Back to Journals » Vascular Health and Risk Management » Volume 13
Novel stroke risk reduction in atrial fibrillation: left atrial appendage occlusion with a focus on the Watchman closure device
Authors Alipour A, Wintgens LIS, Swaans MJ , Balt JC, Rensing BJWM, Boersma LVA
Received 21 March 2016
Accepted for publication 12 October 2016
Published 6 March 2017 Volume 2017:13 Pages 81—90
DOI https://doi.org/10.2147/VHRM.S89213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Arash Alipour, Lisette I S Wintgens, Martin J Swaans, Jippe C Balt, Benno J W M Rensing, Lucas V A Boersma
Department of Cardiology, St. Antonius Hospital, Nieuwegein, the Netherlands
Abstract: Atrial fibrillation (AF) remains an important clinical problem with severe complications such as stroke, which especially harms those with risk factors as calculated by the CHADS2 or CHA2DS2-VASc. Until now, no therapy has proven 100% effective against AF. Since the left atrial appendage (LAA) is the most prominent nonvalvular AF-related thromboembolic source and (novel) oral anticoagulant [(N)OAC] carries the hazard of bleeding, LAA occlusion may be an alternative, especially in patients who are ineligible for (N)OAC therapy. In this review, we discuss several LAA occlusion techniques with a focus on the Watchman device since this device is the most thoroughly studied device of all.
Keywords: left atrial appendage, atrial fibrillation, ischemic stroke
Introduction
Atrial fibrillation (AF) is the most common type of sustained arrhythmia. The lifetime risk for AF is high. Data derived from the Framingham Heart Study estimate the risk of AF to be one in four for subjects over 40 years of age.1 With improved life expectancy, the prevalence of AF will increase. The fact that approximately two of three AF patients receive at least one cardioversion forces us to recognize that AF is associated with a tremendous rise in health care resources and costs.2,3
AF would not have been such a big health issue if therapy required to treat it was 100% effective. Several antiarrhythmic drugs (AADs) with different mechanistic approaches have been used, but none of them resulted in complete freedom from AF. Moreover, AADs with significant favorable effect on restoring sinus rhythm (such as amiodarone) come with significant side effects. Landmark trials such as the Rate Control vs Electrical Cardioversion (RACE) trial and the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) trial have indeed shown that even rhythm control strategies are not able to sustain sinus rhythm in half of the AF patients.4,5 Catheter ablation (CA) has been shown to be more effective than AADs in the short term, but long-term freedom from AF is still disappointing with dramatic success rates of about 29% after 5 years in patients, especially those with nonparoxysmal AF.6,7 Despite the fact that the (minimally) invasive surgical approaches have shown higher success rates, these procedures also do not guarantee longstanding persistent or permanent sinus rhythm. Moreover, these procedures are more invasive and have increased complication rates when compared to AADs in CA approach.8
Besides symptoms such as palpitations, weakness, and dyspnea, AF also may result in serious sequelae. The most striking of these is ischemic stroke, which accounts for 15%–20% of all strokes.9 To reduce the stroke risk in high stroke risk patients, (novel) oral anticoagulant [(N)OAC] therapy is mandatory. However, (N)OACs also have disadvantages such as an increased propensity to cause bleeding. The purpose of this paper is to review the risk of stroke in patients with AF as well as therapeutic strategies to prevent stroke and oral anticoagulation-related bleeding in these patients. Special attention will be given to the Watchman left atrial appendage (LAA) closure device, which is designed to prevent AF-related stroke. For the sake of clarity, we use the term “AF” solely for nonvalvular AF. Valvular AF is another entity where the LAA is a less prominent source for thromboembolism; other therapeutic strategies are required for treating this and will not be discussed here.
Current therapeutic strategies to prevent stroke in AF
Mechanism of stroke in AF
Ischemic stroke is a direct result of thromboembolic events predominantly originating from the left atrium (LA) or the LAA.10 The pathophysiology is stasis of blood in the LA giving rise to thrombus, which embolizes to the arterial circulation. The features are similar to those described as the triad of Virchow, consisting of 1) slow blood flow (stasis) in the LA which may be visible as spontaneous echo contrast on an echocardiogram, 2) dilatation of the LA indicating structural abnormalities, and 3) increased susceptibility to thrombus formation due to the activation of coagulation elements and hyperactive platelets.11 The effect of stasis is the strongest for LAA. Indeed, it has been shown that LAA is the source of thrombi in >90% of patients with AF.10 Therefore, anticoagulation therapy is given to these patients in order to keep the LAA free of thrombi or to resolve preexisting thrombi. The LAA morphology may be highly variable, which could affect the likelihood of LAA-related stroke. The chicken wing morphology may be less thrombogenic, whereas windsock and cauliflower morphologies seem to show a higher tendency for stroke/transient ischemic attack (TIA).12,13 The relevance for the need to use anticoagulation has not yet been determined.
