Back to Journals » Nature and Science of Sleep » Volume 11
Novel non-pharmacological insomnia treatment – a pilot study
Authors Pavlova MK , Latreille V, Puri N, Johnsen J, Batool-Anwar S, Javaheri S, Mathew PG
Received 29 December 2018
Accepted for publication 10 June 2019
Published 11 September 2019 Volume 2019:11 Pages 189—195
DOI https://doi.org/10.2147/NSS.S198944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sutapa Mukherjee
Milena K Pavlova,1 Véronique Latreille,1 Nirajan Puri,1 Jami Johnsen,1 Salma Batool-Anwar,2 Sogol Javaheri,2 Paul G Mathew1
1Department of Neurology, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA; 2Department of Medicine, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA
Correspondence: Milena K Pavlova
Department of Neurology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA
Tel +1 617 983 7580
Fax +1 617 983 7582
Email [email protected]
Objective: The objective of this prospective pilot study was to examine the effects of a novel non-pharmacological device (BioBoosti) on insomnia symptoms in adults.
Methods: Subjects with chronic insomnia were instructed to hold the device in each hand for 8 mins for 6 cycles on a nightly basis for 2 weeks. Outcomes tested included standardized subjective sleep measures assessing sleep quality, insomnia symptoms, and daytime sleepiness. Sleep was objectively quantified using electroencephalogram (EEG) before and after 2 weeks of treatment with BioBoosti, and wrist actigraphy throughout the study.
Results: Twenty adults (mean age: 45.6±17.1 y/o; range 18–74 y/o) were enrolled in the study. No significant side effects were noted by any of the subjects. After 2 weeks of BioBoosti use, subjects reported improved sleep quality (Pittsburgh Sleep Quality Index: 12.6±3.3 versus 8.5±3.7, p=0.001) and reduced insomnia symptoms (Insomnia Severity Index: 18.2±5.2 versus 12.8±7.0, p<0.001). Sleepiness, as assessed by a visual analog scale, was significantly reduced after treatment (5.7±2.8 versus 4.0±3.3, p=0.03).
Conclusion: BioBoosti use yielded an improvement in insomnia symptoms. Larger placebo-controlled studies are needed to fully assess efficacy.
Keywords: insomnia, non-pharmacological treatment, sleep quality, actigraphy, electroencephalography
Introduction
Approximately one-third of the general population has experienced at least transient insomnia, while 10% of the population has chronic insomnia.1 Insomnia is frequently associated with loss of productivity, poor health outcomes, and high health care utilization.2 Although a wide range of pharmacological treatments are available for the treatment of insomnia, many patients develop tolerance or find them either ineffective or intolerable due to side-effects. Moreover, treatment of older individuals or patients with multiple comorbidities can be even more challenging, due to drug–drug interactions and adverse effects.
The use of a safe, non-pharmacologic treatment for insomnia has gained much attention in recent years. In addition to improving insomnia, such treatments may also better align with patient safety and preferences to avoid pharmacological therapies.
Cognitive behavioral therapy for insomnia (CBTi) is commonly recommended as first-line treatment, as it is effective and avoids the hazards of pharmacological treatment. Several recent meta-analyses have evaluated efficacy and confirm its efficacy3,4 both for short term and for long term. While it is the first-line treatment in our practice, a sizable number of patients refuse, citing either lack of insurance coverage, or variable logistics reasons related to the travel to appointments, technical challenges with online systems, long wait for appointments with a therapist, lack of time, etc. Others report insufficient/partial response. Due to the large prevalence of insomnia, many patients still need other nonpharmacological treatment methods.
