Back to Journals » OncoTargets and Therapy » Volume 8
NRBP1 is downregulated in breast cancer and NRBP1 overexpression inhibits cancer cell proliferation through Wnt/β-catenin signaling pathway
Authors Wei H, Wang H, Ji Q, Sun J, Tao L, Zhou X
Received 3 June 2015
Accepted for publication 21 October 2015
Published 14 December 2015 Volume 2015:8 Pages 3721—3730
DOI https://doi.org/10.2147/OTT.S89779
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Hong Wei,1 Hongbin Wang,2 Qiao Ji,1 Jiawei Sun,1 Lin Tao,1 Xianli Zhou1
1Department of Ultrasonography, The Second Affiliated Hospital of Harbin Medical University, 2Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang, People’s Republic of China
Abstract: Nuclear receptor binding protein 1 (NRBP1) is a highly conserved protein that is ubiquitously expressed across cell types in humans. NRBP1 has been recently identified as an adaptor protein. It has been suggested that it plays important roles in cellular homeostasis and the pathophysiology of cancer. To determine whether NRBP1 is involved in the pathophysiology of breast cancer, we performed a correlation study between the expression level of NRBP1 and the clinicopathological features in 92 breast cancer patients. A strong correlation was detected between NRBP1 expression and advanced histopathology grades, tumor, node, and metastasis stage, tumor diameter, lymph node involvement, as well as the recurrence of breast cancer in 92 tested patients. The tumor tissues from patients also expressed lower NRBP1 than did adjacent healthy tissues. Furthermore, we overexpressed NRBP1 in MCF-7 and MDA-MB-231 breast cancer cell lines and found NRBP1 upregulation-inhibited cell proliferation by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Blocking the autocrine Wnt signaling pathway by LGK974 could remove the NRBP1-overexpression-induced inhibition in breast cancer cells. The results of this study suggest that NRBP1 plays a tumor-suppressive role in breast cancer pathophysiology, which likely acts through the Wnt/β-catenin signaling pathway.
Keywords: nuclear receptor binding protein 1 (NRBP1), breast cancer, clinicopathological features, tumor suppression Wnt/β-catenin pathway, cell proliferation
Introduction
Worldwide, breast cancer is the second most common cancer and the leading cause of death by cancer in women. The incidence rates of breast cancer are increasing, and the global burden of breast cancer exceeds that of all other cancers.1 Despite great advances in early detection and progress in treatment with systemic agents such as hormones, most breast cancers develop resistance to drugs.2 Understanding the underlying biological mechanisms and altered molecular events of carcinogenesis could lead to the identification of novel molecular targets and to the development of targeted therapies. In recent years, landmark discoveries in the field of breast cancer research and the use of gene expression profiling to identify distinct subtypes of breast cancer have contributed to the significant improvement in the survival rate and treatment outcome in breast cancer patients.3–5 Targeting the pathways that promote or sustain the growth and invasion of carcinoma cells is critical to the effective treatment of breast cancer.
We investigated nuclear receptor binding protein 1 (NRBP1) as a potential breast cancer treatment target and studied its involvement in the Wnt/β-catenin signaling pathway. NRBP1 is a ubiquitously expressed adapter protein, recently described to have a tumor-suppressive role in cancer.6,7 Human NRBP1 contains 535 amino acid residues. Its gene is located on human chromosome 2p23 and is expressed across cell types. Between species, NRBP1 is highly conserved, which suggests a conserved function for NRBP1 in cellular activities. It has been suggested that it functions as an adaptor protein and has been shown to carry two putative nuclear receptor binding motifs, a putative binding domain for Src homology 2 domain-containing proteins. NRBP1 also carries nuclear export signals, a nuclear localization signal, a myeloid leukemia factor 1 binding region, a BC-binding box, and a transforming growth factor beta 1 (TGFβ1)-stimulated clone 22 binding region. NRBP1 has been detected both in cytoplasm and nucleus and has been demonstrated to predominantly localize in the cytoplasm. In vitro studies have postulated that NRBP1 shuttles between the nucleus and cytoplasm, regulating protein localization, and transcription factor activity.