Anticoagulation as cornerstone therapy for stroke prevention
Anticoagulation therapy has been used for decades to prevent thrombus formation. The guidelines are clear on this topic as they recommend the use of (N)OAC in patients at higher stroke risk, which is calculated by the CHADS2 or the CHA2DS2-VASc.14,15 Until a few years ago, vitamin K antagonists (VKAs) were used as the preferred OAC. VKAs such as warfarin are able to reduce the risk of ischemic stroke by one-half to two-thirds in AF patients with a moderate-to-high thromboembolic risk.16 However, there are several disadvantages to the VKAs including bleeding, intolerance, interaction with food and drugs, and noncompliance.17–19 Also, the therapy has to be monitored continuously by determining international normalized ratio values at regular intervals and keeping this value within a narrow therapeutic range. The use of aspirin is not recommended any longer by the guidelines since the evidence for effective stroke prevention by aspirin is weak and the risk of major bleeding is substantial, especially in the elderly.20–22
In the past few years, the NOACs have found their way into the guidelines and have since become common practice. From the currently used NOACs, dabigatran, a factor IIa inhibitor, was the first anticoagulant to be compared directly with warfarin in a randomized clinical trial (RCT). For the primary efficacy end point of stroke and systemic embolism, a dosage of 150 mg twice a day was superior to warfarin, with no significant difference in major bleeding. The dosage of 110 mg twice a day was noninferior to warfarin, with 20% fewer major bleeding.23 The first factor Xa inhibitor that was compared to warfarin in an RCT in AF patients with moderate to high risk for stroke was rivaroxaban. In the ROCKET-AF trial, the investigators showed noninferiority in terms of stroke or mortality of rivaroxaban 20 mg once a day (15 mg daily for those with estimated creatinine clearance 30–49 mL/min) compared to warfarin in a double-blind prospective randomized manner.24 The primary end point (stroke or systemic embolism) occurred in 88 patients in the rivaroxaban group (1.7% per year) and in 241 in the warfarin group (2.2% per year). A significant reduction in hemorrhagic stroke and intracranial hemorrhage was also observed in the rivaroxaban arm. Apixaban is another factor Xa inhibitor that was studied compared to warfarin in the ARISTOTLE trial, a randomized, double-blind, double-dummy phase III trial. The regular dosage is 5 mg twice a day, with a dose adjustment to 2.5 mg twice a day in patients ≥80 years, weight ≤60 kg, or with a serum creatinine ≥1.5 mg/dL (133 µmol/L). The primary efficacy outcome of stroke or systemic embolism was reduced by 21% in the apixaban group compared to warfarin group, with a 31% reduction in major bleeding and a 11% reduction in all-cause mortality. A significant reduction in rates of hemorrhagic stroke and intracerebral hemorrhage, but not of ischemic stroke, was also observed in the apixaban group.25 Finally, also for edoxaban (another factor Xa inhibitor), favorable effects on stroke were found; the prospective RCT ENGAGE AF-TIMI 48 showed that hemorrhagic stroke was decreased by 46% for high-dose (60 mg once a day) and 53% for low-dose (30 mg once a day) edoxaban compared with warfarin. For ischemic stroke, high-dose edoxaban tended to be superior when compared with warfarin.26
Despite the fact that these factor IIa and Xa inhibitors have overcome some disadvantages of VKA therapy, they may still lead to bleeding, especially in susceptible patients.27 Other limitations include high medication cost, lack of antidote in case of bleeding, and limited long-term data on cardiovascular end points.23–25,28 Moreover, there are clinically relevant drug–drug interactions with NOACs making their use less than straightforward.
