Back to Journals » OncoTargets and Therapy » Volume 12
Numb inhibits cell proliferation, invasion, and epithelial–mesenchymal transition through PAK1/β-catenin signaling pathway in ovarian cancer
Authors Liang J, Han B, Zhang Y, Yue Q
Received 15 November 2018
Accepted for publication 29 January 2019
Published 29 April 2019 Volume 2019:12 Pages 3223—3233
DOI https://doi.org/10.2147/OTT.S194725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr William C. Cho
Jiabin Liang,1 Bingbing Han,2 Yunhe Zhang,3 Qingfen Yue1
1Department of Gynaecology, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang, Henan 471009, People’s Republic of China; 2Department of Reproductive Medicine, Luoyang Central Hospital Affiliated to Zhengzhou University, Luoyang, Henan 471009, People’s Republic of China; 3Department of Obstetrics and Gynecology, China Meitan General Hospital, Beijing 100028, People’s Republic of China
Objective: The present study aimed to investigate the expression of Numb in ovarian cancer tissues and to assess the effect of Numb on cell proliferation, invasion, and EMT in ovarian cancer.
Methods: Real-time PCR and Western blotting were used to detect the mRNA and protein expression of Numb, PAK1, β-catenin, and epithelial–mesenchymal transition (EMT)-related proteins. MTT was employed to check the effect of Numb on proliferation of ovarian cancer cells. Transwell assay was performed to examine the functions of Numb and PAK1 on migration and invasion of ovarian cancer cells.
Results: The Numb expression was significantly downregulated while PAK1 and β-catenin were significantly upregulated in both ovarian cancer tissues and cell lines. Silencing of Numb promoted cell proliferation, migration, invasion, and EMT in ovarian cancer cell lines while overexpressed Numb reversed the above effects. Moreover, the EMT process induced by the inhibition of Numb was regulated through Numb-mediated PAK1/β-catenin signaling pathway.
Conclusion: Numb was downregulated and associated with cell proliferation, invasion, and EMT in ovarian cancer through regulating PAK1/β-catenin signaling, providing a novel potential biomarker and potential therapeutic target for ovarian cancer.
Keywords: Numb, ovarian cancer, PAK1, β-catenin, invasion, EMT
Introduction
Ovarian cancer is one of the leading causes of lethal gynecological malignancy-related morbidity and mortality among women worldwide.1 According to relevant statistics in 2018, ~22,240 new cases were diagnosed and 14,070 deaths were estimated in the United States.2 Although advanced progress in therapeutic approaches including surgery, radiotherapy, and neoadjuvant chemotherapy as the primary methods for treating ovarian cancer, the long-term prognosis of patients is still poor, mainly due to infinite proliferation and strong metastatic ability of tumor cells.2,3 Evidence suggests that invasiveness and migration capability in ovarian cancer cells are enhanced by loss of epithelial features and gain of mesenchymal phenotype, known as epithelial–mesenchymal transition (EMT).4–6 Therefore, it is critical to uncover molecular mechanisms underlying proliferation, invasion, and EMT in ovarian cancer and develop more effective therapeutic strategies accordingly.
Numb is an endocytic adaptor protein that localizes to the basement layer of polarized epithelial cells and mediates endocytosis and endocytic transport of cell membrane proteins.7 Numb has been implicated in diverse array of cellular processes that contributes to cell polarity maintenance, cell migration, and EMT.8–12 It is widely considered to be a tumor suppressor and is involved in a variety of cancer progressions, including lung cancer,13 head and neck squamous cell carcinoma,14 prostatic cancer,15 breast cancer,16 cervical cancer,17 and so on. Colaluca et al indicated that Numb functions as a suppressor by inhibiting notch signaling and stabilizing p53 in breast cancer.16 However, the role and underlying mechanism of Numb in the tumorigenesis and progression of ovarian cancer are rarely investigated.