Given its conserved structure, binding interactions with a number of key transcription factors, and ubiquitination machinery, it is not surprising that NRBP1 plays an important role in cellular function. However, only recently has NRBP1 been proposed to play a role in cancer progression. When NRBP1 was conditionally deleted in adult somatic tissue, profound effects, including widespread crypt elongation, reduced differentiation, and increased proliferation of intestinal progenitor cells were observed. The NRBP1-deleted tissue showed an enhanced Wnt activation signature8–11 consistent with the intestinal progenitor cell phenotype. Mosaic somatic deletion of NRBP1 circumvented lethality and resulted in accelerated tumorigenesis. These results all indicate that the somatic loss of NRBP1 increased the risk of tumorigenesis and strongly indicate that NRBP1 might have a tumor-suppressive role. We hypothesize that NRBP1 expression is related to breast cancer and that upregulating NRBP1 expression could suppress breast cancer development. We also hypothesize that NRBP1 is involved in regulating breast cancer development through the Wnt signaling pathway. To test our hypotheses, we investigated the correlation between NRBP1 expression levels and breast cancer clinicopathological features. We also overexpressed NRBP1 in two breast cancer cell lines – MCF-7 and MDA-MB-231 – to determine the tumor-suppressive role of NRBP1 in breast cancer progression. Finally, Wnt signaling pathway inhibitor LGK974 was applied to both NRBP1-overexpressed and control breast cancer cell lines (MCF-7 and MDA-MB-231).
Materials and methods
Cancer tissue sample collection
Samples were obtained from 92 breast cancer patients who underwent surgery at The Second Affiliated Hospital of Harbin Medical University. None of the patients received adjuvant chemotherapy or radiotherapy before surgery. Fresh cancer tissues and their matched normal tissues were collected and stored at -80°C immediately after resection. Two pathologists examined the cancer tissues and the matched normal tissue. Tumors were staged based on Union Internationale Contre Le Cancer tumor, node, and metastasis (TNM) classification. The presence of lymph node metastasis was determined by histological examination. The Medical Ethics Committee of The Second Affiliated Hospital of Harbin Medical University approved the protocol, and written informed consent was obtained from all participants.
Follow-up
Physical examination and laboratory investigation of these patients were carried out every 4–6 months for the first 4 years and every 12 months thereafter during the follow-up period. All these patients were followed until the study closing date (January 31, 2014) or death. Overall survival was calculated in months from the diagnosis until the date of death, last known to be alive, or the study closing date. The median follow-up time in this study was 42 months (range: 14–102).
Cell culture, plasmid construction, and plasmid transfection
Breast cancer cell lines MCF-7 and MDA-MB-231 were purchased from the Cell Library of the Chinese Academy of Sciences (Wuhan, Hubei, People’s Republic of China). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Gibco BRL), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (Thermo Fisher Scientific), at 37°C, under a humidified atmosphere of 5% CO2 and 95% air. NRBP1 gene was polymerase chain reaction (PCR)-amplified from MCF-7 genomic DNA. The PCR product containing NRBP1 was constructed into pcDNA3.1 vector (Promega Corporation, Fitchburg, WI, USA). Lipofectamine™ 2000 (Thermo Fisher Scientific) was used for NRBP1-pcDNA3.1 and siRNA transfection according to the manufacturer’s instructions.
qRT-PCR
Total RNA was extracted using TRIzol® (Thermo Fisher Scientific) according to the manufacturer’s instructions. RNase-free DNase I was used to remove DNA contamination. RNA concentration was determined by measuring absorption at 260 nm. RNA was reversely transcribed using first-strand cDNA Synthesis Kits (Thermo Fisher Scientific). The resulting cDNAs underwent real-time quantitative RT-PCR analysis. The following primers were used to evaluate the relative gene expression levels in real-time quantitative RT-PCR: 5′-AATGAAAAGGCTTGGAAACG-3′ (F) and 5′-CAGGTCGTCCATGAGGTTTT-3′ (R) for NRBP1; 5′-GGATGCTGGAGGTCTGCGAGGAAC-3′ (F) and 5′-GAGAGGAAGCGTGTGAGGCGGTAG-3′ (R) for Cyclin D1; 5′- AAACACAAACTTGAACAGCTAC-3′ (F) and 5′-ATTTGAGGCAGTTTACATTATGG-3′ (R) for c-Myc; and 5′-TGGCACCCAGCACAATGAA-3′ (F) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (R) for β-actin mRNA. Quantitative real-time PCR was done using the 7500 Real-Time PCR System (Thermo Fisher Scientific) with SYBR Green® PCR Master Mix (Thermo Fisher Scientific) according to the manufacturer’s instructions. The housekeeping genes, β-actin, were used to normalize for RNA loading. The data were analyzed using the comparative threshold cycle (2−ΔΔCT) method.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissues were sectioned at a 5 μm thickness, deparaffinized, and rehydrated in gradients of high percentage ethanol to distilled water. Sections were immersed in 3% hydrogen peroxide for 15 minutes at room temperature to quench endogenous peroxidase activity. Sections were then incubated with the NRBP1 primary antibody (GeneTex, Beijing, People’s Republic of China, at a 1:100 dilution) at 4°C overnight, washed three times in phosphate buffered saline (PBS) for 5 minutes, and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Beyotime, Beijing, People’s Republic of China) at room temperature for 30 minutes, followed by 3-amino-9- ethylcarbazole staining. Sections were lightly counterstained with hematoxylin.