Various other methods for treatment of insomnia have been researched. For example, eye movement desensitization reprocessing has been tried for treatment of insomnia associated with posttraumatic stress disorder.5 Another recently used method used temperature regulation.6 Yet another method uses direct current stimulation.7
BioBoosti is a device that emits pulsed electromagnetic fields.8 According to the information from the manufacturing company, Bioboosti’s main intended use is to systemically increase microcirculation across the entire body. The device pulsed electromagnet field (PEMF) to which stimulates endothelial cells and red blood cells by changing the cell surface charges and thus improves perfusion.9 Having been found to be safe, the Bioboosti went to market in China as a State Food and Drug Administration (SFDA)-approved Class 2 device based on the clinical studies carried out on hypertension from 2011 to 2014. After 2014, 28,000 units have been sold for treatment of hypertension without any significant risks associated with the use of the device. Improvement of sleep was volunteered as a subjectively reported observation among participants in this intramural research, as well as customers who have purchased the device. These reposts were not collected systematically, however, and we are the first to actually examine this issue systematically for either subjective effect and any objective sleep data. Thus, this study was guided mainly by empiric report and designed to screen for effect. Given the complexity of insomnia, it would not have been realistic to perform any hemodynamic measurements that explain the mechanism of action step by step.
Despite the lack of specific data of the mechanism by which BioBoosti affects sleep, this was considered a priority for investigation, due to the high prevalence and comorbidity of insomnia, which sometimes makes treatment challenging either due to contraindication of a specific medication class or even elements of CBTi. Examples of medications contraindications include use of benzodiazepines in a patient with cognitive impairment,10 or some due to concerns for interactions, such as potential for some antidepressants to provoke manic symptoms in a patient with depression,11 or interaction with antidepressants.12,13 Furthermore, there are many examples of serendipity leading to therapeutic advances. For instance, the therapeutic effects of valproic acid were discovered after an observation that different antiepileptic medications that were diluted in this substance were all effective. It was subsequently confirmed that the therapeutic agent is not any of the investigated drugs, but the diluting agent (valproic acid).14 With other therapeutic interventions, the proposed mechanism of action is broad or nonspecific – such as with vagal nerve stimulation.15 Many such treatments have become widely accepted as medical standards over the years, partly because the need for one more treatment superseded the need for a mechanistic explanation. Therefore, although we did not have any mechanism-based hypothesis, we designed the current study as a prospective pilot trial, with the aim of obtaining preliminary efficacy results, and determine treatment feasibility and any effect size to guide a more thorough investigation if indicated. With this aim, we tested the efficacy of BioBoosti on insomnia symptoms in adults with chronic insomnia.
Methods
This study was approved by the Brigham and Women’s Hospital Institutional Review Board (Human Subjects Committee). Participants provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. After informed consent, the subjects with a diagnosis of chronic insomnia based on the International Classification of Sleep Disorders-3 criteria1 were recruited for the study. The diagnosis was confirmed by a board-certified sleep medicine physician, who also confirmed that prospective participants met inclusion and exclusion criteria. The participants with shift work disorder, recent transmeridian travel, untreated medical or psychiatric disorders, untreated sleep apnea, and pregnancy were excluded, as the safety of BioBoosti in these conditions is unknown. Since this is a pilot study and we did not have any preliminary data of any subtype of insomnia more likely to benefit from this treatment, we did not limit the participation to any insomnia subtype and did not exclude comorbid sleep disorders, provided they were already treated and controlled at the time of enrollment. We also did not have adequate preliminary data to determine the optimal sample size. Thus, our efforts were focused on determining any effect size in a non-blinded manner to adequately conduct future placebo-controlled studies.
