Back to Journals » Clinical Ophthalmology » Volume 11
Novel positioning sensor with real-time feedback for improved postoperative positioning: pilot study in control subjects
Authors Brodie FL, Ramirez DA , Pandian S , Woo K, Balakrishna A, De Juan E, Choo H, Grubbs RH
Received 19 February 2017
Accepted for publication 13 April 2017
Published 19 May 2017 Volume 2017:11 Pages 939—944
DOI https://doi.org/10.2147/OPTH.S135128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Frank L Brodie,1 David A Ramirez,2,* Sundar Pandian,3,* Kelly Woo,3 Ashwin Balakrishna,3 Eugene De Juan,1 Hyuck Choo,3 Robert H Grubbs3
1Department of Ophthalmology, University of California San Francisco, San Francisco, CA, USA; 2School of Medicine, University of California San Francisco, San Francisco, CA, USA; 3Department of Medical Engineering, California Institute of Technology, Pasadena, CA, USA
*These authors contributed equally to this work
Introduction: Repair of retinal detachment frequently requires use of intraocular gas. Patients are instructed to position themselves postoperatively to appose the intraocular bubble to the retinal break(s). We developed a novel wearable wireless positioning sensor, which provides real-time audiovisual feedback on the accuracy of positioning.
Methods: Eight healthy volunteers wore the wireless sensor for 3 hours while instructed to maintain their head tilted toward the 2 o’clock meridian with no audiovisual feedback. Positioning accuracy was recorded. The subjects repeated the experiment for 3 hours with the audiovisual feedback enabled.
Results: With no audiovisual feedback, the percentage of time greater than 10° out of position varied from 8.9% to 93.9%. With audiovisual feedback enabled, these percentages ranged from 9.4% to 65%. Three subjects showed significant improvement in their time out of position (P<0.01, Fisher’s exact test). Four subjects demonstrated a nonsignificant improvement, and one subject had a significant increase in time out of position with feedback (P<0.01). When pooled, all subjects demonstrated a statistically significant decrease in degrees out of position (P<0.001, Wilcoxon test) and a statistically significant improvement in total time out of position (P<0.001).
Conclusion: The novel positioning sensor showed improved positioning compliance in half of the healthy volunteers during our short pilot study. Other subjects derived little benefit from the feedback. The causes for this observation are unclear. However, given the significant improvement as a group, this new technology could be beneficial to patients who struggle with postoperative positioning.
Keywords: retinal detachment, pneumatic retinopexy, intraocular gas, device, postoperative positioning, vitrectomy, macular hole
Introduction
Vitreoretinal surgery for retinal detachment and macular hole frequently uses gas as an intraocular tamponade. Specific postoperative positioning is required to position the gas bubble in apposition to the retinal break in order to achieve anatomic closure. There has been some debate in the literature as to the need to position, especially after macular hole surgery,1 but a recent Preferences and Trends survey from the American Society of Retinal Surgeons found that over 90% of retinal surgeons instruct their patients to position themselves postoperatively.2 In addition to increasing the likelihood of a successful surgical outcome, correct positioning can reduce the incidence of postoperative complications, such as glaucoma and cataract.3
Despite its importance, correct positioning is challenging for patients to maintain. Several studies utilizing sensors worn on patients’ heads have shown that patients maintained correct face-down positioning on average between 18%–48% of the time over a 24-hour period.4,5 Similarly, a study of admitted patients who were instructed to position themselves, found that only one-third of patients positioned themselves correctly, as observed by nurses throughout the day.6 Attempts to improve positioning compliance include the “tennis ball technique” in which patients who are instructed to remain prone (face-down) have a tennis ball attached to their back to make supine positioning uncomfortable. While this method has been shown to be very effective for prone positioning, it is not applicable to other types of positioning.7
In an effort to improve compliance with postoperative positioning, we developed a novel, small device that can be embedded in a headband and which provides real-time feedback on positioning accuracy. The device contains a 6-axis position sensor as well as a Bluetooth transmitter which connects to a custom iPod application (app). After the surgeon has entered the correct position into the app, the patient not only receives real-time visual feedback on positioning, but the device also has an audible alarm if the patient is out of position for too long. Our device differs from previous head sensors4,5,7 used for positioning, in that it provides visual and audio feedback to the patient to improve compliance.
