002/oby.22378
23. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(9):714–724. doi:10.1016/S2213-8587(18)30137-2
24. Ji T, Zhang L, Tang Z, Sun F, Li Y, Ma L. Prevalence of normal-weight obesity in community-dwelling Chinese older adults: results from the Beijing longitudinal study of aging. Diabetes Metab Syndr Obes. 2020;13:1611–1617. doi:10.2147/DMSO.S246884
25. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. doi:10.1038/s41580-018-0093-z
26. Sobreira DR, Joslin AC, Zhang Q, et al. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science. 2021;372:1085–1091. doi:10.1126/science.abf1008
27. Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017;2(2):251–260. doi:10.1016/j.ekir.2017.01.007
28. Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol. 2015;26(2):258–269. doi:10.1681/ASN.2014030278
29. Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278(5):F817–F822. doi:10.1152/ajprenal.2000.278.5.F817
30. Denic A, Glassock RJ. Obesity-related glomerulopathy and single-nephron GFR. Kidney Int Rep. 2020;5:1126–1128. doi:10.1016/j.ekir.2020.05.017
31. Edwards A, Christensen EI, Unwin RJ, Norden AG. Predicting the protein composition of human urine in normal and pathological states: quantitative description based on Dent1 disease (CLCN5 mutation). J Physiol. 2021;599:323–341. doi:10.1113/JP280740
32. Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004;43:699–706. doi:10.1161/01.HYP.0000121881.77212.b1
33. Gómez-Hernández A, Beneit N, Díaz-Castroverde S, Escribano Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int J Endocrinol. 2016;2016:1–15. doi:10.1155/2016/1216783
34. Moreno-Navarrete JM, Fernandez-Real JM. The complement system is dysfunctional in metabolic disease: evidences in plasma and adipose tissue from obese and insulin resistant subjects. Semin Cell Dev Biol. 2019;85:164–172. doi:10.1016/j.semcdb.2017.10.025
35. Al Haj Ahmad RM, Al-Domi HA. Complement 3 serum levels as a pro-inflammatory biomarker for insulin resistance in obesity. Diabetes Metab Syndr. 2017;11(Suppl 1):S229–S232. doi:10.1016/j.dsx.2016.12.036
36. Vlaicu SI, Tatomir A, Boodhoo D, Vesa S, Mircea PA, Rus H. The role of complement system in adipose tissue-related inflammation. Immunol Res. 2016;64(3):653–664. doi:10.1007/s12026-015-8783-5
37. Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013;25(1):47–53. doi:10.1016/j.smim.2013.04.003
38. Rhee CM, Ahmadi SF, Kalantar-Zadeh K. The dual roles of obesity in chronic kidney disease: a review of the current literature. Curr Opin Nephrol Hypertens. 2016;25:208–216. doi:10.1097/MNH.0000000000000212
39. Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol. 2013;33(1):54–65. doi:10.1016/j.semnephrol.2012.12.005
40. Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci. 2020;21:1219. doi:10.3390/ijms21041219
41. Xu X, Huang X, Zhang L, Huang X, Qin Z, Hua F. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol. 2021;22(1):218. doi:10.1186/s12882-021-02391-1
42. Wu Y, Liu Z, Xiang Z, et al. Obesity-related glomerulopathy: insights from gene expression profiles of the glomeruli derived from renal biopsy samples. Endocrinology. 2006;147(1):44–50. doi:10.1210/en.2005-0641
43. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi:10.1016/j.cell.2010.01.022
44. Hughes MM, O’Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281(1):88–98. doi:10.1111/imr.12608
45. Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi:10.1038/nature04946
46. Wani K, AlHarthi H, Alghamdi A, Sabico S, Al-Daghri NM. Role of NLRP3 inflammasome activation in obesity-mediated metabolic disorders. Int J Environ Res Public Health. 2021;18:511. doi:10.3390/ijerph18020511
47. He J, Yuan G, Cheng F, Zhang J, Guo X. Mast cell and M1 macrophage infiltration and local pro-inflammatory factors were attenuated with incretin-based therapies in obesity-related glomerulopathy. Metab Syndr Relat Disord. 2017;15(7):344–353. doi:10.1089/met.2017.0057
48. Jarad G, Miner JH. Update on the glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18(3):226–232. doi:10.1097/MNH.0b013e3283296044
49. Camici MGF, Abraham N, Carpi A. Obesity-related glomerulopathy and podocyte injury: a mini review. Front Biosci. 2012;4:1058–1070.
