Back to Journals » Cancer Management and Research » Volume 11
Nutritional and clinical outcomes of chemoradiotherapy for clinical T1N0M0 esophageal carcinoma
Authors Sakanaka K , Fujii K , Ishida Y , Miyamoto S , Horimatsu T, Muto M, Mizowaki T
Received 9 October 2018
Accepted for publication 7 March 2019
Published 26 April 2019 Volume 2019:11 Pages 3623—3630
DOI https://doi.org/10.2147/CMAR.S189518
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Katsuyuki Sakanaka,1 Kota Fujii,1 Yuichi Ishida,1 Shin’ichi Miyamoto,2 Takahiro Horimatsu,3 Manabu Muto,3 Takashi Mizowaki1
1Department of Radiation Oncology and Image-applied Therapy, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 2Department of Gastroenterology and Hepatology, Graduate School of Medicine, Kyoto University, Kyoto, Japan; 3Department of Therapeutic Oncology, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Purpose: Whether nutritional assessment and management improves clinical outcomes in patients with clinical T1N0M0 esophageal squamous cell carcinoma (ESCC) who undergo chemoradiotherapy remains to be demonstrated. This study aimed to determine the nutritional status of such patients pre- and post-chemoradiotherapy and its clinical outcomes.
Patients and methods: This single institutional retrospective study included patients who underwent chemoradiotherapy for clinical T1N0M0 ESCC using serum albumin concentrations and body weights evaluated pre- and post-chemoradiotherapy from January 2005 to December 2016. The nutritional risk index (NRI) score was used to quantify the nutritional status: NRI score ≥100: no risk, 97.5–100: mild risk, 83.5–97.5: moderate risk, and <83.5: major risk. NRI categories pre-and post-chemoradiotherapy were compared using Wilcoxon signed rank test. Local-regional control (LRC), overall survival (OS), and cause-specific survival (CSS) rates were calculated using Kaplan–Meier method. The effect of prechemoradiotherapy NRI score and decreased NRI category during chemoradiotherapy on OS was evaluated using log-rank test.
Results: Among the 492 patients with ESCC who underwent chemoradiotherapy, 44 were included in this study. Among these, 21 patients exhibited a pre-chemoradiotherapy NRI score of <0.0001). With a median follow-up of 72 months, the 5-year LRC, OS, and CSS rates were 79.8%, 88.9%, and 96.8%, respectively. The pre-chemoradiotherapy NRI score of <100. During chemoradiotherapy, the NRI score decreased from 100.5 to 94.5, and
the median NRI category significantly decreased by 2 (p<0.0001). With a median follow-up of 72 months, the 5-year LRC, OS, and CSS rates were 79.8%, 88.9%, and 96.8%, respectively. The pre-chemoradiotherapy NRI score of <100 and decreased NRI category during chemoradiotherapy did not significantly affect OS.
Conclusion: Patients with clinical T1N0M0 ESCC without dysphagia were at risk of undernutrition pre- and post-chemoradiotherapy. The current study’s results justify further clinical nutritional research for this population, and that clinicians consider nutritional support for this population.
Keywords: esophageal neoplasms, radiotherapy, conformal, chemotherapy, nutritional status
Introduction
Patients with esophageal carcinoma are prone to body weight loss and undernutrition due to dysphagia, early satiety, and cachexia.1 Overall, 70% of the patients with esophageal carcinoma showed body weight loss and undernutrition before treatment.2 Chemoradiotherapy is one of the definitive treatments for patients with esophageal carcinoma, which takes 5–7 weeks to complete. During chemoradiotherapy, adverse events such as esophagitis, anorexia, and nausea occur. These chemoradiotherapy-related symptoms cause body weight loss and undernutrition. Both the disease itself and chemoradiotherapy-related symptoms worsen this body weight loss and nutritional status in patients with esophageal carcinoma.
The body weight and nutritional status were reportedly associated with the clinical outcomes of esophageal carcinoma treated with chemoradiotherapy. The ancillary study using multi-institutional phase II/III data3 demonstrated that undernutrition management at pre-chemoradiotherapy improved survival in patients with locally advanced esophageal carcinoma.4 One retrospective study reported that body weight and serum albumin concentrations declined progressively during chemoradiotherapy for locally advanced esophageal carcinoma and well-nourished patients during chemoradiotherapy exhibited better clinical outcomes than those who were undernourished.5 However, these reports had limitations in that they did not focus on patients with superficial esophageal carcinoma and only included those with locally advanced esophageal carcinoma.