As seen in the study schema (shown in Figure 2), treatment consisted of placing the BioBoosti device at the center of the palm for 8 mins using the devices’ built-in timer. Participants were instructed to hold the device for six 8-min cycles prior to initiation of habitual sleep. Participants were instructed to make no change in any medication intake or schedule during the study. Outcomes tested were subjective measures including a sleep log (nightly) and the following standardized instruments: The Insomnia Severity Index (ISI) which is composed of seven items assessing the perceived severity of difficulties initiating sleep, staying asleep, and early morning awakenings, satisfaction with current sleep pattern, interference with daily functioning, noticeability of impairment attributed to the sleep problem, and degree of distress or concern caused by the sleep problem.16,17 The Pittsburgh Sleep Quality Inventory (PSQI) is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval. Nineteen individual items generate seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The sum of scores for these seven components yields one global score.17,18 Karolinska Sleepiness Scale (KSS) measures the subjective level of sleepiness at a particular time during the day. On this scale, subjects indicate which level best reflects the psycho-physical state experienced in the last 10 mins. The KSS is a measure of situational sleepiness.19,20 The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire with 8 questions. Respondents are asked to rate, on a 4-point scale (0–3), their usual chances of dozing off or falling asleep while engaged in 8 different activities. The ESS score (the sum of 8 item scores, 0–3) can range from 0 to 24. The higher the ESS score, the higher that person’s average sleep propensity in daily life (ASP) or their “daytime sleepiness”.21 A visual analog scale for sleepiness is used as well which is essentially a diary card that measures changes in patients’ sleep quality in clinical trials and practice. They are useful due to their ability to measure the correlation between objective measurements and subjective complaints in a patients’ sleep quality.22
Objective assessments
Sleep was quantified using ambulatory EEG recordings (24‒72 hrs, with standard 10–20 EEG electrodes, and added chin electromyography and electrooculography channels), performed before and after BioBoosti treatment. Sleep stages were scored visually according to current criteria.23 For each subject, sleep architecture variables, including total sleep time, wake after sleep onset, and sleep stage duration, were averaged over the 24‒72 hrs for the baseline and follow-up periods separately.
We also examined whether there is a “first night effect” (the first night of sleep is generally lighter and more fragmented compared to the following nights) by comparing sleep architecture measures on the first and third nights of recording. A subset of subjects (n=14) also wore a wrist actigraphy device throughout the entire study period. Actigraphy-based sleep measures included total sleep time, sleep latency and efficiency, wake after sleep onset, and number of awakenings.
As a screening measure, we measured 24-hr cortisol and catecholamine excretion.
Statistical analysis
Objective and subjective sleep measures at baseline and after 2 weeks of treatment with BioBoosti were compared using paired sample t-test or Wilcoxon paired test. Spearman correlation was also performed to examine the relationships between the improvement in insomnia symptoms and objective sleep measures (actigraphy and EEG sleep architecture). To assess the possibility of a “first night effect”, we compared sleep architecture variables between the first and third night of the 72 hrs recording using paired sample t-tests or their non-parametric equivalent (Wilcoxon paired test). Statistical significance was set at p<0.05.
Results
The recruitment process is presented in Figure 3.
Of 202 subjects who expressed interest, 20 subjects were enrolled in the study and 17 completed all component including both baseline and post-treatment ambulatory sleep EEG, and all questionnaires and wrist actigraphy. Twenty individuals with chronic insomnia (mean age: 45.6±17.1 y/o; range 18–74 y/o; 16 women) completed the questionnaires and sleep log portion of the study, as well as baseline EEG. Three participants did not complete the post-treatment EEG because of discomfort from the electrodes, and thus 17 subjects were included in the EEG sleep architecture analysis. Eleven subjects (55%) had previously failed more than one hypnotic medications. Comorbid sleep disorders included obstructive sleep apnea (on CPAP) in six, Restless legs syndrome in two (on treatment). None reported any adverse effects related to device use.
After 2 weeks of treatment with BioBoosti, subjects reported significant improvements in several insomnia measures (Figure 1), including a statistically significant decrease in insomnia severity scores (ISI) by 5.4 points (p<0.001) and improvement of sleep quality scores (PSQI) by 4.1 points (p=0.001) after treatment. Subjective sleepiness, as measured by a visual analog scale, significantly improved by a mean of 1.7 points (p=0.03) following BioBoosti use, while changes on the ESS did not reach statistical significance. Mean total sleep time, as measured by the sleep logs, increased on average by 30 mins (p=0.03) in the second week of treatment.
 |
Figure 1 Bioboosti device. |
As delineated in Figure 1, actigraphy-based sleep measures demonstrated a trend for improvement in time spent awake after sleep onset (WASO), with a mean reduction of 12 mins (p=0.07) following treatment. EEG sleep measures also indicated a trend for increased percentage of slow wave sleep (p=0.06) after treatment.