Western blot
The cultured cells were treated with RIPA lysis buffer (50 mm Tris-HCl, pH 8.0, 1 mm ethylenediaminetetraacetic acid, 0.1% sodium dodecyl sulfate, 150 mm NaCl, 1% NP-40, 0.1% sodium deoxycholate) including cOmplete™ protease inhibitor mixture (Boster, Wuhan, Hubei, People’s Republic of China). The lysates were cleared by centrifugation (14,000 rpm) at 4°C for 20 minutes, and supernatants were collected as protein samples. Approximately 20–30 μg of each protein sample was run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA). After blocking the nonspecific binding sites for 60 minutes with 5% nonfat milk, the membranes were incubated with primary antibodies against NRBP1 (GeneTex, at a 1:1,000 dilution), Cyclin D1 (Santa Cruz Biotechnology Inc., Dallas, TX, USA, at a 1:1,000 dilution), c-Myc (Santa Cruz Biotechnology Inc., at a 1:1,000 dilution), or β-actin (Boster, at a 1:10,000 dilution) overnight at 4°C. The membranes were then incubated with HRP-conjugated secondary antibody (at a 1:10,000 dilution) for 60 minutes at room temperature after three 10-minute washes with Tris-buffered saline and Tween 20 (TBST). The membranes were washed three more times with TBST and developed using an enhanced chemiluminescence system (Beyotime).
MTT assay
MCF-7 or MDA-MB-231 cells were seeded in 96-well plates at a density of 3,000 cells/well and measured daily for 6 days after cultivating. Twenty microliters of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL; Genebase, Shanghai, People’s Republic of China) was added into each well, and the cells were incubated for 4 hours at 37°C. The culture medium in each well was removed after 4 hours of incubation and replaced by 100 μL of dimethyl sulfoxide.
After shaking at a low speed (suggest to add the exact speed) for 10 minutes to fully homogenize and aspirate the remaining MTT, the supernatant was measured for its optical density at 550 nm wavelength by using a microplate reader (BioTek, Winooski, VT, USA).
Luciferase assay
Breast cancer cells were plated in 24-well plates at a density of 1×105 cells/well in complete growth medium and incubated overnight after NRBP1 plasmid transfection. Plasmid mixtures containing 2 μg TopFlash luciferase construct (Addgene, Cambridge, MA, USA) and 0.05 μg Renilla reporter plasmid were transfected into cells overnight using the Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Media were changed, and cells were left to recover from transfection for 6 hours. After 48 hours incubation, cells were lysed, and the luciferase activity was measured using the dual luciferase assay kit (Promega Corporation, Fitchburg, WI, USA) to evaluate the evolvement of the Wnt/β-catenin signaling pathway. Values for TopFlash luciferase activity were normalized with Renilla activity.
Statistical analyses
The present results were analyzed using SPSS Statistical Software for Windows (version 18.0.0, SPSS Inc., Chicago, IL, USA) and represented as mean ± standard error of the mean of three experiments performed in triplicate. Two-tailed Student’s t-test or one-way ANOVA was used to assess the statistical significance of differences in groups. For all statistical analyses, P<0.05 was considered statistically significant.
Results
Correlation between NRBP1 levels and clinicopathological features
Table 1 presents the clinicopathological features of all 92 breast cancer patients who participated in this study. Among these 92 participants, 49 (53%) were younger than 50 years of age and 35 (38%) patients were pre-menopausal. Based on histologic grade analysis, 23 (25%) patients were classified as G1 (well differentiated), 38 (41.3%) were G2 (moderately differentiated), and 31(33.7%) were G3 (poorly differentiated). The majority of the patients (84.8%) had ductal carcinoma and eleven (12%) patients had lobular carcinoma. Sixteen patients (17.4%) were classified as stage I, 21 (22.8%) as stage II, and 55 (59.8%) as stage III + IV, according to the TNM staging system. Tumor diameter in 43 (82.6%) patients was more than 2 cm. Based on the presence of lymph node metastasis, 60 (65.2%) patients were classified as node-positive. Twenty-four (26%) patients had a recurrence.
qRT-PCR analysis was then carried out to evaluate the NRBP1 expression frequency in 92 breast cancer samples. High NRBP1 expression was confirmed in 46 (50%) patients, whereas 46 patients (50%) had low NRBP1 expression. Histopathology, TNM stage, tumor diameter, lymph node involvement, and the presence of recurrence were all significantly correlated with NRBP1 expression levels. The relationships between clinicopathological factors and NRBP1 expression levels are listed in Table 1.
