Back to Journals » Cancer Management and Research » Volume 10
NSAID consumption and risk of acute myeloid leukemia: a national population-based case-control study
Authors Østgård LSG , Nørgaard M , Pedersen L, Østgård R , Friis LS, Schöllkopf C, Severinsen MT , Marcher CW, Medeiros BC, Jensen MK
Received 14 February 2018
Accepted for publication 29 May 2018
Published 29 October 2018 Volume 2018:10 Pages 5043—5051
DOI https://doi.org/10.2147/CMAR.S165498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Lene Sofie Granfeldt Østgård,1–3 Mette Nørgaard,2 Lars Pedersen,2 René Østgård,4 Lone Smidstrup Friis,5 Claudia Schöllkopf,6 Marianne Tang Severinsen,7,8 Claus Werenberg Marcher,9 Bruno C Medeiros,10 Morten Krogh Jensen11
1Department of Hematology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark; 3Department of Medicine, Holstebro Regional Hospital, Holstebro, Denmark; 4Diagnostic Center, Silkeborg Regional Hospital, Silkeborg, Denmark; 5Department of Hematology, The University Hospital Rigshospitalet, Copenhagen, Denmark; 6Department of Hematology, Herlev University Hospital, Herlev, Denmark; 7Department of Hematology, Aalborg University Hospital, Aalborg, Denmark; 8Department of Clinical Medicine, Aalborg University, Aalborg, Denmark; 9Department of Hematology, Odense University Hospital, Odense, Denmark; 10Stanford University, School of Medicine, Stanford, CA, USA; 11Department of Hematology, Roskilde University Hospital, Roskilde, Denmark
Background: Most cases of acute leukemia arise without identifiable risk factors. Studies investigating the impact of autoimmune diseases and infections on leukemogenesis have revealed conflicting results. If inflammation increases the risk of acute myeloid leukemia (AML), nonsteroidal anti-inflammatory drug (NSAID) use may decrease the risk of leukemia.
Methods: We conducted a case-control study of 3,053 patients with AML diagnosed between 2000 and 2013, who were registered in the Danish National Acute Leukemia Registry, and 30,530 population controls matched on sex and age. We identified prescriptions through the Danish National Health Service Prescription Database. We used conditional logistic regression analysis to compute ORs associating AML with NSAID use overall, in patients with inflammatory diseases, and for specific AML subtypes (de novo AML, AML related to previous hematological disease, ie, secondary AML [sAML], or therapy-related AML [tAML; exposed to previous cytotoxic therapy]).
Results: Overall, NSAID use was not associated with a lower risk of AML (OR 1.1, 95% CI=1.0–1.2), de novo AML (OR 1.0, 95% CI=0.9–1.1), and sAML/tAML (OR 1.3, 95% CI=1.1–1.5). In addition, in patients with known inflammatory diseases, NSAIDs did not affect AML risk (OR 0.9, 95% CI=0.5–1.6). Number of prescriptions, type of NSAID, age, or sex did not influence the results.
Conclusion: In line with our recent findings that showed no association between autoimmune diseases and infections and de novo AML, NSAID use was not found to reduce the risk of AML.
Keywords: acute myeloid leukemia, population controls, population-based, risk, NSAIDs, inflammation
Introduction
The most important risk factors for acute myeloid leukemia (AML) are prior cytotoxic therapy (therapy-related AML [tAML]) and/or antecedent hematological diseases (secondary AML [sAML]).1 Still, most cases arise without identifiable risk factors (de novo AML). Studies have found an association between infections,2–5 autoimmune diseases,2,6 and AML, suggesting that acute and chronic inflammation may influence leukemogenesis. We recently demonstrated that, in general, this association is confined to tAML and sAML.7
It is well accepted that inflammation plays a carcinogenic role in lymphomas and solid cancers.8 In line with this, nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to reduce the risk of cancers such as colon cancer, likely due to prostaglandins and Cox-2 inhibition of the Wnt signaling pathway, a protein signal transduction pathway demonstrated to play an important role in cell regeneration and carcinogenesis.9
Furthermore, the Wnt pathway affects nonmalignant proliferation of the bone marrow, and aberrant activation of Wnt signaling involving genes implicated in leukemogenesis has been demonstrated in AML (eg, AML1-ETO and mutated FLT3).10–12 If inflammation plays an important role in the development of AML (in particular sAML and tAML), it is important to determine if use of anti-inflammatory drugs reduces the risk of AML.