In this study we present data from eight healthy volunteers who were instructed to maintain positioning similar to that required in pneumatic retinopexy, with a 10 o’clock retinal break (head tilted toward 2 o’clock). Additionally, participants had a cash incentive for adherence – to simulate the desire of a postoperative patient to position correctly.
Methods
Device
The device is compromised of the MetaWear C sensor board (MbientLab, San Francisco, CA, USA) (Figure 1). The board uses a 6-axis sensor (3-axis accelerometer and 3-axis gyroscope) connected to a Bluetooth driver for connection with smartphones. Using the MetaWear iOS app program interface, an iPod or iPhone (Apple, Cupertino, CA, USA) app can access the raw sensor data over a Bluetooth connection. The gyroscope senses angular velocity in terms of roll, pitch, and yaw. The current head angles can then be calculated using Riemann sums. The accelerometer values are compared to the constant gravitational force of the Earth. This provides a stable calculation of the head position angles.
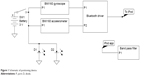  | Figure 1 Schematic of positioning device. |
User interface: patient
The app uses Objective-C (Apple) for data processing and Bluetooth connection. The final angle values are written to a text file which is continuously updated. The app also uses a Swift module (Apple) that accesses this text file and outputs a 3D visual display. The display uses a simple cube to represent the patient’s head (Figure 2A). A solid cube represents the current head position, while a superimposed translucent cube represents the proper head position (Figure 2B). In addition to visual feedback, text commands guide the patient to the correct head position when incorrect; for example, displaying “turn right” or “tilt down” (Figure 2B). To alert the patient of poor head positioning, the app has an audio feature that rings after 2 minutes of continuous incorrect positioning. This allows patients some freedom to adjust when necessary. Once the alarm is triggered, it continues at 30-second intervals until the patient corrects his/her head position. This alarm is enabled even when the smartphone screen is locked, for use during sleep and other daily activities. The patient also has the option to snooze the alarm for 5 minutes for use during eating, etc. All other features of position tracking continue, while the patient has the liberty of moving without noise for a short amount of time.
Headgear sensor device
The current device design uses soft headgear, composed of a headband with a vertical strip crossing the top of the head (Figure 3). This strip contains the bulk of the circuitry which is sewn inside to maximize comfort. The headgear can be worn comfortably throughout the duration of positioning, both day and night, to maximize adherence to positioning. The sensor board is modified and stripped of all battery components to minimize thickness. A 25 mm radius copper plate is used to protect the board from physical damage. The sensor is then connected in parallel with two light-emitting diodes as power indicators. There is also a small power switch that then connects to a replaceable battery pack consisting of four CR2032 batteries in parallel, to maximize charge.
  | Figure 3 (A) Wearing the sensor embedded in the headband. (B) The sensor without casing next to a US quarter for size reference. |
User interface: surgeon
The app saves the patient head angle data when out of position. The data are stored in spherical angle coordinates of pi and phi. This represents rotation off the z-axis along both the xz and yz planes. These two values can cover all possible head positions in vector form. The surgeon can input the sensitivity in degrees within which the alarm does not ring and data are not recorded.
Experiment
Study approval was obtained by the University of California San Francisco Institutional Review Board. Healthy controls were identified and recruited by study staff using word of mouth. Informed consent was obtained for all participants. Participants were excluded if medically unable to position for the study, or if unable to operate the handheld digital device.
Subjects were outfitted with the head positioning sensor and asked to maintain head position at a tilt of 30° away from vertical midline in the coronal plane, to simulate postoperative positioning after pneumatic retinopexy. This position was then set as the baseline sensor location within the digital device app, and any deviation >10° in any axis was recorded. Subjects were compensated for their time and received a significant additional payment if they were out of position <10% of the time.
Volunteers were instructed to maintain this head position for two consecutive 3-hour blocks: block 1 was performed with no alarm feedback from the connected digital device, and block 2 was performed with real-time visual feedback on the app screen and audio alarm feedback after 2 minutes out of correct head position, as previously described. No restrictions were placed on their activity during the experiment beyond instructions on correct head positioning.
Subjects were extensively educated beforehand on digital device use, including appropriate sensor placement on top of the head, navigating the digital device and associated app, and alarm silencing.
We used the Fisher’s exact test to calculate improvement in minutes out of position depending on feedback status for each participant, as well as for pooled participant values. We used the Wilcoxon test to assess for a change in mean degree out of position and mean time spent in or out of position for each alarm status.