NRBP1 expression and survival
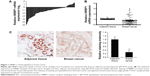
To investigate the function of NRBP1 in breast cancer, we first compared the expression of NRBP1 in cancer tissue with that in adjacent normal tissues. NRBP1 levels were lower in the majority of cancer tissues (76 of 92) than in adjacent normal tissues (P<0.001) (Figure 1A and B). Immunohistochemistry staining further confirmed the downregulation of NRBP1 in breast cancer tissues compared with normal tissues (Figure 1C). When 92 patients were tested at the median follow-up of 42 months (range: 14–102), the median overall survival time of patients with low NRBP1 expression was significantly shorter than that of patients with high NRBP1 expression (Figure 2, P=0.002). Taken together, our results suggest that NRBP1 is downregulated in breast cancer tissues, and NRBP1 may play a negative role in cancer development.
NRBP1 regulates breast cancer cell proliferation through Wnt/β-catenin signaling pathway
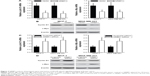
To further explore the function of NRBP1 in breast cancer tumorigenesis, we overexpressed NRBP1 in breast cancer cell lines (MCF-7 and MDA-MB-231) by transfecting NRBP1 plasmid (Figure 3A). As expected, MTT assays revealed that NRBP1 inhibited breast cancer cell proliferation in both MCF-7 and MDA-MB-231 cells (Figure 3B). Furthermore, we knocked down NRBP1 in both MCF-7 and MDA-MB-321 cells (Figure 3C). Downregulation of NRBP1 increased breast cancer cell proliferation (Figure 3D).
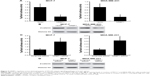
Previous studies showed that NRBP1 is functionally associated with Wnt/β-catenin signaling pathway in intestinal progenitor cells, and Wnt/β-catenin signaling pathway plays a pivotal role in breast cancer development.12,13 We sought to determine whether NRBP1 affected breast cancer cell proliferation through Wnt/β-catenin signaling pathway by analyzing Wnt/β-catenin signaling pathway downstream gene expression in control and NRBP1-overexpressed cells. TopFlash luciferase reporter activities and nuclear β-catenin levels were also evaluated. mRNA and protein expression of Wnt/β-catenin signaling pathway downstream genes Cyclin D1 and c-Myc were significantly reduced with ectopic NRBP1 overexpression (Figure 4A and B). Moreover, downstream genes of Wnt/β-catenin signaling pathway were upregulated when NRBP1 was knocked down (Figure 4C and D), indicating that NRBP1 can inhibit Wnt/β-catenin signaling in breast cancer cells. TopFlash luciferase reporter assays also showed that NRBP1 overexpression reduced canonical Wnt signaling transcriptional activity in breast cancer cells (Figure 5A). Furthermore, β-catenin level was decreased in the nuclei of NRBP1-overexpressed MCF-7 and MDA-MB-231 cells (Figure 5B). Additionally, inhibition of NRBP1 promoted Wnt signaling transcriptional activity and β-catenin nuclear translocation (Figure 5C and D).
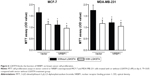
We further examined whether NRBP1-mediated inhibition of Wnt/β-catenin activity actually contributes to suppression of breast cancer cell proliferation. Control or NRBP1-overexpressed MCF-7 and MDA-MB-231 cells were incubated with or without LGK974,14 a porcupine inhibitor of autocrine Wnt signaling. Our results showed that LGK974 inhibited breast cancer cell proliferation. Interestingly, overexpression of NRBP-1 did not add any growth inhibitory effects in combination with LGK947, which indicates that NRBP1 and LGK947 act on the same signaling axis (Figure 6). Taken together, our results strongly indicate that NRBP1 inhibits breast cancer cell growth via downregulating Wnt/β-catenin signaling pathway activity.
Discussion
NRBP1 has been proposed to have a role in cancer progression only very recently, and its precise functions still remain largely unknown. This study is the first analysis of NRBP1 expression in breast cancer clinical cases as well as in cultured breast cancer cell lines. Breast cancer tissue samples were collected from 92 breast cancer patients at different ages and different stages of breast cancer. Low NRBP1 expression in tissue samples collected from breast cancer patients was found to be strongly correlated with more advanced histological grades (G2 and G3), the late TNM stage, bigger tumor diameter, high lymph node involvement, as well as the high presence of recurrence of breast cancer in 92 tested patients. Tumor tissue samples collected from the breast cancer patients also featured a significantly low NRBP1 expression level compared with the adjacent normal tissues.