Limited studies have investigated the effects of NSAID use in AML patients. Ross et al found a chemo-preventive effect of regular- to extra-strength doses of aspirin on AML in only women; however, no effects of ibuprofen and Cox-2 inhibitors were shown.13 Consistent with this finding, Kasum et al found a reduced risk of AML in aspirin users in a cohort of older women,14 whereas Pogada et al demonstrated that long-term use of NSAID reduces the overall risk of AML.15 Small study samples, selected patients, and use of self-reported data limit previous reports. In a larger study using prescription data, Bhayat et al did not find any association between AML and use of NSAIDs.16 However, the AML diagnoses were not validated, no clinical information was available, and results were only given for unspecified NSAIDs, which may be important in light of previous findings.
To further investigate whether NSAID use reduces the risk of AML, we designed a national population-based case-control study among all Danish AML patients diagnosed between 2000 and 2013 and matching population controls. We stratified results by NSAID subtypes, sex, age, inflammatory disease, and by whether AML developed de novo or following antecedent hematological diseases or cytotoxic therapy.
Methods
The source population for this case-control study was Danish residents ≥15 years of age (Danish population 5.7 million)17 observed between 2000 and 2013. All Danish residents are provided with tax-supported medical care, including deduction on prescription medication.
AML cases
We included all nonpromyelocytic AML cases 16 years or older diagnosed in Denmark between January 1, 2000, and December 31, 2013, identified through the Danish National Acute Leukemia Registry, which has been described in details elsewhere.18,19 We included information on date of diagnosis as well as data on AML type (de novo, sAML, and tAML).20 The definition was based on the WHO classification system and was based on information on prior exposure to chemotherapy or radiotherapy for an unrelated disease (tAML), and/or preceding myeloid hematological malignancy (sAML; eg, myelodysplastic syndrome and myeloproliferative neoplasms).21 From the Danish National Registry of Patients (DNRP), we retrieved information on autoimmune diseases and five chronic autoimmune inflammatory disease categories were elected to retrieve the patient’s information (Table S1).
Selection of population controls
The Civil Registration System22 and the DNRP23 were used to select 10 controls for each AML case. Controls had to be alive at the index date (day of AML diagnosis of their matched patient) and without a history of AML (ICD-10: DC 92.0–92.9, ICD-8: 205.0 registered in the DNRP). Risk set sampling24 was used to match controls with cases on sex and date of birth.
Use of NSAID
We obtained data on NSAID prescriptions through the Danish National Health Service Prescription Database.25 The database is maintained by the Danish Medicines Agency and includes information on all prescribed drugs dispensed from Danish pharmacies. Information includes the unique civil registration number, date of prescription, and type of drug prescribed according to the Anatomical Therapeutic Chemical (ATC) classification system. We used ATC codes included in the M01A category comprising systemic nonsteroidal anti-inflammatory and antirheumatic products to identify prescriptions for NSAIDs. We categorized NSAIDs into nonselective NSAIDs, Cox-2 inhibitors, and others (specific ATC codes used to define NSAID subgroups; Table S2, online only). Only higher priced small packages of low-dose (20 pills, 200 mg) ibuprofen can be bought over the counter, and therefore, long-time users are likely to redeem prescriptions.26 Prescription data are only available after 1995, which allowed for a minimum 5-year opportunity for exposure to NSAIDs before AML diagnosis. We excluded prescriptions <3 years prior to AML diagnosis to minimize the risk of reverse causality and detection bias. In the overall and NSAID subgroup analysis, we defined NSAID use as more than one prescription redeemed.
Statistical analysis
We computed the frequencies and proportions of cases and controls by categories of drug exposure and covariates.
We used conditional logistic regression analysis to estimate the ORs associating AML with NSAID use. Given the density sampling of controls, ORs in conditional logistic regression are valid estimates of the corresponding incidence rate ratios.
