Back to Journals » Drug Design, Development and Therapy » Volume 8
Novel nanocrystal formulation of megestrol acetate has improved bioavailability compared with the conventional micronized formulation in the fasting state
Authors Jang K , Yoon S , Kim S, Cho J , Yoon SH, Lim KS, Yu K , Jang I , Lee H
Received 10 February 2014
Accepted for publication 15 March 2014
Published 25 June 2014 Volume 2014:8 Pages 851—858
DOI https://doi.org/10.2147/DDDT.S62176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Kyungho Jang, Seonghae Yoon, Sung-Eun Kim, Joo-Youn Cho, Seo Hyun Yoon, Kyoung Soo Lim, Kyung-Sang Yu, In-Jin Jang, Howard Lee
Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, Republic of Korea
Background: Megestrol acetate is an effective treatment for improving appetite and increasing body weight in patients with cancer-associated anorexia. However, Megace® oral suspension (OS), a micronized formulation of megestrol acetate, has low bioavailability in the fasting state. To overcome this limitation, a nanocrystal formulation has been developed. This study was performed to evaluate the pharmacokinetics and tolerability of the nanocrystal formulation and to compare them with those of Megace® OS in the fed and fasting states.
Methods: A randomized, open-label, two-treatment, two-period, two-sequence, crossover study was performed in three parts in 93 healthy subjects. A single 625 mg/5 mL oral dose of a nanocrystal formulation was administered in the fasting and fed states (part I). In parts II and III, a single 625 mg/5 mL oral dose of the nanocrystal formulation or Megace® OS 800 mg/20 mL was given in the fed and fasting states, respectively. Blood samples were collected for up to 120 hours post dose for pharmacokinetic analysis. Tolerability was evaluated throughout the entire study period.
Results: The nanocrystal formulation of megestrol acetate was rapidly absorbed in both the fed and fasting states. In the fed state, systemic exposure was comparable between the nanocrystal formulation of megestrol acetate and Megace® OS. In the fasting state, however, the peak plasma concentration and area under the plasma concentration-time curve to the last measurable concentration of megestrol acetate was 6.7-fold and 1.9-fold higher, respectively, for the nanocrystal formulation than for Megace® OS. No serious adverse events were reported.
Conclusion: Systemic exposure to megestrol acetate is less affected by lack of concomitant food intake when it is administered using the nanocrystal formulation. The nanocrystal formulation of megestrol acetate could be more effective in treating patients with cachexia or anorexia.
Keywords: megestrol acetate, nanocrystal formulation, food, anorexia
Introduction
Megestrol acetate, a synthetic progesterone, has been used as an antineoplastic agent in the treatment of breast, endometrial, and prostate cancers.1 Megestrol acetate is also effective in improving appetite and increasing body weight in patients with cancer-associated anorexia. For example, a meta-analysis of nine trials involving 994 patients showed a significant weight gain effect of megestrol acetate over the control (relative probability 2.17, 95% confidence interval [CI] 1.59–2.97).2 Furthermore, in the same study, improvement in appetite was 3.7 times higher in the megestrol acetate group than in the control group. Therefore, megestrol acetate would be of benefit to patients with pre-cachexia or anorexia in whom weight loss and inadequate nutrition negatively affects their survival.
Megace® oral suspension (OS) (Boryung Pharmaceutical Co., Ltd., Seoul, Republic of Korea), a micronized formulation of megestrol acetate (particle size 1–10 μm), is inadequately absorbed, likely because of its poor water solubility.3 Because the solubility of Megace® OS is further reduced in the fasting state, it is recommended to take the drug after a meal.4 However, given that most patients with anorexia take medications without food, the clinical utility of Megace® OS has been limited.
To overcome the limitations of Megace® OS, a nanocrystal formulation has been developed, 625 mg (in 5 mL) which is bioequivalent to 800 mg of Megace® OS (in 20 mL) in the fed state.5 This new formulation is expected to enhance the biopharmaceutical performance of megestrol acetate by optimizing the drug delivery system, which may be further augmented due to its smaller volume per dose compared with the micronized formulation. In other words, nanocrystalline particles increase the surface area per unit mass, leading to markedly improved solubility and more rapid dissolution than micronized Megace® OS.6 For example, >95% of the nanocrystal formulation was dissolved within 10 minutes, whereas 3 hours were necessary for coarse megestrol acetate powder.7 Furthermore, in preclinical experiments with Beagle dogs, the peak concentration and time to reach peak concentration was higher and shorter, respectively, for the nanocrystal formulation of megestrol acetate than for the micronized suspension.8 In addition, although a high-fat meal increased the bioavailability of both formulations, systemic exposure to megestrol acetate in the fasting state was much higher for the nanocrystal formulation than for Megace® OS.8 These preclinical results suggest that the pharmacokinetic behavior of the nanocrystal formulation was not affected much by lack of concomitant food intake. Collectively, these favorable pharmacokinetic findings support the notion that the nanocrystal formulation of megestrol acetate could be more effective in patients with cachexia or anorexia treated even in the fasting state.