Results
We recruited eight healthy volunteers with a mean age of 26. Five subjects showed improvement in their time correctly positioned with the alarm function enabled, ranging from 4–139 more minutes spent in the correct position during the 3-hour period. This corresponded to 4%–82% improvement in positioning as compared to their positioning compliance without the alarm activated. Three of these demonstrated statistical significance. One subject showed no change in position compliance, and two subjects had worse positioning (Table 1).
The change in total time spent in position for pooled participants without feedback was not statistically significant. When taken in aggregate, there was a significant improvement in time spent in the correct position when participants were given feedback on head position (Figure 4).
We measured the degree of deviation from ideal position in both groups. There was a statistically significant decrease in the magnitude of degrees out of position for those receiving feedback, compared to those without (Figure 5).
  | Figure 5 The magnitude of degrees out of position for each subject. P<0.001 for magnitude of degree out of position between each group. |
Discussion
This pilot study demonstrated the use of a positioning sensor with companion smartphone app to improve postoperative positioning. We instructed healthy volunteers to maintain strict positioning that mimicked the positioning required after pneumatic retinopexy. For the first 3 hours subjects received no feedback on the accuracy of their positioning, during the next 3 hours they had both visual and audio feedback on the accuracy of their positioning. Their head position was recorded throughout the experiment and the positioning accuracy with and without the feedback functions were compared.
Our data showed a split in the response to our positioning sensor. Half the participants derived significant benefit from the feedback and on average their positioning improved by over 40%, ranging from 23%–82%. However, three participants showed little-to-no change in their positioning accuracy (range: −6% to +4%).
For those volunteers who did not benefit, one had excellent positioning (>90% accuracy) at baseline so there was little room for improvement.
In speaking with the subjects who did not show a difference in their time out of position, they related that they became reliant on the alarm to adjust their position after audiovisual feedback, and thus stopped trying to actively maintain position. Given a 2-minute delay was used, it is not surprising that in such a short study period (180-minute blocks) this had a meaningful impact. Undue reliance on the technology will be a concern moving forward as it could ultimately lead to worse compliance than if patients were self reliant.
A limitation of this study is its small sample size. Additionally, the participants in this study, all aged <30 years, are not representative of the typical postsurgical patient, and the duration of positioning measured (6 hours) is far shorter than the duration typically instructed by retinal surgeons (3–7 days). This suggests that our control subjects did not experience the range of positions typical of postsurgical patients, including the stress of maintaining correct position overnight. Finally, the monetary incentives used in this study may be less compelling than and therefore incomparable to the threat of loss of vision, for which actual retinopexy patients will be positioning.
This study shows the feasibility and potential benefit of a novel, wireless digital positioning feedback device. Key to the success of this and other technologies is identifying which patients will derive the most benefit. Future work will test this device in postoperative patients who have undergone retinal surgery requiring intraocular gas that necessitates postoperative positioning.
Acknowledgments
Support from That Man May See, Research to Prevent Blindness and Chartrand Foundation. The authors acknowledge the University of California, San Francisco Open Access Publishing Fund.
Intellectual property has been filed by California Institute of Technology on behalf of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
Solebo AL, Lange CA, Bunce C, Bainbridge JW. Face-down positioning or posturing after macular hole surgery. Cochrane Database Syst Rev. 2011;(12):CD008228. | ||
Stone TW. Preferences and Trends Membership Survey. 2015. | ||
Mohamed S, Lai TY. Intraocular gas in vitreoretinal surgery. Hong Kong J Ophthalmol. 2010;14(1):8–13. | ||
Verma D, Jalabi MW, Watts WG, Naylor G. Evaluation of posturing in macular hole surgery. Eye (Lond). 2002;16(6):701–704. | ||
Leitritz MA, Ziemssen F, Voykov B, Bartz-Schmidt KU. Usability of a gravity- and tilt-compensated sensor with data logging function to measure posturing compliance in patients after macular hole surgery: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2014;252(5):739–744. | ||
Seno Y, Shimada Y, Mizuguchi T, Tanikawa A, Horiguchi M. Compliance with the face-down positioning after vitrectomy and gas tamponade for rhegmatogenous retinal detachments. Retina. 2015;35(7):1436–1440. | ||
Forsaa VA, Krohn J. Postoperative positioning in macular hole surgery: an objective evaluation of nonsupine positioning and the effect of the “tennis ball technique”. Retina. 2016;36(6):1081–1086. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