The p21-activated kinases (PAKs) family of serine/threonine protein kinases has been found to be associated with cellular morphology, cytoskeletal organization, and survival, and the members of PAKs have significant effect on various diseases including cancers and neurological disorders. The PAK1 is a downstream effector of Rac/Cdc42 GTPases that has been implicated in the process of cellular processes such as cytoskeletal reorganization, cell growth, motility, morphogenesis, and gene regulation.18,19 Mounting evidence reveals that migration potential and abnormal mitotic activity are increased by overexpression of PAK1 in many malignancies, such as carcinomas of breast, ovary, thyroid, and colon.20 In addition, Pak1 and p-Pak1 expression levels were associated with poor overall prognosis and enhanced ovarian cancer cell migration and invasion.21 Nevertheless, whether PAK1 is able to interact with Numb in ovarian cancer remains to be explored.
Aberrant activation of the Wnt/β-catenin signaling pathway has been reported to be involved in the initiation and progression of human cancer22 and has been proven to regulate a variety of biological processes, including cell proliferation, apoptosis, invasion, and EMT in ovarian cancer.23,24 Moreover, β-catenin is a key protein in the Wnt/β-catenin pathway that forms adherens junctions to affect EMT together with N-cadherin, E-cadherin, and Snail-1.25 Furthermore, given the established role of β-catenin binding to the Numb promoter and accelerated Numb expression in breast cancer cells,16 we examined the unknown relationship between Numb and β-catenin in ovarian cancer.
In the present study, we found that Numb was decreased and acted as a tumor suppressor by interaction with PAK1/β-catenin in ovarian cancer. Furthermore, we explored the biological roles of Numb in ovarian cancer cells to participate in various pathological processes, including cell proliferation, migration, invasion, and EMT. Based on the above discussion, our study elaborated a novel Numb/PAK1/β-catenin regulatory network in the development and progression of ovarian cancer, providing a potential biomarker and therapeutic target for ovarian cancer.
Materials and methods
Tissue samples
In the present study, specimens of 60 epithelial ovarian cancer tissues and 60 matched adjacent normal tissues were obtained from patients (age range 32–68 years) at the Luoyang Central Hospital Affiliated to Zhengzhou University during March 2013 to August 2014. The ovarian cancer tissues were surgically resected, and the matched adjacent normal tissues were normal tissues from distance >2 cm from the cancer tissues. Tissue samples were collected and immediately snap-frozen in liquid nitrogen and stored at −80°C for further analysis. Tissues were divided according to TNM stage I–II and III–IV. All tissues were confirmed as ovarian cancer by pathological analysis, and the pathological types were also recorded. No patients had received chemotherapy or radiotherapy before the surgery. All patients gave their informed written consent for the use of these clinical materials for research purposes. The study was approved by the Luoyang Central Hospital Affiliated to Zhengzhou University Research Ethical Committee. All experiments were in accordance with the Declaration of Helsinki.
Cell lines and culture
The human ovarian cancer cell lines 3AO, HEY, HO8910, SKOV3, and OVCAR3 cells, as well as normal ovarian ISOE80 cells, were purchased from American Type Culture Collection (Manassas, VA, USA). Ovarian cancer lines were maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Thermo Fisher Scientific). Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C.
Cell transfection
SKOV3 and OVCAR3 were transiently transfected with pcDNA3.1-Numb, pcDNA3.1-PAK1 plasmids using lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Numb siRNA26 and PAK1 siRNA27 were used as described previously. All siRNAs and plasmids were synthesized by GenePharma (Shanghai, China). siRNA sequences for Numb and PAK1 were as follows: si-Numb: 5′-CAGCCACUGAACAAGCAGA-3′; si-PAK1: 5′-GAGTGTGGGCGATCC TAAGAAGAAA-3′.