The nanocrystal formulation of megestrol acetate was approved in 2005 by the US Food and Drug Administration,8 and another nanocrystal formulation has been developed for marketing approval in the Republic of Korea (Boryung Pharmaceutical Co, Ltd, Seoul). This study aimed to evaluate the pharmacokinetics and tolerability of a nanocrystal formulation of megestrol acetate in healthy subjects in the fasting and fed states (part I), and compare them with those of Megace® OS in the fed (part II) and fasting (part III) states.
Materials and methods
Subjects
Males aged 20–55 years were enrolled in the study if they had a body mass index of 19–27 kg/m2 and were in good general health based on a detailed medical history, physical examinations, vital signs, electrocardiography, and clinical laboratory evaluations, including hematology (hemoglobin, blood cell count, platelet count), liver function tests (alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and bilirubin), renal function tests (blood urea nitrogen, serum creatinine), blood glucose, urinalysis (including urine drug screening), and seroimmunology (hepatitis B surface antigen, anti-hepatitis C virus antibody, and anti-human immunodeficiency virus antibody). Subjects were excluded if they had: a history of significant gastrointestinal, hepatic, renal, respiratory, cardiovascular, metabolic, immunological, or hormonal disease; a history of drug or food allergy; taken any prescription medication within 2 weeks prior to the first administration of the study drug; a diet that would affect the absorption, distribution, metabolism, and/or elimination of the drug; a positive drug or alcohol screen; smoked 10 or more cigarettes per day within the previous 3 months; participated in a clinical trial during the last 3 months prior to the start of the study.
Study design
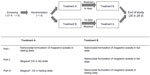
This study consisted of three parts, each of which was conducted using a randomized, open-label, two-treatment, two-period, two-sequence, crossover design. Subjects were screened for eligibility within 1–21 days before the first administration of the study drug in each part of the study, followed by two treatment periods that were separated by a 14-day washout. In each part of the study, subjects were admitted to the clinical trials center at Seoul National University Hospital on the night before the day of administration of the study drug (day 1) and stayed for two further days. In addition, subjects visited the clinical trials center on days 3–6 for the planned study procedures (Figure 1).
In part I, after overnight fasting, subjects received a single oral dose of the nanocrystal formulation of megestrol acetate at 625 mg in the fasting state or within 30 minutes of a high-fat meal of 950 calories (calories from fat, 57.2%) in a random sequence to evaluate the effect of food on the nanocrystal formulation. In parts II and III, subjects randomly received a single oral dose of the nanocrystal formulation at 625 mg or Megace® OS at 800 mg after a high-fat meal (part II) or in the fasting state (part III). In each part of the study, the trial medication was taken with approximately 240 mL of water. The nanocrystal formulation of megestrol acetate and Megace® OS were both manufactured by Boryung Pharmaceutical Co, Ltd.
For pharmacokinetic analysis, blood samples were collected at 0 (ie, predose), 1, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96, and 120 hours post dose. Tolerability was evaluated throughout the study period by examining the incidence and type of adverse events, and changes in clinical laboratory test values, physical examinations, vital signs, and 12-lead electrocardiograms.
The study protocol was approved by the institutional review board of Seoul National University Hospital (clinicaltrials.gov registry, NCT01397214). All subjects provided their written informed consent prior to any study procedure. The study was performed in accordance with the Declaration of Helsinki as implemented in the Good Clinical Practice guidelines.
Pharmacokinetic assessments and statistical analysis
Blood samples were centrifuged within 30 minutes of collection at ≥750× g for 15 minutes. The separated plasma was then aliquoted into three tubes and immediately stored below −70°C until use.9 Plasma concentrations of megestrol acetate were determined using a validated liquid chromatography tandem mass spectrometry method with a limit of quantification of 2 ng/mL.10 The standard curve was linear over the range of 2–5,000 ng/mL with a coefficient of determination ≥0.9952. Accuracy ranged from 92.61% to 103.2% and the interassay precision was ≤4.192%.