Superficial esophageal carcinoma accounted for 26.9% of all esophageal carcinomas.6 Unlike the locally advanced esophageal carcinoma, the superficial esophageal carcinoma does not cause malignant esophageal stricture. Therefore, patients with superficial esophageal carcinoma do not experience dysphagia and can orally consume foods without any difficulty. In addition, the small tumor volume of this early stage carcinoma unlikely causes any or at least severe anorexia and early satiety due to the metabolic changes mediated by pro-inflammatory cytokine activity observed in advanced carcinoma. Thus, patients with clinical T1N0M0 esophageal carcinoma seem to be at low risk of undernutrition. Although the European Society of Clinical Nutrition and Metabolism guideline indicated that all patients with cancer undergoing radiation in the gastrointestinal tract or the head and neck regions are recommended to receive thorough nutritional assessment, adequate nutritional counseling, and, if necessary, nutritional support according to symptoms and nutritional status,7 based on our literature search, no study has investigated the nutritional status and clinical outcomes in patients with clinical T1N0M0 esophageal carcinoma.
The current study retrospectively reviewed the nutritional status and clinical outcomes in patients with clinical T1N0M0 esophageal squamous cell carcinoma (ESCC) who underwent chemoradiotherapy from our institutional database. The study aimed to reveal the evidence whether the nutritional assessment and management is necessary or not for such patients.
Material and methods
Trial design
This was a single institutional retrospective study. Written informed consents to use the clinical data were obtained from all patients before initiating radiotherapy. The Institutional Review Board of Kyoto University Hospital approved this study on March 30, 2018 (R1468).
Consort diagram
Our institutional database was reviewed to identify all patients who underwent definitive chemoradiotherapy for esophageal carcinoma between January 2005 and December 2016. Patients who meet the following inclusion criteria were included in this study: those (1) with superficial ESCC without lymph nodal or distant metastasis; (2) who were concurrently undergoing chemotherapy; (3) who fulfilled the planned radiotherapy with 50–66 Gy prescribed for the primary tumor; (4) without comorbidities causing esophageal stenosis except for ESCC; (5) with serum albumin concentration, C-reactive protein (CRP), and body weight measured within 28 days before the initial day of radiotherapy; and (6) with serum albumin concentration, CRP, and body weight measured within 14 days from the last day of radiotherapy. A total of 44 patients satisfied the above criteria and were finally included in the study. Our institution has not provided any regular nutritional care (counseling or supports) for patients with cT1N0M0 ESCC during chemoradiotherapy except for the patients with severe dysphagia or odynophagia. If patients experienced a dysphagia or odynophagia, the administration of the intravenous drip or the prescription of analgesics were considered depending on the degree of severity of those symptoms. Figure 1 demonstrates the consort diagram of this study.
 | Figure 1 Consort diagram. Abbreviation: N, number of patients. |
Collected information
Information collected from the medical records of the included patients were as follows: age, gender, Eastern Cooperative Oncology Group performance status, history of chemoradiotherapy or surgery for head and neck or gastric malignancy, history of alcohol intake, body weight, height, clinical stage based on the eighth edition of the TNM classification for esophageal cancer in the Union for International Cancer Control criteria, serum CRP concentration, serum albumin concentration, computed tomography images, esophagogastroduodenoscopy images, and acute adverse event. The depth of tumor invasion was diagnosed by magnifying endoscopy ± endoscopic ultrasonography. The alcohol intake per day was estimated to be the beverage volume (in mL) multiplied by its percentage of alcohol. The percentage of alcohol was considered as 5% in beer, 15% in Japanese sake, 43% in whiskey, 35% in distilled spirits, and 12% in wine. Table 1 summarizes the details of patient characteristics.