Quantitative reverse transcription-PCR (qRT-PCR)
Total RNA was extracted from clinical tissues or cultured cells using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. First-strand cDNA was obtained using reverse transcriptase kit (Takara, Otsu, Japan). qRT-PCR was performed using SYBR Green Real-time PCR Kit (TOYOBO Co. Ltd., Osaka, Japan) on a Bio-Rad Real-Time PCR instrument. The mRNA expression of Numb, PAK1, and β-catenin was standardized to control values of β-actin. The PCR primer sequences used for analyses were as follows: Numb: forward: 5′-AGGCCAGTCGTCCACATCA-3′ and reverse: 5′-GGTACTTAACCGGGAAGCTACAT-3′; PAK1: forward: 5′-AGCAAAAGAGGCAACCAAGA-3′ and reverse: 5′-GACGGTTTCCAAGGATCAAA-3′; β-catenin: forward: 5′-GGAAGGGACAGTATCGTTTGTT-3′ and reverse: 5′-GCCTCAGCATCTACCAGCATAG-3′; E-cadherin: forward: 5′-CTCCCAATACATCTCCCTTCAC-3′ and reverse: 5′-CGCCTCCTTCTTCATCATAGTAA-3′; N-cadherin: forward: 5′-GGCATACACCATGCCATCTT-3′ and reverse: 5′-GTGCATGAAGGACAGCCTCT-3′; Snail 1: forward: 5′-CAAGGAATACCTCAGCCTGG-3′ and reverse: 5′-ATTCACATCCAGCACATCCA-3′; β-actin: forward: 5′-TGGTATCGTGGAAGGACTCAT-3′ and reverse: 5′-TGGGTGTCGCTGTTGAAGTC-3′. The relative change in expression of mRNA was calculated by the 2−ΔΔCT method. All qRT-PCR experiments were replicated at least three times.
MTT assay
Cells were harvested and seeded at a density of 1×104 cells/well into 96-well flat-bottomed plates in 100 μL of complete medium. The cells were then incubated overnight to allow cell attachment and recovery, after they were transfected with si-negative control or si-Numb as for 12, 24, 48, and 72 hours. Then, 30 μL MTT solution (5 mg/mL; Sigma-Aldrich Co., St Louis, MO, USA) was added into each well, and the cells were incubated for 4 hours at 37°C. Next, 150 μL DMSO was added into each well to dissolve the formazan crystal. The optical density was measured at 490 nm by spectrophotometry using microplate reader (Bio-Tek, Winooski, VT, USA). Three independent experiments were performed in triplicate.
Transwell assay
The migratory and invasive abilities of ovarian cancer were evaluated by using transwell chambers (Corning Incorporated, Corning, NY, USA). For cell invasion assay, the membrane was coated with Matrigel (BD, Franklin Lakes, NJ, USA) to form a matrix barrier. Transfected cells were suspended in serum-free medium and added to the upper chamber. The matched lower chamber was filled with complete medium containing 10% FBS as a chemoattractant. After incubation for 24 hours, cells on the upper surface were removed with a cotton swab, and cells that had migrated to the bottom surface of the filter membrane were fixed with 4% formaldehyde, then stained with 0.1% crystal violet (Cat # C-3886; Sigma-Aldrich Co.). The number of migration and invasion cells were photographed and counted in five randomly selected fields using a light microscope (Olympus BX61; Olympus Corporation, Tokyo, Japan) at a magnification of ×100. Experiments were performed in triplicate.
Western blotting
Cells were harvested and lysed in RIPA buffer supplemented with protease inhibitors (50 mM Tris-HCl pH8, 50 mM NaCl, 0.5% NP-40). The protein concentrations were determined by the Bio-Rad (Bradford) protein assay (Bio-Rad Laboratories Inc., Hercules, CA, USA), and a total of 50 μg of protein was separated by denaturing 12% SDS-PAGE and transferred to a PVDF membrane (EMD Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk in a 0.1% TBST solution for 1 hour at room temperature, membranes were then incubated with rabbit polyclonal anti-Numb (1:1,000; Abcam, Cambridge, UK), anti-E-cadherin (1:1,000; Cell Signaling Technology Inc., Danvers, MA, USA), anti-N-cadherin (1:1,000; Cell Signaling Technology), anti-Snail 1 (1:1,000; Abcam), anti-β-catenin (1:1,000; Abcam), and anti-β-actin (1:10,000; Abcam) overnight at 4°C. After washing, blots were incubated with HRP-conjugated goat anti-rabbit secondary antibodies (1:2,000; Abcam) for 1 hour. The immunoreactive proteins were visualized using ECL detection system (Pierce Biotechnology, Rockford, IL, USA). Protein levels were determined by normalization against β-actin. All experiments were conducted in triplicate.