There is a big challenge in daily practice regarding the decision-making process of prescribing (N)OACs to those with both high stroke risk and high bleeding risk. As stated earlier, the guidelines are clear on the indications for the use of (N)OACs in AF patients at stroke risk, which is calculated using the CHADS2 or the CHA2DS2-VASc. The problem arises when such a patient has had one or more severe bleedings in the past or has a condition that is expected to be unsuitable for (N)OAC therapy, for example, in those with hereditary hemorrhagic telangiectasia. Other strategies such as LAA closure provide an elegant solution. In the very recent 2016 ESC guidelines on AF management, a multidisciplinary team approach is proposed to facilitate such a decision-making process.29
LAA exclusion to prevent stroke
As mentioned, the LAA was found be the source of thrombi in >90% of patients with AF.10 Therefore, excluding the LAA from the systemic circulation by excision or occlusion may be an effective alternative to (N)OAC. Indeed, several percutaneous and surgical techniques have been developed to reach this goal. Surgical LAA resection to prevent recurrent arterial emboli was described in 1949 by Madden.30 Prophylactic LAA excision during open heart surgery showed such good results on prevention of AF-related stroke that the authors partly named their article “the left atrial appendage: our most lethal human attachment!” and concluded that routine LAA excision is safe and should be considered whenever the chest is opened.31 Nowadays, concomitant surgical ligation of the LAA as part of open heart surgery for structural heart disease and/or coronary artery bypass grafting is widely accepted. Prospective randomized data, however, are scarce. The Left Atrial Appendage Occlusion Study (LAAOS)32 and LAAOS II33 both included a small number of patients. Despite the fact that both trials demonstrated the safety of the procedure, no benefit was observed on the clinical end points of death, myocardial infarction, stroke, or major bleeding. LAAOS III is ongoing and is expected to give definite answers on whether the procedure is beneficial regarding the abovementioned end points and whether there are safety issues.34
LAA ligation by means of thoracoscopy has also been shown. In 1996, Odell et al35 described the first thoracoscopic obliteration of the LAA in dogs and human cadavers (five with a stapler and five with an Endoloop) and showed the feasibility of this method. After this, several studies have been published showing the feasibility of this minimally invasive method, most of them using a stapler or a loop snare.31,36,37 One technique has been developed to clip the LAA.38 This device is constructed from two stainless steel strips covered with a knit braided polyester fabric (AtriCure, Inc., West Chester, OH, USA) and can be implanted at the base of the LAA.37,39,40 One small study showed thoracoscopic LAA occlusion in conjunction with the minimally invasive MAZE procedure for ablation of AF.41
Because of its effectiveness and relatively low risks, percutaneous LAA closure has become increasingly popular. In 2002, the first results of a percutaneous, catheter-based method to occlude the LAA using the PLAATO device (eV3, Plymouth, MN, USA) were described.42,43 This device is no longer available. Amplatzer cardiac plug (ASO, AGA Medical/St. Jude Medical, St. Paul, MN, USA) is another device used to occlude the LAA. The development was based on the Amplatzer double-disk septal occluders, which were designed for closure of atrial septal defects and patent foramen ovale. This device consists of a distal lobe with stabilizing wires (retaining hooks) and a proximal disc connected by a central waist. The disc seals the outer circumference of the LAA orifice by what has been termed the “pacifier principle”. The device is available in eight diameter sizes with respect to the lobe, ie, 16–30 mm, increasing stepwise by 2 mm. The diameter of the disc is 4 or 6 mm larger than the lobe for the 16–22 mm or 24–30 mm devices, respectively. The appropriate size is chosen to be 10–20% larger than the narrowest measured diameter 1–2 cm distal to the LAA orifice. The purpose of this ‘oversizing’ is to have sufficient device fixation.44 In the initial European experience including 143 patients, LAA occlusion was successfully performed in 96%. Major adverse cardiac events occurred in ten patients (7.0%), including three patients with an ischemic stroke, two patients with device embolization, and five patients with clinically significant pericardial effusion.45 Despite the fact that larger multicenter experiences have been published with more favorable data;46 until now, no RCTs are available on this device.