 | Table 1 Patient characteristics |
Details of radiotherapy and concurrent chemotherapy
In planning the radiotherapy, all patients underwent computed tomography simulation. The esophagus with superficial tumors was identified as gross tumors based on endoscopic findings. To indicate the gross tumors in the computed tomography, radiopaque markers were endoscopically placed on the cranio-caudal esophageal mucosa of the gross superficial tumor before computed tomography simulation. The clinical target volume (CTV) for gross tumors was defined as the volume of the primary tumor with a 2-cm craniocaudal margin along the esophagus plus a 0.5-cm cross-sectional margin based on the CTV margin of the radiotherapy for clinical T1b ESCC adopted by the multi-institutional phase II study.8 The extent of subclinical regional lymph nodes was defined depending on the location of the primary tumor: upper thoracic primary tumor, from the supraclavicular to superior mediastinal lymph nodes; middle thoracic primary tumor, from the mediastinal to perigastric lymph nodes; and lower thoracic primary tumor, from the mediastinal to celiac axis lymph nodes. The CTV with a 0.5-cm margin and the volume of subclinical regional lymph nodes with a 0.5-cm margin were defined as the planning target volume (PTV) and subclinical PTV, respectively.9
A dose of 1.8–2.0 Gy per day was administered 5 days per week without a planned split by a linear accelerator using 6–15-MV photon beams. All patients underwent three-dimensional conformal radiotherapy. The dose was prescribed based on the International Commission on Radiation Units and Measurements point. A median dose of 60 Gy (range, 50–60 Gy) was prescribed as the PTV for all patients. Clinical T1bN0M0 esophageal squamous cell carcinoma had the risk of lymph nodal recurrences. The multi-institutional observational study of lymph nodal dissection for clinical T1bN0M0 showed the latent lymph nodal metastasis existed in 20% of patients.10 To decrease the local-regional recurrences, our institution performed subclinical irradiation for the non-frail patients but omitted it for frail-patients. Fifteen non-frail patients with clinical T1bN0M0 ESCC underwent the subclinical irradiation. They received subclinical irradiation of 40–41.4 Gy for subclinical PTV, and a 9–20-Gy booster dose was added to the PTV.11,12 The other 29 patients did not undergo any subclinical irradiation. The median overall treatment time was 43 (range, 37–45) days.
Concurrent chemotherapy was administered twice every 4 weeks during radiotherapy by continuous infusion of 5-fluorouracil ± slow drip of platinum. Thirty-five patients received the regimen of cisplatin 70 mg/m2/day (days 1 and 29) with 5-fluorouracil 700 mg/m2/day (days 1–4, 29–32). Seven patients received 5-fluorouracil 700 mg/m2/day (days 1–4, 29–32). One patient received 75 mg/m2/day (days 1 and 29) with 5-fluorouracil 1000 mg/m2/day (days1–4, 29–32). One patient received nedaplatin 90 mg/m2/day (days 1 and 29) and 5-fluorouracil 800 mg/m2/day (days 1–5, 29–33).
Nutritional risk index
To quantify the changes in nutritional status during chemoradiotherapy, this study used the nutritional risk index (NRI). It can be calculated from height, weight, and serum albumin concentration. Therefore, we could retrospectively recalculate it with minimal data lacking. In addition, the ancillary study based on the phase III study of chemoradiotherapy for locally advanced esophageal carcinoma has shown that the NRI differentiated the prognosis of those who underwent chemoradiotherapy.4 NRI was then calculated at each time point using the following formula: NRI = (1.519× albumin g/dl) +41.7 (present body weight/ideal body weight).13,14 The ideal body weight was calculated using the Lorentz formula.15 The nutritional status of each patient was stratified according to the calculated NRI: NRI score ≥100: no risk; 97.5–100: mild risk; 83.5–97.5: moderate risk; and <83.5: major risk.4
Toxicity and efficiency of chemoradiotherapy
Non-hematological toxicity including changes in body weight during chemoradiotherapy was graded based on the criteria of the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). This study used the serum CRP concentration to estimate the degree of inflammation related to chemoradiotherapy and hypercatabolic state related to ESCC. Local-regional control (LRC) events were locoregional progressions based on the date of its most recent verification. Any esophageal tumors that progressed within the irradiation fields were considered as locoregional progression events, whereas metachronous superficial esophageal tumors outside the radiation fields were not considered as locoregional progression events. Overall survival (OS) events included death from any cause determined at the final follow-up. The causes of death were divided into death from ESCC progression and others. The cause-specific survival (CSS) events included death from ESCC progression identified at the final follow-up. LRC, OS, and CSS rates were estimated using Kaplan–Meier method.