Immunohistochemistry assay
The clinical tissues were collected and fixed overnight in 4% paraformaldehyde, embedded in paraffin, and then sectioned at a width of 4 μm. The sections mounted on the glass slides were deparaffinized and rehydrated. For immunofluorescence assay, the sections were incubated with antibodies for anti-Numb antibody (1:500; Abcam), anti-PAK1 antibody (1:100; Abcam), and anti-β-catenin (1:500; Abcam) at 4°C overnight and then washed in PBS and incubated with the secondary HRP-conjugated anti-rabbit antibody was incubated for 1 hour at room temperature, then stained with a DAB staining solution and counterstained with hematoxylin for 2 minutes. The stained images were captured using an Olympus IX73 microscope (Olympus Corporation, Tokyo, Japan). Immunohistochemistry scores were calculated by the product of staining intensity 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining) and the percentage scores of the stained area, 0 (none); 1 (<10%); 2 (10%–25%); 3 (26%–50%); 4 (51%–75%); and 5 (>75%).
Statistical analysis
The data were exhibited as mean ± SD of three independent experiments and processed using the Statistical Package for Social Sciences version 17.0 (SPSS 17.0; SPSS, Inc., Chicago, IL, USA). Student’s t-test was used to compare differences between the two groups. A value of P<0.05 was considered to be statistically significant. All experiments were performed at least three times.
Results
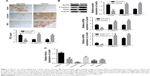
Numb was downregulated while PAK1 and β-catenin were upregulated in ovarian cancer tissues
To elucidate the expression pattern of Numb, immunohistochemistry was used to estimate the expression of Numb in ovarian cancer tissues and matched adjacent normal tissues. As shown in Figure 1A, Numb expression was obviously reduced in ovarian cancer tissues compared to normal tissues (P<0.05). However, PAK1 and β-catenin expression levels in ovarian cancer tissues were dramatically upregulated compared to normal tissues (P<0.05). Further qRT-PCR and Western blotting experiments verified the above results (P<0.05, Figure 1B). Moreover, we used qRT-PCR to see if the expression of Numb, PAK1, and β-catenin was associated with the TNM stage. Results showed that expression of Numb was significantly lower in tissues of TNM stage III–IV (n=13) than in tissues of TNM stage I–II (n=47), and the expression of PAK1 and β-catenin showed the opposite trend (P<0.05). Among the tissue samples, 36 cases were serous type, 20 cases were mucous type, and four cases were endometrioid type. However, the expression of Numb, PAK1, and β-catenin showed no significant difference in different pathological types (data not shown). Expression of Numb in different ovarian cancer cell lines was also determined. Results showed that Numb was significantly downregulated in all cancer cell lines (P<0.05, Figure 1C). As mentioned above, we suspected that Numb may be associated with PAK1 and β-catenin and can play a regulatory role in ovarian cancer.
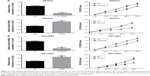
Numb inhibited cell proliferation in ovarian cancer cell lines
To explore the influence of Numb on ovarian cancer cell lines, we conducted the MTT assays to measure cell proliferation in both SKOV3 and OVCAR3 cell lines with loss-of-function and gain-of-function assays. The transfection efficiency was detected by qRT-PCR as shown in Figure 2A, and the expression of Numb was obviously increased by transfection with pcDNA3.1-Numb vector and conversely decreased with transfection of si-Numb (P<0.05). MTT assay demonstrated that interference of Numb promoted the proliferation vitality of both SKOV3 and OVCAR3 cells in a time-dependent manner (P<0.05, Figure 2B). Meanwhile, opposite results were observed in cells with overexpressed Numb, indicating Numb could inhibit cell proliferation in ovarian cancer cell lines.