50. Grahammer F. New structural insights into podocyte biology. Cell Tissue Res. 2017;369(1):5–10. doi:10.1007/s00441-017-2590-3
51. Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi:10.1146/annurev-physiol-020911-153238
52. Tagawa A, Yasuda M, Kume S, et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic. Diabetes. 2016;65:755–767. doi:10.2337/db15-0473
53. Mastrocola R, Aragno M, Alloatti G, Collino M, Penna C, Pagliaro P. Metaflammation: tissue-specific alterations of the NLRP3 inflammasome platform in metabolic syndrome. Curr Med Chem. 2018;25(11):1294–1310. doi:10.2174/0929867324666170407123522
54. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi:10.1038/nm.2279
55. Chang A, Ko K, Clark MR. The emerging role of the inflammasome in kidney diseases. Curr Opin Nephrol Hypertens. 2014;23(3):204–210. doi:10.1097/01.mnh.0000444814.49755.90
56. Shi Y, Wang C, Zhou X, et al. Downregulation of PTEN promotes podocyte endocytosis of lipids aggravating obesity-related glomerulopathy. Am J Physiol Renal Physiol. 2020;318(3):F589–F599. doi:10.1152/ajprenal.00392.2019
57. Zhao J, Rui HL, Yang M, Sun LJ, Dong HR, Cheng H. CD36-mediated lipid accumulation and activation of NLRP3 inflammasome lead to podocyte injury in obesity-related glomerulopathy. Mediators Inflamm. 2019;2019:3172647.
58. Ye Y, Zhong X, Li N, Pan T. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-α-mediated NF-κB and MAPK pathway. Obes Res Clin Pract. 2019;13(4):385–390. doi:10.1016/j.orcp.2019.03.003
59. Li Y, Xia T, Li R, Tse G, Liu T, Li G. Renal-protective effects of the peroxisome proliferator-activated receptor-γ agonist pioglitazone in ob/ob mice. Med Sci Mon. 2019;25:1582–1589. doi:10.12659/MSM.913461
60. Zhu JJ, Chen YP, Yang M, et al. Aldosterone is involved in the pathogenesis of obesity-related glomerulopathy through activation of Wnt/β-catenin signaling in podocytes. Mol Med Rep. 2018;17(3):4589–4598.
61. Zhao Y, Li G, Wang Y, Liu Z. Alteration of Connexin43 expression in a rat model of obesity-related glomerulopathy. Exp Mol Pathol. 2018;104(1):12–18. doi:10.1016/j.yexmp.2017.11.017
62. Hou XX, Dong HR, Sun LJ, Yang M, Cheng H, Chen YP. Purinergic 2X7 receptor is involved in the podocyte damage of obesity-related glomerulopathy via activating nucleotide-binding and oligomerization domain-like receptor protein 3 inflammasome. Chin Med J. 2018;131(22):2713–2725. doi:10.4103/0366-6999.245270
63. Guo YP, Jiang HK, Jiang H, Tian HY, Li L. Lipoxin A4 may attenuate the progression of obesity-related glomerulopathy by inhibiting NF-κB and ERK/p38 MAPK-dependent inflammation. Life Sci. 2018;198:112–118. doi:10.1016/j.lfs.2018.02.039
64. Guo H, Wang B, Li H, Ling L, Niu J, Gu Y. Glucagon-like peptide-1 analog prevents obesity-related glomerulopathy by inhibiting excessive autophagy in podocytes. Am J Physiol Renal Physiol. 2018;314(2):F181–f189. doi:10.1152/ajprenal.00302.2017
65. Wang XX, Edelstein MH, Gafter U, et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. 2016;27(5):1362–1378. doi:10.1681/ASN.2014121271
66. Luo M, Luo P, Zhang Z, et al. Zinc delays the progression of obesity-related glomerulopathy in mice via down-regulating P38 MAPK-mediated inflammation. Obesity. 2016;24(6):1244–1256. doi:10.1002/oby.21463