Another device currently under investigation is the LARIAT Suture Delivery Device (SentreHEART, Inc., Palo Alto, CA, USA). It uses the combination of a transseptal placement of a temporary 15 mm compliant occlusion balloon in the LAA, two magnet-tipped guide wires inserted into the LAA and the pericardial space, and a closure snare device (using a 40 mm pre-tied suture loop) for ligation/exclusion of the LAA. The procedure involves four basic steps: 1) pericardial and transseptal access; 2) placement of the endocardial magnet-tipped guide wire in the apex of the LAA with balloon identification of the LAA ostium; 3) connection of the epicardial and endocardial magnet-tipped guide wires for stabilization of the LAA; and 4) snare capture of the LAA with closure confirmation and release of the pretied suture for LAA ligation. Because of the need for pericardial access, patients with a history of coronary artery bypass surgery or pericarditis who may have adhesions in this space are not suitable candidates for the LARIAT procedure. Unlike endocardial procedures, the LARIAT procedure does not require the use of immediate postprocedural anticoagulation therapy with warfarin. Because of the unavoidable irritation of the pericardium associated with the pericardial access used in the LARIAT procedure, most patients develop pericarditis after the procedure. The feasibility of the device was first shown in 2010 in a canine model.47 A larger series in humans was published in 2013 in which 89 patients with AF were enrolled to undergo percutaneous ligation of the LAA with the LARIAT device.48 LAA ligation was successful in 85 (96%) of the patients; of these, 81 patients had a complete closure immediately. There were no device-related complications, but there were access-related complications in two patients during the pericardial access and one patient during the transseptal puncture. Adverse events included severe pericarditis postoperatively (n=2), late pericardial effusion (n=1), unexplained sudden death (n=2), and late strokes thought to be nonembolic (n=2). At 1 month (81 of 85) and 3 months (77 of 81) postligation, 95% of the patients had complete LAA closure as shown by transesophageal echocardiogram (TEE). Among the patients undergoing 1-year TEE (n=65), 98% patients had complete LAA closure, including patients with previous leaks. An advantage of the LARIAT device is that successful implantation may also lead to the elimination of electrical foci from the LAA. This may be beneficial in patients in whom the LAA is a source of ectopic triggers leading to AF. Indeed, it has been shown that successful occlusion of LAA using the LARIAT device resulted in a decrease of AF burden in patients with proven LAA ectopy.49 However, the US Food and Drug Administration issued a safety communication for the off-label use of the LARIAT device in the USA since procedural safety may be an issue. Indeed, a significant amount of serious adverse events have been described, eg, cardiac tamponade and bleeding needing urgent surgery, and even one death.50 A recent multicenter evaluation showed decreased rates of cardiac perforation after the introduction of a micropuncture needle for pericardial access.51 Moreover, the use of periprocedural colchicine significantly decreased the risk of pericarditis. The ongoing aMAZE trial comparing pulmonary vein isolation in combination with LAA ligation vs pulmonary vein isolation alone will address the safety issues as well as LARIAT’s effectiveness on maintenance of sinus rhythm in the difficult patient category of persistent and longstanding persistent AF.52 The device has also been investigated in AF patients who were ineligible for OAC therapy.53 In this trial in 139 patients, 99% acute successful LAA closure was observed, and at follow-up, 100% of the patients showed successful closure including leaks <5 mm. The adverse events (11.5%) included two cardiac perforations and even one death due to pulmonary embolus. Over a mean follow-up of 2.9 years, the rate of stroke and systemic embolism was 1.0% per year, which is low. No RCTs have been published so far to compare the effectiveness/hazards of the LARIAT device vs (N)OACs.
Review of design, insertion, and mode of action of Watchman LAA closure device
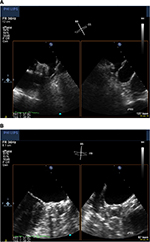
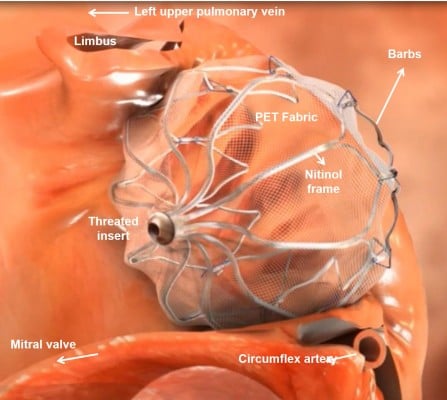
The Watchman (Atritech, a subsidiary of Boston Scientific, Plymouth, MN, USA) is the LAA closure device that has been investigated most thoroughly. It has a self-expanding nitinol frame with fixation barbs with a polyester fabric cover (Figure 1). In contrast to the PLAATO device, blood can initially pass the porous Watchman device, and therefore OAC is needed for at least 45 days until endothelialization occurs. Five sizes are available (21, 24, 27, 30, and 33 mm), and selection depends on the varying anatomy and size of the LAA. The proper size is chosen as 10%–20% above the largest measured diameter of the LAA, which is taken 1–2 cm distally to the orifice. The oversizing is important so as to have sufficient fixation in the LAA for stable positioning. An example of device positioning in the LAA is demonstrated in Figure 2A and B.
  | Figure 1 Image of the positioning of the Watchman device in the left atrial appendage. Note: Different components and the relevant structures are noted. Abbreviation: PET, polyethylene terephthalate. |
Clinical efficacy, safety, and tolerability
The implant feasibility of this device for stroke prevention was shown in the study by Sick et al.54 Device implantation was successful in 66 of the 75 enrolled patients (88%). At 45 days, 93% devices showed successful sealing of LAA according to protocol. Two patients experienced device embolization, both successfully retrieved percutaneously. No embolizations occurred in 53 patients enrolled after modification of fixation barbs. There were two cardiac tamponades, one air embolism, and one delivery wire fracture (first generation) with surgical explantation, but no long-term sequelae for the patient. At 6 months follow-up, four patients developed a flat thrombus layer on the device that resolved with additional anticoagulation. During a mean follow-up of 740±341 days, two patients experienced a TIA, one without visible thrombus on the device, and there were also two non-device-related deaths. No strokes occurred during follow-up period despite the fact that >90% of patients discontinued anticoagulation therapy.