These findings are consistent with the work from Wilson et al, which showed that NRBP1 is downregulated in several diverse cancer types, including colorectal and lung tissue. NRBP1 has been identified as a novel enhancer of the oncogenic Ras;6 the conditional knockout of NRBP1 could activate Ras as well as induce an upregulation of Wnt reporter activity. It is interesting to note that many cancers, including breast cancers, show a deregulation of Wnt signaling without activating mutations in β-catenin. It is possible that NRBP1 downregulation may contribute to this. Breast cancer and prostate cancer are the two most common invasive cancers in women and men with high biological similarities, respectively.15 Interestingly, NRBP1 was reported to be highly expressed and increase the cell growth in prostate cancer.16 NRBP1 might function in prostate cancer through different signaling pathway.
Given that NRBP1 cooperates with oncogenic Ras to modulate Wnt signaling and our finding that low expression of NRBP1 is strongly correlated with more severe breast cancer clinicopathological features, we hypothesized that NRBP1 acts as a breast cancer suppressor through the Wnt signaling pathway. To further investigate our hypothesis, we overexpressed NRBP1 in MCF-7 and MDA-MB-231 breast cancer cell lines. NRBP1 overexpression successfully inhibited the proliferation of MCF-7 and MDA-MB-231 cells, validating the breast cancer-suppressive function of NRBP1. LGK974 was then applied to block porcupine, a Wnt-specific acyltransferase in both NRBP1-overexpressed and control breast cancer cells. The administration of LGK974 failed to further affect breast cancer cell proliferation in NRBP1-overexpressed cells, indicating that NRBP1 could suppress breast cancer tumorigenesis by regulating the Wnt signaling pathway.
Conclusion
We discovered that NRBP1 levels were reduced in breast cancer tumor tissues as well as the correlation between the NRBP1 expression level and breast cancer clinicopathological features in patients. We also determined the cancer-suppressive role of NRBP1 in cultured breast cancer cell lines. These results validated the suggestion made in many previous studies that NRBP1 may play a very critical role as tumor suppressor. We also showed that the Wnt signaling pathway could regulate NRBP1-induced cancer cell proliferation. Together, our results indicate that NRBP1 could be a potential therapeutic target for suppressing breast cancer progression.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. | ||
Munagala R, Aqil F, Gupta RC. Promising molecular targeted therapies in breast cancer. Ind J Pharmacol. 2011;43:236–245. | ||
Eniu A. Integrating biological agents into systemic therapy of breast cancer: trastuzumab, lapatinib, bevacizumab. J BUON. 2007;12 Suppl 1:S119–S126. | ||
Green MD, Francis PA, Gebski V, et al. Gefitinib treatment in hormone-resistant and hormone receptor-negative advanced breast cancer. Ann Oncol. 2009;20:1813–1817. | ||
Ishida T, Kiba T, Takeda M, et al. Phase II study of capecitabine and trastuzumab combination chemotherapy in patients with HER2 overexpressing metastatic breast cancers resistant to both anthracyclines and taxanes. Cancer Chemother Pharmacol. 2009;64:361–369. | ||
Wilson CH, Crombie C, van der Weyden L, et al. Nuclear receptor binding protein 1 regulates intestinal progenitor cell homeostasis and tumour formation. EMBO J. 2012;31:2486–2497. | ||
Kerr JS, Wilson CH. Nuclear receptor-binding protein 1: a novel tumour suppressor and pseudokinase. Biochem Soc Trans. 2013;41:1055–1060. | ||
Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. | ||
Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. | ||
Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. | ||
Finch AJ, Soucek L, Junttila MR, Swigart LB, Evan GI. Acute overexpression of Myc in intestinal epithelium recapitulates some but not all the changes elicited by Wnt/beta-catenin pathway activation. Mol Cell Biol. 2009;29:5306–5315. | ||
Tan EH, Sansom OJ. A new tumour suppressor enters the network of intestinal progenitor cell homeostasis. EMBO J. 2012;31:2444–2445. | ||
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. | ||
Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. | ||
Risbridger GP, Davis ID, Birrell SN, Tilley WD. Breast and prostate cancer: more similar than different. Nat Rev Cancer. 2010;10: 205–212. | ||
Ruiz C, Oeggerli M, Germann M, et al. High NRBP1 expression in prostate cancer is linked with poor clinical outcomes and increased cancer cell growth. Prostate. 2012;72:1678–1687. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.