67. Fang Q, Deng L, Wang L, et al. Inhibition of mitogen-activated protein kinases/nuclear factor κB-dependent inflammation by a novel chalcone protects the kidney from high fat diet-induced injuries in mice. J Pharmacol Exp Ther. 2015;355(2):235–246. doi:10.1124/jpet.115.226860
68. Yan Z, Ni Y, Wang P, et al. Peroxisome proliferator-activated receptor delta protects against obesity-related glomerulopathy through the P38 MAPK pathway. Obesity. 2013;21(3):538–545. doi:10.1002/oby.20103
69. Solini A, Menini S, Rossi C, et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol. 2013;231(3):342–353. doi:10.1002/path.4237
70. Chintam K, Chang AR. Strategies to treat obesity in patients with CKD. Am J Kidney Dis. 2021;77(3):427–439. doi:10.1053/j.ajkd.2020.08.016
71. Kim BY, Kang SM, Kang JH, et al. 2020 Korean society for the study of obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr. 2021;30(2):81–92. doi:10.7570/jomes21022
72. Oppert JM, Bellicha A, van Baak MA, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of Obesity Physical Activity Working Group. Obesity Rev. 2021;22(Suppl 4):e13273. doi:10.1111/obr.13273
73. Muniraj T, Day LW, Teigen LM, et al. AGA clinical practice guidelines on intragastric balloons in the management of obesity. Gastroenterology. 2021;160(5):1799–1808. doi:10.1053/j.gastro.2021.03.003
74. Liu Y, Tao L, Zhang J, et al. Impact of commuting mode on obesity among a working population in Beijing, China: adjusting for air pollution. Diabetes Metab Syndr Obes. 2020;13:3959–3968. doi:10.2147/DMSO.S265537
75. Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi:10.2147/IJNRD.S39739
76. Hall JE, Hall ME. Cardiometabolic surgery for treatment of hypertension? Hypertension. 2019;73(3):543–546. doi:10.1161/HYPERTENSIONAHA.118.12369
77. Heffron SP, Parham JS, Pendse J, Aleman JO. Treatment of obesity in mitigating metabolic risk. Circ Res. 2020;126(11):1646–1665. doi:10.1161/CIRCRESAHA.119.315897
78. Serra A, Esteve A, Navarro-Diaz M, Lopez D, Bancu I, Romero R. Long-term normal renal function after drastic weight reduction in patients with obesity-related glomerulopathy. Obes Facts. 2015;8(3):188–199. doi:10.1159/000431027
79. Vilarrasa N, San Jose P, Rubio MA, Lecube A. Obesity in patients with type 1 diabetes: links, risks and management challenges. Diabetes Metab Syndr Obes. 2021;14:2807–2827. doi:10.2147/DMSO.S223618
80. Liu J, Sun Y, Zheng H, et al. Emodin attenuated the kidney damage of high-fat-diet mice via the upregulation of glucagon-like peptide-1 receptor. Biomed Res Int. 2021;2021:6662704.
81. Ye L, Hu X, Hu X, et al. Curcumin analogue C66 attenuates obesity-induced renal injury by inhibiting chronic inflammation. Biomed Pharmacother. 2021;137:111418. doi:10.1016/j.biopha.2021.111418
82. Jiang YH, Jiang LY, Wu S, et al. Proteomic analysis reveals the renoprotective effect of tribulus terrestris against obesity-related glomerulopathy in rats. Biol Pharm Bull. 2018;41(9):1430–1439. doi:10.1248/bpb.b18-00304
83. Ren Y, Wang D, Lu F, et al. Coptidis Rhizoma inhibits NLRP3 inflammasome activation and alleviates renal damage in early obesity-related glomerulopathy. Phytomedicine. 2018;49:52–65. doi:10.1016/j.phymed.2018.05.019
84. Sheng X, Zhu X, Zhang Y, et al. Rhein protects against obesity and related metabolic disorders through liver X receptor-mediated uncoupling protein 1 upregulation in brown adipose tissue. Int J Biol Sci. 2012;8(10):1375–1384. doi:10.7150/ijbs.4575
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