All results were given for NSAID overall, by subgroups (nonselective, Cox-2, and other NSAIDs), and by number of NSAID prescriptions (0, 1–2, 3–5, 6–10, >10). All results were given overall and stratified by sex, age (<60 years, ≥60 years), type of AML (de novo, sAML, or tAML), and in patients with known inflammatory diseases (connective tissue disease; ICD-10). Results were provided with 95% CIs.
We repeated all analyses using 1-year exclusion of prescription history. In addition, we repeated the overall analysis using >1 redeemed prescription as exposure.
Analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). The study protocol was approved by the Danish Data Protection Agency (jr. nr. 2013-41-1570).
Results
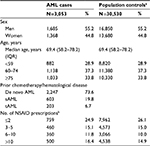
The final study population included 3,053 AML cases and 30,530 sex- and age-matched population controls. The median age was 69.4 years (15–99). The prevalence of AML was stable during the study period with 73.6% de novo AML, 19.8% sAML, and 6.7% tAML. Fifty-five percent of the patients were men. Descriptive characteristics are shown in Table 1.
The number of patients and controls redeeming a prescription for NSAID during the years 1995 and 3 years prior to their AML diagnosis by number of prescriptions are shown in Table 1.
The number of AML patients who redeemed >1 prescription (not accounting patients who only redeemed prescriptions <1 year before the AML diagnosis) was 2,079 (68.1%). Among the 30,530 controls, 20,139 (66.0%) had redeemed at least 1 NSAID prescription during the years 1995–2013 and >1 year before their index date. Ignoring prescriptions <3 years before AML diagnosis or index date, the numbers were 1,869 (61.2%) for cases and 18,128 (59.4%) for controls.
The associations between NSAID use and risk of AML are shown in Table 2.
Overall, any use of NSAID prior to AML diagnosis was not associated with the risk of AML (OR 1.1, 95% CI=1.0–1.2). Similar results were seen across NSAID subtypes. In addition, long-term use did not reduce the risk of AML when the risk of AML was compared between patients with different numbers of prescriptions (1–2 prescriptions, OR 1.0, 95% CI=0.9–1.1) vs those with >10 prescriptions (OR 1.1, 95% CI=0.9–1.2).
When repeating the analysis for patients with de novo AML and sAML/tAML separately (Table 2), an increased risk of AML in NSAID users across NSAID subtypes and numbers of prescriptions was observed in patients with sAML/tAML (OR 1.3, 95% CI=1.1–1.5), whereas use of NSAIDs was not associated with the risk of de novo AML (OR 1.0, 95% CI=0.9–1.1). Stratification by sex or age (<60 vs ≥60 years) did not change the interpretation of results, although lower estimates were found in women without any evidence of protective effect (Table 3).
Restricting analysis to patients with known inflammatory conditions measured as autoimmune diseases, NSAID consumption did not reduce the overall risk of AML (Table 4). In a subgroup analysis, NSAID use did not affect the risk of AML in patients with connective tissue disease (OR 0.9, 95% CI=0.5–1.6) compared with non-NSAID users. In contrast, we did observe a lower risk of AML in patients with inflammatory gastrointestinal conditions; however, results were imprecise (OR 0.6, 95% CI=0.2–1.2).
To test the robustness of our results, we repeated all analyses ignoring prescriptions only 1 year before AML diagnosis or index date (results not shown). In general, the estimates were slightly higher when using only 1 year as an exclusion criterion (NSAID overall: 1 year: OR 1.0, 95% CI=0.9–1.1 vs 3 years: OR 1.1, 95 CI=1.0–1.2). Thus, our overall conclusions did not change based on these results. In addition, we repeated analysis defining NSAID users as subjects with 1 or more prescriptions. This did not change the estimates of the overall analysis (> 1 prescription: OR 1.1, 95% CI= 1.0–1.2 vs ≥ 1 prescription: OR 1.1, 95% CI= 1.0–1.2).
Discussion
In this large population-based case-control study, we demonstrated that NSAID use does not protect against AML overall or in patients with known inflammatory conditions.