Data from the subjects who completed the study as scheduled were included in the pharmacokinetic and statistical analyses. The actual sampling times were used to derive the pharmacokinetic parameters of megestrol acetate based on a noncompartmental method implemented in Phoenix® WinNonlin® version 6.3 (Certara, St Louis, MO, USA). The pharmacokinetic parameters included: maximum observed plasma concentration (Cmax); time taken to reach the maximum plasma concentration (Tmax); area under the plasma concentration-time curve from time zero to the time of the last quantifiable concentration (AUClast); area under the plasma concentration-time curve from time zero to infinite time (AUCinf); and the elimination half-life (t1/2). AUCinf was calculated as the sum of AUClast and the last quantifiable concentration divided by the slope of the final decline portion of the individual log-linear concentration-time curve.
The data were summarized using descriptive statistics. A paired t-test was used to compare the pharmacokinetic parameters of megestrol acetate between the fasting and fed conditions (part I) and the different formulations (parts II and III). In addition, using the natural logarithm-transformed Cmax, AUClast, and AUCinf, a mixed effects analysis of variance model was fit, where treatment regimen, period, and sequence were the fixed effects, and subject nested for sequence was the random effect. Based on the analysis of variance model, the geometric mean ratio and an associated 90% CI was constructed for the pharmacokinetic parameters.
Results
Study participants
A total of 103 subjects were randomized throughout parts I–III, 93 (90.3%) of whom completed the study. Twenty-nine subjects were enrolled in part I, 27 (93.1%) of whom completed the study. One subject had an abnormal electrocardiogram before administration of the study drug in period 1 and another withdrew consent after period 1. In part II, 45 subjects were randomized, 39 (86.7%) of whom completed the study. Three and two subjects dropped out of the study prior to study drug administration during and after period 1, respectively. Furthermore, one subject discontinued from the study because he took a prohibited concomitant medication before period 2. Twenty-nine subjects were enrolled in part III, which was completed by 27 (93.1%) subjects. One subject was discontinued because of smoking before administration of the study drug in period 1 and another withdrew consent prior to period 2. Those who completed the study as scheduled and had plasma concentrations available in both periods comprised the population used for pharmacokinetic analysis (n=93), whereas the tolerability analysis population consisted of any subject who took at least one single oral dose of megestrol acetate (n=98).
The mean ± standard deviation for age, body weight, and body mass index was 26.0±4.3 years, 68.2±7.4 kg, and 22.7±2.1 kg/m2, respectively. No significant differences were noted in baseline characteristics between subjects who took the study drugs following the two sequences in each part of the study.
Pharmacokinetics of megesterol acetate
Part I
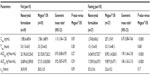
After a single oral dose of the nanocrystal formulation, megestrol acetate was rapidly absorbed both in the fasting and fed states (median Tmax one hour, Table 1) although its systemic exposure was 35% lower in the fasting state than in the fed state (geometric mean ratio for AUCinf without and with food: 0.65, 90% CI 0.60–0.71, Table 1).
Parts II and III
The concentration-time profiles for megestrol acetate in the fed state were comparable between the nanocrystal formulation of megestrol acetate and Megace® OS (Figure 2, left). Furthermore, the point estimate and its 90% CI of the geometric mean ratio for Cmax, AUClast, and AUCinf fell entirely within the conventional bioequivalence range of 80%–125% (Table 2).
In the fasting state, megestrol acetate in the nanocrystal formulation was rapidly absorbed, whereas Megace® OS was slowly and inadequately absorbed (Figure 2, right). As a result, the Cmax for megestrol acetate was 6.7-fold higher with the nanocrystal formulation than with Megace® OS (1,374.8 ng/mL versus 207.1 ng/mL, see Table 2). Likewise, the AUClast and AUCinf values were 1.90 and 1.86 times greater, respectively, for the nanocrystal formulation than for Megace® OS in the fasting state (Table 2).
When combining the results from parts II and III, the changes in Cmax and AUClast for megestrol acetate between the fed state and the fasting state were of much smaller magnitude for the nanocrystal formulation than for Megace® OS (Figure 3).