We found a significant positive correlation between changes in ISI scores and number of awakenings post-treatment as measured by actigraphy, indicating that improved subjective insomnia complaints are associated with a more consolidated sleep following BioBoosti use (r =0.49, p=0.04; Table 1).
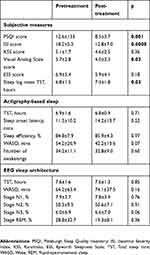 |
Table 1 Insomnia symptoms and sleep outcomes before and after treatment with BioBoosti |
In the subset of subjects with 72 hr of EEG recordings (n=9), there was no significant difference in any sleep architecture measures from night 1 to 3, indicating no first night effect.
Cortisol and catecholamine excretion did not change after treatment.
Discussion
Although this was a pilot study, we found a considerable improvement of insomnia symptoms and of sleep quality following 2 weeks of treatment with BioBoosti in 20 adults with chronic insomnia. Sleep time, as measured by sleep logs increased by 30 mins on average in the second week of treatment, a clinically significant increase that compares to hypnotics, such as Ambien24 and cognitive behavioral therapy for insomnia.25 Further, there were both clinically and statistically significant improvements in insomnia symptoms as measured by the ISI and by the PSQI that were comparable to improvements seen by web-based cognitive behavioral therapy for insomnia.26 Additionally, there was a statistically significant improvement in insomnia symptoms, as measured by the ISI, that was comparable to improvements seen with web-based cognitive behavioral therapy for insomnia27 as well as by sleep coaches.25 Finally, BioBoosti treatment led to improved daytime sleepiness, as assessed by a visual analog scale. Sleepiness scores also appeared improved on the ESS and numerical KSS but did not reach statistical significance. This is likely due to the nature of the visual analog scale, which may be more sensitive to day-to-day change, while the KSS and ESS consist of numeric estimations of the level of sleepiness or likelihood to sleep in different situations.
Objective sleep measures also demonstrated trends towards an increase in slow wave sleep and a reduction of nocturnal awakenings after BioBoosti use. There was a great variation in the proportion of slow wave sleep, both at baseline and follow-up, which may be influenced by the relatively large age range of the subjects. As aging is associated with decreased deep sleep, this may have contributed to the loss of statistical power to reach significance.
The major limitations of this pilot study included the small sample size and lack of a placebo control device. Moreover, most of the significant findings were found for subjective measures. While sleep logs, ISI, PSQI, and KSS are all widely used and validated, they are subjective instruments. Thus, our findings indicate effect on the subject’s symptoms, do not confirm objective change in sleep duration. However, there was a significant correlation between improved subjective insomnia symptoms and number of awakenings on objective actigraphy testing following BioBoosti use, suggesting that the reduction of insomnia symptoms at least partially reflects improved sleep. Furthermore, a trend toward improvement of slow wave sleep proportion was seen, and it is likely that this pilot study was underpowered to assess these changes.
Other limitations should be addressed as well. We also do not have any explanations of the mechanisms driving any sleep improvement by electromagnetic stimulation. Our participant group was heterogeneous, as we did not have any preliminary indication as to what type of patients may benefit. Furthermore, the treatment period may be too short to detect longitudinal improvement. We also could not assess for the potential for a change in behavior (such as more regularity or relaxation time, related to holding the device on a nightly basis) to be the actual reason for symptomatic improvement, nor account for placebo effect.
Finally, there was a large age range in our subjects, and as mentioned earlier, since older individuals typically have less slow wave sleep, this slightly limits interpretation of findings. As we found a trend toward increased slow wave sleep with BioBoosti use, it would be of great interest to investigate in future studies whether the device could help “restore” the age-related reduction in deep sleep in older adults with insomnia.