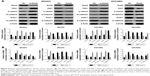
Numb decreased the migration and invasion ability of ovarian cancer cell lines
To obtain deeper insights into the potential role of Numb for migration and invasion ability in ovarian cancer cell lines, we conducted transwell assay in SKOV3 and OVCAR3 cells with Numb depletion and Numb overexpression. As shown in Figure 3A, Numb knockdown could significantly increase the number of migrated and invaded cells of both SKOV3 and OVCAR3 cell lines (P<0.05). On the contrary, overexpressing Numb could remarkably suppress migration and invasion abilities of both SKOV3 and OVCAR3 cell lines (P<0.05, Figure 3B), suggesting Numb could inhibit cell invasion and migration of ovarian cancer cell lines.
Numb suppressed EMT in ovarian cancer cell lines
To further explore whether Numb could regulate EMT in ovarian cancer cell lines, the mRNA and protein expression levels of EMT markers were assessed by qRT-PCR and Western blotting. When Numb was overexpressed in both SKOV3 and OVCAR3 cell lines, the expression of mesenchymal markers N-cadherin and Snail 1 was significantly attenuated, and the epithelial marker E-cadherin was significantly upregulated, both in mRNA and protein levels (P<0.05, Figure 4A and B). In contrast, the mRNA and protein expression level of the mesenchymal markers N-cadherin and Snail 1 were dramatically increased, and the epithelial maker E-cadherin was reduced when Numb was knocked down in both SKOV3 and OVCAR3 cell lines (P<0.05, Figure 4A and B). These results indicated that Numb could reverse EMT to MET in ovarian cancer cell lines.
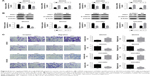
Numb regulated EMT through PAK1/β-catenin in ovarian cancer cell lines
As shown in the above experimental results in Figure 1, based on the correlation of expression differences, it is suggested that PAK1 and β-catenin may be involved in the mechanism of Numb regulation of EMT in ovarian cancer cell lines. To confirm this hypothesis, PAK1 was overexpressed or knocked down in both SKOV3 and OVCAR3 cell lines and the cells’ invasion and migration were also determined. Results showed expression of β-catenin was significantly increased when PAK1 was overexpressed and was significantly decreased when PAK1 was inhibited (P<0.05, Figure 5A and B). Furthermore, both the migration and invasion abilities were significantly promoted in PAK1-overexpressing cells while knocking down PAK1 yielded the opposite results (P<0.05, Figure 5C). These results suggested Numb could regulate EMT through PAK1-mediated regulation of β-catenin in ovarian cancer cell lines.
Discussion
Recent studies have identified Numb has an anti-oncogene role in lung cancer, prostate cancer,28 endometrial cancer,26,29 and breast cancer.30 Accumulating evidence indicated that Numb was found to stabilize p53 and inhibit Notch signaling.26,31–34 Further analysis elucidated that Numb had been frequently downregulated in human breast cancer resulting in p53 inactivation and an aggressive disease course regulation.30 In addition, it has been demonstrated that Numb correlates with a worse survival in multiple independent lung and ovarian cancer datasets and prevents a complete EMT by modulating Notch signaling in lung cancer. Currently, the role and underlying mechanism of Numb in cell proliferation, migration, invasion, and EMT in ovarian cancer is largely unclear. In our study, Numb was significantly decreased in ovarian cancer tissues, and the decrease was even worse in tissues of TNM III–IV stages, which was in accordance with the above reports. We found that cell proliferation in ovarian cancer was obviously constrained in the gain-of-function experiment of Numb. Based on these facts, we concluded that Numb played a suppressor role in ovarian cancer.
EMT, with a positive relation to migration and invasion, is a crucial factor for cancer progression and is featured by the loss of epithelial markers and the gain of mesenchymal markers.35 In the present study, we found that overexpression of Numb altered the expression of EMT markers, thus inhibiting ovarian cancer cell migration and invasion. Accordingly, our data also validated that the increase in the expression of Numb suppressed the expression of PAK1 and β-catenin.