Statistical analysis
The difference in NRI category distribution between pre- and post-chemoradiotherapy were compared using Wilcoxon signed ranked test. The CRP-values at pre- and post-chemoradiotherapy were compared using paired t-test. The log-rank test was used to compare the OS of the following populations: those with NRI of ≥100 vs <100 at pre-chemoradiotherapy and those with decreased NRI category (at least one) during chemoradiotherapy vs those without. The chi-square test was conducted to evaluate the effects of the history of chemoradiotherapy or surgery for head and neck or gastric malignancy, and the volume of the alcohol intake (current drinker with more than 48 g of the alcohol intake per day vs the other included patients) on the pre-chemoradiotherapy NRI of <100, as well as the effects of acute esophagitis on decreased NRI category. A p-value of <0.05 was considered significant in this study. All analyses were performed using the GraphPad software (ver. 5.03; GraphPad Software, San Diego, CA, USA).
Results
The median interval days from the initiation of radiotherapy to the measurement of body weight and serum chemistries were 1 (range, 0–21) and 1 day (range, 0–28), respectively. The median interval days from the last day of radiotherapy to the measurement of body weight and serum chemistries were −3 days (range, −11–9) and 0 day (range, −3–13), respectively. Regarding non-hematological ≥grade 2 acute adverse events, grades 2 and 3 acute esophagitis were observed in 3 (7%) and 2 (4%) patients, respectively. Aside from the adverse events related to esophagitis, such as a dysphagia or odynophagia, the increase of grade 2 serum creatinine concentration was observed in two patients. As a severe acute hematological adverse event, one patient experienced the grade 4 neutropenia; however, the blood cells recovered without causing any critical symptoms.
This study found a decreased NRI score (<100) in 21 (48%) of the 44 patients at pre-chemoradiotherapy. Nutritional parameters further declined during chemoradiotherapy (Table 2). Overall, 13 (29%) patients lost ≥5% to <10% of body weight (CTCAE grade 1) and 2 (5%) lost ≥10% to <20% of body weight (CTCAE grade 2) during chemoradiotherapy. The post-chemoradiotherapy NRI category decreased in 26, remained stable in 16, and improved in 2 patients. The median NRI category significantly decreased by two categories during chemoradiotherapy (p<0.0001). The absolute increase was small; however, the serum CRP concentration significantly increased at post-chemoradiotherapy (0.1 mg/dL vs 0.5 mg/dL, p=0.019). The effect of acute esophagitis on decreased NRI scores was not significant (p=1.00).
 | Table 2 Changes of nutritional parameters during chemoradiotherapy in 44 patients |
The median follow-up period of the included patients was 72 (range, 13–151) months. The initial recurrent sites were as follows: intraluminal recurrence within irradiation fields (7 patients), lymph nodal recurrence (1 patient); and distant recurrence (none). The five-year LRC was 79.8% [95% confidence interval (CI), 63.4%–89.5%] (Figure 2). All intraluminal progressions of ESCC were successfully salvaged by endoscopic treatment. During the follow-up period, 8 patients died. Among them, only 1 patient died from ESCC progression, ie, systemic disease progression of ESCC after salvage lymph nodal dissection for the initial recurrence in the paratracheal lymph node. The remaining 7 patients died without the evidence of ESCC: 4 from metachronous malignancy (2 hematological and 2 lung-related), 2 sudden death without recurrence; and 1 due to aspiration pneumonia. The five-year OS and CSS were 88.9% (95% CI, 73.1%–95.7%) and 96.8% (95% CI, 79.2%–99.5%), respectively (Figure 2).