To our knowledge, this is the first report of pulse electromagnetic treatment of insomnia. Since there were no prior reports of this type of treatment and no preliminary data, our study was not designed as a randomized placebo-controlled trial, but rather as a pilot study, to evaluate any effect size and feasibility of such trial. Thus, the results are not conclusive of efficacy. Furthermore, 2 weeks may be insufficient time to assess effectiveness. Nevertheless, our findings are very encouraging, particularly if consideration is given to the fact that more half of the participants had failed more than one medication treatment. Larger, longer term, placebo-controlled studies will be needed to fully evaluate the effectiveness of BioBoosti as a treatment for insomnia.
The use of hypnotic or sedative medications has been associated with falls, motor vehicle accidents, and dementia.24 Cognitive behavioral therapy can be effective at reducing a patient’s need for hypnotic medications but may not be accessible due to distance, barriers to travel, or time restraints.26 Other non-pharmacological treatments such as BioBoosti may reduce the initiation of hypnotic medications and frequency of use in the treatment of insomnia. Given the personal and societal burden of untreated chronic insomnia, as well as limitations in current therapeutics, BioBoosti may be a promising future option.
Informed consent
Participant provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. We do not indent to publish the data elsewhere, but if requested, de-identified data can be shared, according to current HIPPAA law. We will have to also ask the IRB for approval. Sleep log measures, actigraphy, ISI/PSQI/ESS score, and any adverse event reports may be shared upon request for 3 years following the conclusion of the study. The clinical trial identifier is NCT02924116.
Acknowledgments
The authors wish to thank all the subjects for participation in this study. This work was supported by Biomobie Inc. Part of the findings have been presented in abstract form as posters at the 2018 meetings of the American Academy of Neurology and the American Academy of Sleep Medicine.
Author Contributions
MKP designed and led the study, performed most data interpretation and prepared the first manuscript draft. VL performed data analysis, contributed to interpretation and manuscript preparation (performed technical editing, table preparation, and draft revision). NP was the study coordinator, leading recruitment of subjects, study completion and data organization. PGM, assisted in data analysis, manuscript preparation, and editing. SJ, JJ, and SBA also assisted with data acquisition, manuscript preparation, and editing. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
Research funding for the study included a grant from Biomobie, Inc., to the Brigham and Women’s hospital which included coverage of study cost (testing, subjects remuneration, fees), and partial salary support covering the time involved in the study for Dr Pavlova (principal investigator), Mr Puri (research coordinator), and Dr Latreille (data analysis). Dr Matthew has received non-monetary compensation related to a similar project. Dr Matthew also reports non-financial support from BioMobie, during the conduct of the study. Drs Batool, Javaheri, and Johnsen have no relevant financial relationships. Drs Pavlova and Latreille have received also funding from the Trustee Fund, unrelated to this study. Dr Pavlova has received funding also from Lundbeck, Inc. and Jazz Pharmaceuticals for research unrelated to this study. Dr Latreille has received funding by the Canadian Institute of Health, unrelated to this study. Dr Javaheri is a consultant for Jazz Pharmaceuticals on treatment of type 2 narcolepsy. The authors report no other conflicts of interest in this work.
References
1. American Academy of Sleep Medicine. The International Classification of Sleep Disorders.
2. DiBonaventura M, Richard L, Kumar M, Forsythe A, Flores NM, Moline M. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. Li S, ed. PLoS One. 2015;10(10):e0137117. doi:10.1371/journal.pone.0137117
3. Trauer JM, Qian MY, Doyle JS, Rajaratnam S, Cunnington D. Cognitive behavioral therapy for chronic insomnia a systematic review and meta-analysis. 2015;163(3):191–204. doi:10.7326/M14-2841
4. Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18457234. accessed March 19, 2019.
5. Stickgold R. EMDR: a putative neurobiological mechanism of action. J Clin Psychol. 2002;58(1):61–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11748597. accessed March 19, 2019.