Prior studies have attempted to define the effect of NSAID use on the risk of leukemogenesis, and conflicting results have been reported. For example, in women, a protective effect of nonaspirin anti-inflammatory agents (OR 0.5, 95% CI=0.2–0.9) and that of general NSAID use for more than 4 weeks have been reported in different studies.13,15 These results are in clear contrast to our observations showing a lack of protective or dose–response effect of NSAIDs on leukemogenesis. However, our findings resemble those from 2 separate reports that failed to demonstrate any protective effect of NSAID use on AML risk.14,16 Significant methodological concerns were observed in previous studies, including small cohorts (28–568 cases) and reliance on self-reported data in all but 1 prior report.13–15 Thus, study participants were required to recall medicine intake for up to 10 years and differentiate between different subtypes of NSAIDs. In addition, some studies used proxy responders, which is likely to further increase the risk of recall bias. Participation rates were low (42%–58%), increasing the risk of selection bias and favoring long-term survivors reducing the generalizability.
Overall, our study did not find NSAID to be associated with an increased risk of AML. Patients with sAML and tAML may have a higher consumption of anti-inflammatory drugs and an increased risk of AML mediated through the premalignant disorders or cytotoxic therapy, suggesting against a causal relationship between NSAID use and AML. Our results stratified by type of AML support this theory, as the association between NSAID use and high risk of AML was only seen for sAML/tAML patients, whereas a clear null result was seen for de novo AML.
Although the literature on whether NSAIDs reduce AML risk is sparse, studies have found an association between autoimmune diseases, infections, and AML.2–4,6 However, study designs and lack of clinical data prevented investigation of whether inflammation or bias could explain their findings. The finding that NSAID overall did not reduce the risk of AML supports our hypothesis that at least for de novo AML, inflammation is unlikely to be responsible for initiation of leukemogenesis7 but may be associated with progression to AML in patients with an underlying condition such as prior hematological disorder or selection of Clonal Hematopoiesis of Indeterminate Potential following cytotoxic therapy.
In patients with known inflammatory disease, patients are likely to consume NSAIDs at higher doses and longer periods of time. In patients with connective tissue disease such as rheumatoid arthritis, use of NSAIDs did not influence the risk of AML. Interestingly, though results were imprecise, we did observe a lower risk of AML in NSAID users with gastrointestinal inflammatory conditions. In line with this, we recently found an association between both gastrointestinal infections and gastrointestinal autoimmune conditions and risk of AML, suggesting that inflammation in the gastrointestinal tracts causes immune-related/inflammation-driven leukemogenesis. Therapeutic immune-modulating agents (eg, azathioprine) increase both risk of infections and AML and may explain prior findings. However, the observed tendency toward a lower AML risk in patients with gastrointestinal inflammatory disease with NSAID consumption calls for further investigation of the potential role of gastrointestinal inflammation in leukemogenesis.
To our knowledge, this is the largest study investigating the association between NSAID use and risk of AML. The national population-based design and the use of matched population-based controls reduce selection bias and increase the generalizability of the study results. The use of prescription information prospectively collected of purposes independent of the study aims reduces the risk of recall bias. Both AML coverage and accuracy of the diagnosis are high (>99%).19 Furthermore, our findings were consistent across age and sex strata, thus strengthening our conclusions.
Our study has a few limitations. It was not known whether patients who were prescribed NSAID actually consumed these agents as prescribed. Defining NSAID users as persons with >1 prescription increased the likelihood that patients who redeemed prescriptions actually were NSAID users. In addition, adherence is expected to be similar between cases and controls. Cases and controls are equally likely to buy over-the-counter drugs and since we did not see a protective effect of patients with higher number of prescription and defined NSAID users as persons with >1 prescription, this is unlikely to have influenced our results. The multiple comparison approach may have resulted in some significant associations simply happening by chance, and results of the stratified analyses must be interpreted with caution. However, the analyses were primarily used to test and confirm a predefined hypothesis that NSAID use is not associated with an increased risk of AML, reducing the likelihood of our conclusions relying on coincidence.
Conclusion
In this large population-based study of 3,165 AML cases and 31,650 controls, we did not find that NSAID reduces the risk of AML. Age, sex, and number of prescriptions did not influence the results. In line with our recent findings of no association between autoimmune diseases and infections and de novo AML, these findings support our hypothesis that inflammation plays a minor role in the initiation of de novo AML.