 | Figure 2 Cause-specific survival, overall survival, and local-regional control. |
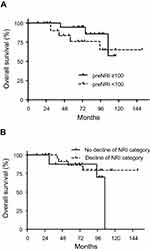
No significant difference was observed in OS between the patients with NRI of <100 and those with NRI of ≥100 at pre-chemoradiotherapy (p=0.33), with five-year OS of 76.0% (95% CI, 46.9%–90.5%) and 94.4% (95% CI, 66.6%–99.2%), respectively (Figure 3A). Similarly, no significant difference was observed in OS between patients who exhibited a decrease in at least one NRI category during chemoradiotherapy (26 patients) and those who did not (18 patients) (p=0.33), with five-year OS of 85.9% (95% CI, 62.2%–95.2%) and 87.5% (95% CI, 58.6%–96.7%), respectively (Figure 3B). The history of chemoradiotherapy or surgery for head and neck or gastric malignancy and the daily alcohol intake did not have a significant effect on the NRI of <100 at pre-chemoradiotherapy (p=0.13, and p=0.25).
Discussion
To our knowledge, this is the first study that investigates the changes of nutritional status in patients with clinical T1N0M0 ESCC who underwent chemoradiotherapy and explores the association between their changes in nutritional status and clinical outcomes. This study demonstrated that half of the patients with clinical T1N0M0 ESCC were at risk of undernutrition at pre-chemoradiotherapy, which worsens the nutritional status and increases the serum CRP concentration. Unexpectedly, the severity of body weight loss was similar in patients with clinical T1N0M0 ESCC who underwent chemoradiotherapy and those with locally advanced ESCC: grades 1, 2, and 3 body weight loss were reported as 31%, 4%, 0%–2%, respectively.3,16 Consistent with the previous reports, chemoradiotherapy achieved an excellent OS in patients with clinical T1N0M0 ESCC. This study did not detect the influence of decreased nutritional status at pre- and post-chemoradiotherapy on OS in patients with clinical T1N0M0 ESCC.
Forty-eight percentage of the patients with clinical T1N0M0 esophageal carcinoma without dysphagia showed that the NRI was under 100 before chemoradiotherapy. That means almost half of this population were at the risk of undernutrition even before chemoradiotherapy. The current study did not find any statistically significant influencing factors for the decline of pre-chemoradiotherapy NRI; however, the previously reported and retrospectively measurable influencing factors on the nutritional status such as the history of chemoradiotherapy or surgery for head and neck carcinoma or total/partial gastrectomy, and alcohol intakes were examined.17,18 One of the reasons for the discrepancy between those previous reports and current one was that the current study suffered the insufficient statistical power to detect the difference. In addition, the current study could not access the information of the calorie intake in each patient before chemoradiotherapy which was one of the retrospectively unmeasurable major influencing factors. The current study indicated that we need to remind the patients with clinical T1N0M0 without dysphagia were at risk of undernutrition even before chemoradiotherapy. Then, the further study is needed to explore the influencing factors on pre-chemoradiotherapy NRI and exam the necessity of the nutritional support for this population with the declined NRI before chemoradiotherapy.
NRI significantly decreased during chemoradiotherapy because of body weight loss and decreased serum albumin concentration. One of the supposed reasons for this progressive undernutrition is decreased oral intake due to esophagitis; however, the statistical analysis did not show any association between esophagitis and decreased NRI scores during chemoradiotherapy. In a phase II study, severe esophagitis due to chemoradiotherapy in superficial ESCC was previously observed in 9% of the patients.8 The incidence of severe esophagitis for these patients was consistent with that of this study, which caused decreased oral intake and undernutrition. In addition, the inflammation induced by chemoradiotherapy was suspected to play an important role in progressive undernutrition. Chemoradiotherapy for such patients induced inflammation within the irradiated area, such as the pleura,19 pericardium, and cardiac muscle,20 other than the esophagus.21 A series of inflammatory reactions then caused the hypercatabolic status and exhaustion,22 which likely resulted in progressive undernutrition in these patients. This study showed a small but significant increase of the serum CRP concentration at post-chemoradiotherapy, which supported our speculation regarding the existence of inflammatory reactions after chemoradiotherapy. Thus, patients with clinical T1N0M0 ESCC should be warned that they were at risk of undernutrition at post-chemoradiotherapy.