PROTECT AF was the first RCT of its kind comparing the Watchman device with OAC (warfarin) in AF patients with CHADS2 of 1 or higher.55 In this trial, which was originally designed for noninferiority, a total of 707 patients were assigned in a random 2:1 ratio to transcatheter LAA closure (n=463 patients) or to warfarin treatment with a target international normalized ratio of 2.0–3.0 (n=244). In the LAA closure group, warfarin was discontinued after a 45-day TEE confirmation that there was either complete or sufficient LAA closure. Sufficient closure was defined as residual flow along the device with a jet width of 5 mm or less. After warfarin discontinuation, clopidogrel and aspirin were used until 6 months. After this, aspirin alone was given lifelong. In 91% of the patients, successful implantation was achieved. After 18 months of evaluation, the primary efficacy (composite end point of stroke, systemic embolism, and cardiovascular death) event rate was similar in both groups (3.0 vs 4.9 events per 100 patient-years) meeting the probability of noninferiority of the intervention by >99.9%. The primary safety (major bleeding, pericardial effusion, and device embolization) events occurred more often in the device group (7.4 vs 4.4 per 100 patient-years). The most significant complications included pericardial effusion and procedural stroke due to air embolism. Because of these important procedural complications, the Continued Access Protocol Registry56 of the PROTECT AF trial was performed and published the impact of training and experience on the complication rates. Reddy et al56 showed that there was a decrease in the number of the abovementioned complications within 7 days of the procedure, most likely due to advanced procedural knowledge and experience. As an example, the rate of serious pericardial effusion in the first week postimplantation, which was the most frequent complication, decreased significantly from 5.0% in the PROTECT AF to 2.2% in the Continued Access Protocol Registry. After dealing with the complications, it has been shown that successful LAA closure by Watchman device not only meets the criteria for noninferiority,55,57 but also demonstrates superiority during longer follow-up time.58 After a follow-up period of 3.8 years in the PROTECT AF study, there were 39 events in 463 LAA closure patients (8.4%) vs 34 events in 244 warfarin-treated patients (13.9%). LAA closure patients had lower rates of cardiovascular mortality (17/463 patients, 3.7% vs 22/244 patients, 9.0%; P=0.005) and all-cause mortality (57/466 patients, 12.3% vs 44/244 patients, 18.0%; P=0.04). The mortality reduction was driven by lower hemorrhagic stroke-related deaths. Interestingly, the rate of ischemic strokes did not differ between the groups (5.2% LAA closure patients vs 4.1% warfarin patients). However, less hemorrhagic strokes were observed in the LAA closure device group (3/463 patients, 0.6%) compared to the warfarin group (10/244 patients, 4.0%). The most important limitation of the PROTECT AF is that NOACs were not included, therefore one cannot generalize the results to the present clinical reality in which both OACs and NOACs are being used. Moreover, referral bias may have been introduced since the patients were referred for the trial because of eligibility for LAA closure. The lack of real-world data makes the results less robust.
Interesting groups of patients are those at high risk of bleeding, especially those using OAC because of AF-derived high stroke risk or those who have suffered from a major bleeding with or without OAC. An important subgroup of patients are those who did not have a major bleeding yet, but are expected to be unsuitable for OAC. Such a group has been included in the AVERROES (Apixaban vs Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trial, which has been terminated early because of a clear benefit in favor of apixaban.59 Interestingly, the investigators excluded patients with serious bleeding events in the past 6 months or those at a high risk of bleeding such as those with a platelet count of <100,000/m3 or hemoglobin level of <10 g/dL, stroke within the previous 10 days, and documented hemorrhagic tendencies. For obvious reasons, no RCT is likely to be undertaken enrolling such patients to receive (N)OACs. Tracing the trail of breadcrumbs allows us to see that these patients must benefit the most from LAA closure as they would no longer need (N)OAC. This patient category was studied in the ASA Plavix Registry study.60 In this prospective nonrandomized registry, 150 patients were included who had contraindications to chronic warfarin treatment. A history of TIA or ischemic stroke was observed in 40% and 93% of the patients suffering from hemorrhagic events. After implantation, no warfarin transition was given, and 6 months clopidogrel treatment and subsequent aspirin lifelong were prescribed. At a mean follow-up time of 14.4±8.6 months, the combined primary efficacy end point (ischemic stroke, hemorrhagic stroke, systemic embolism, and cardiovascular/unexplained death) occurred in eight patients, of whom four were stroke patients. The study-derived rate of ischemic stroke was 1.7%, which was a tremendous reduction when compared to the expected calculated event rates of those treated with aspirin alone (7.3%).