β-catenin, which is located on human chromosome 3p21, plays a vital role in the classic Wnt signaling pathway.36 In addition, β-catenin is increased in malignant cancers37 and has been demonstrated to regulate cell proliferation, differentiation, and invasion and promote EMT in ovarian cancer.38 There was evidence showing that PAK1 was interacted with β-catenin and that PAK1 directly phosphorylates β-catenin proteins and triggers β-catenin transcriptional activity.27,39,40 Given the established role of β-catenin, we first explored whether Numb could regulate EMT through PAK1/β-catenin signaling. PAK1 promoted migration, invasion, and EMT, suggesting that Numb disrupted migration, invasion, and EMT through PAK1-mediated β-catenin signaling in ovarian cancer cell lines. However, whether Numb directly regulated PAK1 or it regulated PAK1 through other signaling pathways still need more studies to confirm.
In conclusion, Numb expression was downregulated while PAK1 and β-catenin were accelerated in ovarian cancer tissues and cells. Mechanically, we demonstrated Numb has an inhibitory effect on cell proliferation, migration, invasion, and EMT in ovarian cancer cell lines. In addition, Numb regulation of EMT could be through regulating PAK1/β-catenin. Therefore, the potential role of Numb in ovarian cancer is first revealed, and it may provide novel insights into the treatment of ovarian cancer.
Highlights
- Numb was significantly downregulated in ovarian cancer.
- Numb inhibited ovarian cancer cell proliferation.
- Numb inhibited ovarian cancer cell migration and invasion.
- Numb inhibited ovarian cancer cell EMT.
- Numb regulated PAK1/β-catenin signaling pathway in ovarian cancer.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Lowe KA, Chia VM, Aliki T, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130(1):107–114. doi:10.1016/j.ygyno.2013.03.026 | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;60(5):277–300. | ||
Basen-Engquist K. Ovarian cancer screening and psychosocial issues: relevance to clinical practice. Gynecol Oncol. 1997;65(2):195–196. | ||
Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31(20):2521–2534. | ||
Lili LN, Matyunina LV, Walker LD, Wells SL, Benigno BB, Mcdonald JF. Molecular profiling supports the role of epithelial-to-mesenchymal transition (EMT) in ovarian cancer metastasis. J Ovarian Res. 2013;6(1):49. | ||
Vergara D, Merlot B, Lucot JP, et al. Epithelial–mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291(1):59–66. | ||
Michel C, Martin R. Asymmetric segregation of Numb: a mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5(12):1265–1269. | ||
Dho SE, French MB, Woods SA, Mcglade CJ. Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem. 1999;274(46):33097–33104. | ||
Alberto G, Lucia DM, Isabella S. The multiple functions of Numb. Exp Cell Res. 2010;316(6):900–906. | ||
Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. Numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58(2):349–360. | ||
Wang Z, Sandiford SC. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. Embo Journal. 2009;28(16):2360–2373. doi:10.1038/emboj.2009.190 | ||
Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13(1):15–28. doi:10.1016/j.devcel.2007.05.003 | ||
Kikuchi H, Sakakibara-Konishi J, Furuta M, et al. Expression of Notch1 and Numb in small cell lung cancer. Oncotarget. 2017;8(6):10348. doi:10.18632/oncotarget.14411 | ||
Chou CH, Tu HF, Kao SY, et al. Targeting of miR-31/96/182 to the Numb gene during head and neck oncogenesis. Head Neck. 2018;40:11. doi:10.1002/hed.25044 | ||
Ji S, Kai W, Teng J, et al. Numb had anti-tumor effects in prostatic cancer. Biomed Pharmacother. 2017;92:108. doi:10.1016/j.biopha.2017.04.134 | ||