6. Roth T, Mayleben D, Feldman N, Lankford A, Grant T, Nofzinger E. A novel forehead temperature-regulating device for insomnia: a randomized clinical trial. Sleep. 2018;41:5. doi:10.1093/sleep/zsy045
7. Frase L, Selhausen P, Krone L, et al. Differential effects of bifrontal tDCS on arousal and sleep duration in insomnia patients and healthy controls. Brain Stimul. 2019. doi:10.1016/j.brs.2019.01.001
8. Ryang We S, Koog YH, Jeong K-I, Wi H. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology. 2013;52(5):815–824. doi:10.1093/rheumatology/kes063
9. Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016. doi:10.1111/jgs.14304
10. Hugo J, Ganguli M. Dementia and cognitive impairment. epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014. doi:10.1016/j.cger.2014.04.001
11. Amitriptyline hydrochloride (amitriptyline hydrochloride) dose, indicuations, adverse effects, interations from PDR.net. Prescribers digital reference. Available from: https://www.pdr.net/drug-summary/Amitriptyline-Hydrochloride-amitriptyline-hydrochloride-1001.5733.
12. Rozerem (ramelteon) dose, indications, adverse effects, interactions … from PDR.net. prescribers digital reference. Available from: https://www.pdr.net/drug-summary/Rozerem-ramelteon-562.646.
13. Fluvoxamine maleate | drug information | PDR.net. Prescribers digital reference. Available from: https://www.pdr.net/drug-information/fluvoxamine-maleate?druglabelid=1970&id=9849.
14. Pinder RM, Brogden RN, Speight TM, Avery GS. Sodium valproate: a review of its pharmacological properties and therapeutic efficacy in epilepsy. Drugs. 1977. doi:10.2165/00003495-197713020-00001
15. Gaskell WH. The electrical changes in the quiescent cardiac muscle which accompany stimulation of the vagus nerve. J Physiol. 1886. doi:10.1113/jphysiol.1886.sp000235
16. Charles M, Morin P. Insomnia Severity Index (ISI). Available from: https://mapi-trust.org/questionnaires/isi/.
17. Chen PY, Jan YW, Yang CM. Are the insomnia severity index and pittsburgh sleep quality index valid outcome measures for cognitive behavioral therapy for insomnia? Inquiry from the perspective of response shifts and longitudinal measurement invariance in their Chinese versions. Sleep Med. 2017. doi:10.1016/j.sleep.2017.04.003
18. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989. doi:10.1016/0165-1781(89)90047-4
19. Shahid A, Wilkinson K, Marcu S, Shapiro CM. Karolinska Sleepiness Scale (KSS). In: STOP, THAT and One Hundred Other Sleep Scales. New York: Springer-Verlag; 2012. doi:10.1007/978-1-4419-9893-4_47
20. Kaida K, Takahashi M, Åkerstedt T, et al. Validation of the karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006. doi:10.1016/j.clinph.2006.03.011
21. Murray Johns M The epworth sleepiness scale. Available from: http://epworthsleepinessscale.com/about-the-ess/.
22. Zisapel N, Tarrasch R, Laudon M. A comparison of visual analog scale and categorical ratings in assessing the patient’s estimate of sleep quality. In: Sleep and Sleep Disorders: A Neuropsychopharmacological Approach. Austin (TX): Landes Bioscience; 2006. doi:10.1007/0-387-27682-3_25
23. Rosenberg RS, Van Hout S. The American academy of sleep medicine inter-scorer reliability program: respiratory events. J Clin Sleep Med. 2014;10(4):447–454. doi:10.5664/jcsm.3630
24. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–2372. doi:10.1016/j.clinthera.2016.09.010
25. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia. JAMA. 2009;301(19):2005. doi:10.1001/jama.2009.682
26. Park KM, Kim TH, Kim WJ, An SK, Namkoong K, Lee E. Cognitive behavioral therapy for insomnia reduces hypnotic prescriptions. Psychiatry Investig. 2018;15(5):499–504. doi:10.30773/pi.2017.11.20
27. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. Ferri R, ed. PLoS One. 2016;11(2):e0149139. doi:10.1371/journal.pone.0149139
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