Recently, the results of the multicenter Registry on WATCHMAN Outcomes in Real-Life Utilization (EWOLUTION) have been published and include real-life data from 1,021 patients of whom 62% were unsuitable for (N)OAC therapy.61 In this registry, a 98.5% rate of successful LAA occlusions was shown, which was the highest success rate of all published Watchman studies to date. Moreover, low procedural and 7-day device-related serious adverse events of 2.8% were demonstrated, which is also the lowest of all published Watchman studies.
Since the LAA has a variable anatomy, residual leaks may be observed around the device. As mentioned earlier, the maximally acceptable residual jet defined in the PROTECT AF was a jet <5 mm. Viles-Gonzalez et al62 showed that this minimal residual flow is a common finding and is not associated with clinically relevant adverse events, most importantly thromboembolic events. The different anatomical morphologies may have an impact on the success rate of LAA closure since some morphologies are more challenging than others. Chicken wing morphology is one of the challenging morphologies because of early intense curving. Despite the difficulties, there are no specific contraindications if the patient is properly prepared before the procedure (ie, thorough evaluation by means of TEE or computed tomography scanning).63
Finally, in these times of a mandatory focus on medical costs, cost-effectiveness is paramount. The cost-effectiveness of the Watchman device has been evaluated recently. It has been demonstrated that transcatheter closure of the LAA saved costs when compared to aspirin after 5 years, warfarin after 7 years, and the NOACs after 5–7 years. Moreover, the expectation is that this cost-effectiveness will remain for the upcoming 20 years.64,65
Patient satisfaction/acceptability
To our knowledge, only one study has been undertaken to assess the quality of life (QoL) after Watchman implantation.66 In this substudy from the PROTECT AF, the QoL was obtained from 547 patients (of whom 361 underwent LAA closure) using the Short Form-12 Health Survey. The investigators demonstrated favorable QoL parameters in the LAA closure group at 1 year follow-up when compared to those on warfarin therapy. The most improvements were seen in the physical parameters.
Watchman implantation in combination with AF ablation
The concept of combining LAA closure and AF ablation in a single procedure is an elegant one for several reasons. Despite its limitations, CA for AF has better outcomes in terms of freedom of AF and/or its symptoms than AADs.67,68 However, as mentioned in the “Introduction” section, the long-term efficacy of AF ablation is still unsatisfactory. Therefore, a strategy in which ablation and LAA occlusion are combined might decrease AF manifestations, lower the LAA-related thromboembolic risk, as well as eliminate the need for OAC. An initial report in 30 patients demonstrated that the combined procedure is feasible and safe.69 The median additional procedural LAA closure time was only 38 minutes without LAA-closure-associated complications. The follow-up data of this registry in 62 patients with a median follow-up time of 38 months also showed that 78% patients who had a mean CHADS2 of 2.5 could discontinue their OAC.70 The total rate of ischemic strokes in this study was 3, corresponding to an “observed” stroke risk of 1.7%, which is lower than the “expected” calculated CHADS2 of 6.5% for those patients. The data on the success of ablation were similar to those observed in the literature; in a heterogeneous cohort of patients consisting of all types of AF, the total rate of freedom from AF was 58.1% after a follow-up time of 38 months. In another study, satisfactory data were shown on freedom of AF and OAC discontinuation, but unfortunately, severe complications in terms of cardiac tamponade occurred in three patients (8.6%).71 In another small study, the feasibility of AF ablation 41–756 days after LAA closure using Watchman or Amplatzer device was shown.72 The major concern here was that in one of the eight studied patients, a device-related thrombus was found despite using NOAC. The difference compared with the other studies is that in those studies, the ablation and LAA closure were done concomitantly. So, the authors concluded that if the LA ablation is done after the LAA closure, regular TEE examinations may be needed.
Conclusion
Several techniques have been tested to exclude the LAA from the LA to reduce the thromboembolic events driven by AF. In experienced hands, the implantation of a LAA closure device is feasible and safe and is associated with good outcomes. The best studied LAA closure device remains the Watchman device, which has been proven to be superior to warfarin in terms of cardiovascular endpoints.