The current study had limitations. First, this was a retrospective study with a small number of included patients. Second, information on influencing factors on NRI such as the caloric intake of each patient at pre- and post-chemoradiotherapy was not accessible because we did not record them. Third, this study did not demonstrate the association between decreased NRI and poor clinical outcome, differently from the reports on locally advanced esophageal carcinoma,4,5 possibly due to the low statistical power of this study. Although the clinical impact of nutritional status on the clinical outcome remains unclear, patients with clinical T1N0M0 ESCC were at risk of undernutrition at pre- and post-chemoradiotherapy. Therefore, we believe that nutritional assessment and management is preferable for such patients. Future studies based on a large number of patients with data on daily calorie intakes will yield a clear evidence on the need for nutritional assessment and management for patients with clinical T1N0M0 ESCC.
Conclusion
Patients with clinical T1N0M0 ESCC were at risk of undernutrition at pre- and post-chemoradiotherapy. The current study’s result justifies the further clinical nutritional research for this population, and the clinicians considered the nutritional support for this population.
Abbreviations list
ESCC, esophageal squamous cell carcinoma; NRI, nutritional risk index; LRC, local-regional control; OS, overall survival; CSS, cause-specific survival; CRP, C-reactive protein; CTV, clinical target volume; PTV, planning target volume; IQR, interquartile range; CTCAE, common terminology criteria for adverse events.
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan under grant number (17 K16434) and the Japan Society of Clinical Oncology Clinical Research Grant Program 2017.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol. 2010;26(2):146–151.
2. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34(4):503–509.
3. Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14(7):627–637. doi:10.1016/S1470-2045(13)70136-0
4. Cox S, Powell C, Carter B, Hurt C, Mukherjee S, Crosby TD. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br J Cancer. 2016;115(2):172–177. doi:10.1038/bjc.2016.129
5. Di Fiore A, Lecleire S, Gangloff A, et al. Impact of nutritional parameter variations during definitive chemoradiotherapy in locally advanced oesophageal cancer. Dig Liver Dis. 2014;46(3):270–275. doi:10.1016/j.dld.2013.10.016
6. Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2010. Esophagus. 2017;14(3):189–214. doi:10.1007/s10388-017-0578-4
7. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. doi:10.1016/j.clnu.2016.07.015
8. Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol. 2009;39(10):638–643. doi:10.1093/jjco/hyp069
9. Sakanaka K, Ishida Y, Itasaka S, et al. Identification of a predictive factor for distant metastasis in esophageal squamous cell carcinoma after definitive chemoradiotherapy. Int J Clin Oncol. 2016;21(5):899–908. doi:10.1007/s10147-016-0967-z
10. Akutsu Y, Kato K, Igaki H, et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg. 2016;264(6):1009–1015.
11. Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81(3):684–690. doi:10.1016/j.ijrobp.2010.06.033
12. Kato K, Eguchi Nakajima T, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43(6):608–615. doi:10.1093/jjco/hyt048
13. Buzby GP, Knox LS, Crosby LO, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47(2 Suppl):366–381. doi:10.1093/ajcn/47.2.366
14.
15. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
16. Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15(3):305–314. doi:10.1016/S1470-2045(14)70028-2
17. Payakachat N, Ounpraseuth S, Suen JY. Late complications and long-term quality of life for survivors (>5 years) with history of head and neck cancer. Head Neck. 2013;35(6):819–825. doi:10.1002/hed.23035
18. Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14(2):144–149. doi:10.1007/s10120-011-0021-9
19. Ito H, Itasaka S, Sakanaka K, Araki N, Mizowaki T, Hiraoka M. Long-term complications of definitive chemoradiotherapy for esophageal cancer using the classical method. J Radiat Res. 2017;58(1):106–113. doi:10.1093/jrr/rrw078
20. Ishida Y, Sakanaka K, Itasaka S, et al. Effect of long fasting on myocardial accumulation in 18F-fluorodeoxyglucose positron emission tomography after chemoradiotherapy for esophageal carcinoma. J Radiat Res. 2018;59(2):182–189. doi:10.1093/jrr/rrx076
21. Aizawa R, Matsumoto S, Uneno Y, et al. Severe esophagitis associated with cytomegalovirus during concurrent chemoradiotherapy for esophageal cancer. Jpn J Clin Oncol. 2017;47(9):885–888. doi:10.1093/jjco/hyx083
22. Laviano A, Meguid MM. Nutritional issues in cancer management. Nutrition. 1996;12(5):358–371.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.