Colaluca IN, Basile A, Freiburger L, et al. A Numb-Mdm2 fuzzy complex reveals an isoform-specific involvement of Numb in breast cancer. J Cell Biol. 2018;217(2):745–762. doi:10.1083/jcb.201709092 | ||
Rong C, Feng Y, Ye Z. Notch is a critical regulator in cervical cancer by regulating Numb splicing. Oncol Lett. 2017;13(4):2465. doi:10.3892/ol.2017.5683 | ||
Chiang YTA, Jin T. p21-Activated protein kinases and their emerging roles in glucose homeostasis. Am J Physiol Endocrinol Metab. 2014;306(7):707–722. doi:10.1152/ajpendo.00506.2013 | ||
Rakesh K, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459. doi:10.1038/nrc1892 | ||
Zhang ZL, Liu G, Peng L, et al. Effect of PAK1 gene silencing on proliferation and apoptosis in hepatocellular carcinoma cell lines MHCC97-H and HepG2 and cells in xenograft tumor. Gene Therapy. 2018;25(4):284. | ||
Siu MKY, Wong ESY, Hoi Yan C, et al. Differential expression and phosphorylation of Pak1 and Pak2 in ovarian cancer: effects on prognosis and cell invasion. Int J Cancer. 2010;127(1):21–31. | ||
Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. | ||
Bodnar L, Stanczak A, Cierniak S, et al. Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J Ovarian Res. 2014;7(1):16. | ||
Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–1473. | ||
Gatcliffe TA, Monk BJ, Planutis K, Holcombe RF. Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer. 2010;18(5):954–962. | ||
Wang C, Feng W, Zhang C. The expression and function of NUMB in endometrial cancer and the interaction with HDM2 and P53. J Cancer. 2015;6(10):1030–1040. | ||
Zhu G, Wang Y, Huang B, et al. A Rac1/PAK1 cascade controls β-catenin activation in colon cancer cells. Oncogene. 2012;31(8):1001. | ||
Flores AN, Niamh MD, Armelle M, Laure M. NUMB inhibition of NOTCH signalling as a therapeutic target in prostate cancer. Nat Rev Urol. 2014;11(9):499–507. | ||
Wang C, Cui T, Feng W, Huashun LI, Lina HU. Role of Numb expression and nuclear translocation in endometrial cancer. Oncol Lett. 2015;9(4):1531–1536. | ||
Tosoni D, Pambianco S, Ekalle SB, et al. Pre-clinical validation of a selective anti-cancer stem cell therapy for Numb-deficient human breast cancers. EMBO Mol Med. 2017;9(5):655. | ||
Colaluca IN, Daniela T, Paolo N, et al. NUMB controls p53 tumour suppressor activity. Nat Neurosci. 2008;451(7174):76–80. | ||
Adams SJ, Aydin IT, Celebi JT. GAB2–a scaffolding protein in cancer. Molecular Cancer Research. 2012;10(10):1265–1270. | ||
Juven-Gershon T, Shifman OT, Elkeles A, et al. The Mdm2 oncoprotein interacts with the cell fate regulator Numb. Mol Cell Biol. 1998;18(7):3974–3982. | ||
Mcgill MA, Dho SE, Gerry WC Jane M. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem. 2009;284(39):26427–26438. | ||
Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18(2):128–134. | ||
Renz H, Kerzel S, Nockher WA. Genetic alteration of the beta-catenin gene (CTNNB1) in human lung cancer and malignant mesothelioma and identification of a new 3p21.3 homozygous deletion. Oncogene. 2001;20(31):4249–4257. | ||
Hans C. Wnt/β-catenin signaling in development and disease. Cell. 2006;127(3):469–480. | ||
Arend RC, Londoño-Joshi AI, Straughn, JM Jr, Buchsbaum DJ. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. | ||
He H, Shulkes A, Baldwin GS. PAK1 interacts with beta-catenin and is required for the regulation of the beta-catenin signalling pathway by gastrins. Biochim Biophys Acta Mol Cell Res. 2008;1783(10):1943–1954. | ||
Lv Z, Hu M, Zhen J, Lin J, Wang Q, Wang R. Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering β-catenin transcriptional activity under high glucose conditions. Int J Biochem Cell Biol. 2013;45(2):255–264. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.