The question in daily practice remains whether we would prescribe (N)OACs to an AF patient at high stroke risk who has had one or more severe bleedings or has a condition that is expected to be unsuitable for (N)OAC therapy, or perform LAA closure without prescribing (N)OAC. In the updated 2012 and 2016 ESC guidelines percutaneous LAA closure has 2B, level of evidence B indication for those patients at high stroke risk with contraindications for longterm oral anticoagulation.29,73 LAA closure as an equal alternative for (N)OACs is not recommended. As rationale for the recommendation, two main reasons are given. First, the lack of adequately powered randomized studies in patients with high stroke risk and long-term follow-up in which NOACs are also studied. Second is the need for lifelong aspirin treatment after LAA closure, which is also associated with bleedings. In our opinion, the patients who will benefit the most from the Watchman implantation are those with AF using (N)OAC who are at high risk of bleeding or those who have suffered from major bleedings while on (N)OACs and others with a contraindication for (N)OACs.74 Since studies on cost-effectiveness of the Watchman device show beneficial economic aspects for the future when compared to warfarin, NOAC, and aspirin. LAA closure might also be considered for other patient categories (other than those at high bleeding risk). These considerations should be seriously examined in an era in which many centers have gained lot of experience making Watchman implantation a relatively safe and simple procedure. Strict reimbursement policies have the potential to negatively impact the use of the Watchman device.
In patients with symptomatic AF, concomitant AF ablation should be considered. This combined procedure of ablation with LAA closure has been shown to be feasible and safe with beneficial long-term outcomes in terms of freedom of AF, lower than expected risk of stroke, and discontinuation of OAC. However, data on this method are still scarce and no RCTs have been published so far.
In conclusion, LAA occlusion is a good alternative for OAC in high stroke risk patients with AF, especially in patients with high bleeding risk. Combining this procedure with AF ablation should be considered in patients who are symptomatic.
Acknowledgment
The Cardiology Department receives proctoring fees for training/educational services from the Boston Scientific Corporation.
Disclosure
The authors report no conflicts of interest in this work.
References
Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. | ||
Nieuwlaat R, Capucci A, Camm AJ, et al; European Heart Survey Investigators. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–2434. | ||
Pisters R, Nieuwlaat R, Prins MH, et al; Euro Heart Survey Investigators. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14(5):666–674. | ||
Van Gelder IC, Hagens VE, Bosker HA, et al; Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. | ||
Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. | ||
Haegeli LM, Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J. 2014;35(3):2454–2459. | ||
Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57(2):160–166. | ||
Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125(1):23–30. | ||
De Backer O, Arnous S, Ihlemann N, et al. Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update. Open Heart. 2014;1(1):e000020. | ||
Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755–759. | ||
Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: virchow’s triad revisited. Lancet. 2009;373(9658):155–166. | ||
Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? results from a multicenter study. J Am Coll Cardiol. 2012;60(6):531–538. | ||
Lupercio F, Carlos Ruiz J, Briceno DF, et al. Left atrial appendage morphology assessment for risk stratification of embolic stroke in patients with atrial fibrillation: a meta-analysis. Heart Rhythm. 2016;13(7):1402–1409. | ||
Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG); Document Reviewers. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–1413. | ||
January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. | ||
Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res. 2006;118(3):321–333. | ||
Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the Randomized Evaluation of Long-Term Anticoagulant Therapy (RE-LY) trial. Circulation. 2011;123(21):2363–2372. | ||
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689–2696. | ||
Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352(9135):1167–1171. | ||
Lip GY. The role of aspirin for stroke prevention in atrial fibrillation. Nat Rev Cardiol. 2011;8(10):602–606. | ||
Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin vs aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. | ||
Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin vs aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing. 2007;36(2):151–156. | ||
Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran vs warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. | ||
Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban vs warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. | ||
Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban vs warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. | ||
Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. | ||
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. | ||
Wallentin L, Yusuf S, Ezekowitz MD, et al; RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376(9745):975–983. | ||
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. Epub August 27, 2016. | ||
Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949;140(9):769–772. | ||
Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg. 2000;17(6):718–722. | ||
Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150(2):288–293. | ||