Acknowledgments
An abstract of preliminary study results was presented at the ASH Annual Meeting 2017 as a poster presentation. The poster’s abstract was published in Blood. 2017;130(Suppl 1), 1,305 (www.bloodjournal.org/content/130/Suppl_1/1305). The study was supported by The Danish National Acute Leukemia Group and the Dagmar Marshall Foundation. LSGØ was supported by research funding from the Danish Cancer Society and MN was supported by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation. None of the funding sources contributed to the design, performance, analysis, or reporting of this study.
Author contributions
Conception and design: LSGØ, MN, LP, and MKJ. Collection and assembly of data: LSGØ, CS, MTS, CWM, LSF, and MKJ. Data analysis and interpretation: LSGØ, MN, RØ, LP, BCM, and MKJ. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Larson RA. Etiology and management of therapy-related myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2007;1:453–459. | ||
Kristinsson SY, Björkholm M, Hultcrantz M, Derolf ÅR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–2903. | ||
Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822–828. | ||
Johnson KJ, Blair CM, Fink JM, et al. Medical conditions and risk of adult myeloid leukemia. Cancer Causes Control. 2012;23(7):1083–1089. | ||
Ramadan SM, Fouad TM, Summa V, Hasan Skh, Lo-Coco F. Acute myeloid leukemia developing in patients with autoimmune diseases. Haematologica. 2012;97(6):805–817. | ||
Titmarsh GJ, Mcmullin MF, Mcshane CM, Clarke M, Engels EA, Anderson LA. Community-acquired infections and their association with myeloid malignancies. Cancer Epidemiol. 2014;38(1):56–61. | ||
Østgård LSG, Nørgaard M, Pedersen L, et al. Autoimmune diseases, infections, use of antibiotics and the risk of acute myeloid leukaemia: a national population-based case-control study. Br J Haematol. 2018;181(2):205-214–214. | ||
Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32(4):1119–1136. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. | ||
Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. | ||
Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–541. | ||
Mikesch JH, Steffen B, Berdel WE, Serve H, Müller-Tidow C. The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia. 2007;21(8):1638–1647. | ||
Ross JA, Blair CK, Cerhan JR, et al. Nonsteroidal anti-inflammatory drug and acetaminophen use and risk of adult myeloid leukemia. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1741–1750. | ||
Kasum CM, Blair CK, Folsom AR, Ross JA. Non-steroidal anti-inflammatory drug use and risk of adult leukemia. Cancer Epidemiol Biomarkers Prev. 2003;12(6):534–537. | ||
Pogoda JM, Katz J, Mckean-Cowdin R, Nichols PW, Ross RK, Preston-Martin S. Prescription drug use and risk of acute myeloid leukemia by French-American-British subtype: results from a Los Angeles County case-control study. Int J Cancer. 2005;114(4):634–638. | ||
Bhayat F, das-Gupta E, Smith C, Hubbard R. NSAID use and risk of leukaemia: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18(9):833–836. | ||
Statistics Denmark. Population and elections: StatBank Denmark—Data and Statistics 2017. Available from: https://www.statbank.dk/statbank5a/defa ult.asp?w=1440. Accessed November, 2017. | ||
Østgård LS, Nørgaard JM, Raaschou-Jensen KK, et al. The Danish National Acute Leukemia Registry. Clin Epidemiol. 2016;8:553–560. | ||
Ostgård LS, Nørgaard JM, Severinsen MT, et al. Data quality in the Danish National Acute Leukemia Registry: a hematological data resource. Clin Epidemiol. 2013;5:335–344. | ||
Swerdlow S, Campo E, Harris N, et al, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. WHO Classification of Tumours, Vol. 2. IARC WHO Classification of Tumours, No. 2. Lyon: International Agency for Research on Cancer; 2017. | ||
Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. | ||
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. | ||
Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. | ||
Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. | ||
Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt M, Pedersen L, Sørensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303–313. | ||
Mellemkjaer L, Blot WJ, Sørensen HT, et al. Upper gastrointestinal bleeding among users of NSAIDs: a population-based cohort study in Denmark. Br J Clin Pharmacol. 2002;53(2):173–181. |
Supplementary material
  | Table S1 Diagnoses and ICD codes included in 5 chronic autoimmune/inflammatory disease categories Abbreviation: ICD, International Classification of Diseases. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.