Whitlock RP, Vincent J, Blackall MH, et al. Left Atrial Appendage Occlusion Study II (LAAOS II) (Left Atrial Appendage Occlusion Study II (LAAOS II). Can J Cardiol. 2013;29(11):1443–1447. | ||
Whitlock R, Healey J, Vincent J, et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg. 2014;3(1):45–54. | ||
Odell JA, Blackshear JL, Davies E, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg. 1996;61(2):565–569. | ||
Blackshear JL, Johnson WD, Odell JA, et al. Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J Am Coll Cardiol. 2003;42(7):1249–1252. | ||
Fumoto H, Gillinov AM, Ootaki Y, et al. A novel device for left atrial appendage exclusion: the third-generation atrial exclusion device. J Thorac Cardiovasc Surg. 2008;136(4):1019–1027. | ||
Salzberg SP, Gillinov AM, Anyanwu A, Castillo J, Filsoufi F, Adams DH. Surgical left atrial appendage occlusion: evaluation of a novel device with magnetic resonance imaging. Eur J Cardiothorac Surg. 2008;34(4):766–770. | ||
Salzberg SP, Plass A, Emmert MY, et al. Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg. 2010;139(5):1269–1274. | ||
Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142(5):1002–1009. | ||
Balkhy HH, Chapman PD, Arnsdorf SE. Minimally invasive atrial fibrillation ablation combined with a new technique for thoracoscopic stapling of the left atrial appendage: case report. Heart Surg Forum. 2004;7(6):353–355. | ||
Nakai T, Lesh MD, Gerstenfeld EP, Virmani R, Jones R, Lee RJ. Percutaneous left atrial appendage occlusion (PLAATO) for preventing cardioembolism: first experience in canine model. Circulation. 2002;105(8):2217–2222. | ||
Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation. 2002;105(16):1887–1889. | ||
Cruz-Gonzalez I, Cubeddu RJ, Sanchez-Ledesma M, et al. Left atrial appendage exclusion using an Amplatzer device. Int J Cardiol. 2009;134(1):e1–e3. | ||
Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011;77(5):700–706. | ||
Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11(10):1170–1179. | ||
Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: initial experience in a canine model. Circ Cardiovasc Interv. 2010;3(3):224–229. | ||
Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62(2):108–118. | ||
Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm. 2015;12(1):52–59. | ||
Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med. 2015;175(7):1104–1109. | ||
Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13(5):1030–1036. | ||
Lee RJ, Lakkireddy D, Mittal S, et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): rationale and design. Am Heart J. 2015;170(6):1184–1194. | ||
Sievert H, Rasekh A, Bartus K, et al. Left atrial appendage ligation in nonvalvular atrial fibrillation patients at high risk for embolic events with ineligibility for oral anticoagulation. Initial report of clinical outcomes. JACCCEP. 2015;1(6):465–474. | ||
Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49(13):1490–1495. | ||
Holmes DR, Reddy VY, Turi ZG, et al; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–542. | ||
Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123(4):417–424. | ||
Reddy VY, Doshi SK, Sievert H, et al; PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127(6):720–729. | ||
Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988–1998. | ||
Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011.364(9):806–817. | ||
Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61(25):2551–2556. | ||
Boersma LV, Schmidt B, Betts TR; EWOLUTION investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37(31):2465–2474. | ||
Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the watchman device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59(10):923–929. | ||
Wunderlich NC, Beigel R, Swaans MJ, Ho SY, Siegel RJ. Percutaneous interventions for left atrial appendage exclusion: options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc Imaging. 2015;8(4):472–488. | ||
Reddy VY, Akehurst RL, Armstrong SO, et al. Cost effectiveness of left atrial appendage closure with the watchman device for atrial fibrillation patients with absolute contraindications to warfarin. Europace. 2016;18(7):979–986. | ||
Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR Jr. Time to cost-effectiveness following stroke reduction strategies in AF: Warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66(24):2728–2739. | ||
Alli O, Doshi S, Kar S, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013;61(17):1790–1798. | ||
Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340–2347. | ||
Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study). Eur Heart J. 2006;27(2):216–221. | ||
Swaans MJ, Post MC, Rensing BJ, Boersma LV. Ablation for atrial fibrillation in combination with left atrial appendage closure: first results of a feasibility study. J Am Heart Assoc. 2012;1(5):e002212. | ||
Alipour A, Swaans MJ, van Dijk VF, et al. Ablation for atrial fibrillation combined with left atrial appendage closure. JACC EP. 2015;1(6):486–495. | ||
Calvo N, Salterain N, Arguedas H, et al. Combined catheter ablation and left atrial appendage closure as a hybrid procedure for the treatment of atrial fibrillation. Europace. 2015;17(10):1533–1540. | ||
Heeger CH, Rillig A, Lin T, et al. Feasibility and clinical efficacy of left atrial ablation for the treatment of atrial tachyarrhythmias in patients with left atrial appendage closure devices. Heart Rhythm. 2015;12(7):1524–1531. | ||
Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–2747. | ||
Vorselaars VM, Velthuis S, Swaans MJ, et al. Percutaneous left atrial appendage closure-An alternative strategy for anticoagulation in atrial fibrillation and hereditary hemorrhagic telangiectasia? Cardiovasc Diagn Ther. 2015;5(1):49–53. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.