Tolerability
Both formulations of megestrol acetate were well tolerated. A total of 57 adverse events were reported in 30 of 98 (30.6%) subjects for the overall study; these were mild to moderate in severity and resolved without sequelae. No serious adverse events were reported.
In part I, 12 adverse events occurred in five of 28 (17.9%) subjects. There was one report each of somnolence and rhinorrhea, respectively, within one hour and 3 hours of drug administration in the fasting state, which were considered related to the study drug. During parts II and III, 13 subjects who received Megace® OS experienced 22 adverse events, while 16 subjects who received the nanocrystal formulation of megestrol acetate had 23 adverse events. Seven of these 45 adverse events were considered to be “possibly related” to the study drug, and comprised somnolence (n=4, 8.9%), feeling abnormal (n=1, 2.2%), headache (n=1, 2.2%), and a nodule on the neck (n=1, 2.2%). The nodule on the neck was noted on day 4 after administration of the study drug in period 2 and disappeared spontaneously. No apparent differences in the frequency of adverse events considered “related to the study drug” were noted between the nanocrystal formulation of megestrol acetate and Megace® OS (Table 3).
Discussion
This study indicates that the nanocrystal formulation of megestrol acetate is rapidly and adequately absorbed, not only in the fed state but also in the fasting state, whereas the bioavailability of the micronized (Megace® OS) formulation is limited in the fasting state. In this study, megestrol acetate reached its peak concentration one hour after administration of the nanocrystal formulation both in the fasting and fed states, although its systemic exposure was 35% lower in the fasting state than in the fed state (Table 1). In contrast, systemic exposure to and peak concentration of megestrol acetate for Megace® OS in the fasting state were only 53.8% and 14.9% those of the nanocrystal formulation, respectively (Table 2 and Figure 2). Collectively, these findings support the notion that, unlike Megace® OS, the nanocrystal formulation of megestrol acetate can be effectively used to increase body weight in patients who are likely to be anorexic or in the fasting state for various reasons. However, fasting healthy volunteers might not be fully representative of cancer patients with anorexia or cachexia, which is a complex metabolic syndrome resulting from underlying illness. Therefore, future studies are warranted to determine if the improved bioavailability of the nanocrystal formulation seen in the present study can be replicated in cancer-associated anorexia and cachexia.
Megestrol acetate is a lipophilic drug with poor water solubility. When administered with a high-fat meal, both the micronized and nanocrystal formulations of megestrol acetate become more soluble, and were completely absorbed, most likely in the small intestine. This would be due to the fat content in food and increased bile excretion in response to food intake.11 This may explain why there was no obvious difference in the Cmax and AUC for megestrol acetate between the nanocrystal formulation and Megace® OS in the fed state, although the time to peak concentration (ie, Tmax) was slightly delayed (Table 2 and Figure 2). In contrast, the Tmax for Megace® OS remained slow in the fasting state due to its low solubility, although fasting may have shortened the gastric emptying time.
Our results are in good agreement with other studies reporting better biopharmaceutical performance for nanocrystal formulations than for conventional formulations. For example, Huang et al conducted a pharmacokinetic study in rats to evaluate the bioavailability of a nanosuspension formulation of SKLB610, a potent inhibitor of vascular endothelial growth factor receptor 2.12 In that study, the nanosuspension formulation resulted in a 3.8-fold and 2.6- fold higher Cmax and AUClast, respectively, than the coarse suspension. Wu et al also reported that systemic exposure to MK-0869, a treatment option for chemotherapy-induced nausea and vomiting, was less affected by food intake when a nanoparticle formulation was administered in Beagle dogs.13 The improved biopharmaceutical performance of the nanocrystal formulation is mainly attributable to its faster dissolution rate as a result of its increased surface area. In addition, the nanocrystal formulation may increase absorption by high adhesiveness of drug particles to the epithelial gut wall, thereby prolonging the absorption time.14
A previous study of the oral suspension of megestrol acetate (800 mg/20 mL, administered once daily in the fasting state) in cachectic patients with acquired immune deficiency syndrome reported that earlier body weight gain on megestrol acetate is related to the extent of in vivo drug exposure, ie, a threshold concentration of >300 ng/mL was required.15 In the present study, the plasma concentration of megestrol acetate were maintained at >300 ng/mL until 12 (fed) and 8 (fasting) hours after a single dose of the nanocrystal formulation (Figure 2). Therefore, a multiple daily regimen, ie, administration 2–3 times daily, may be required for the nanocrystal formulation of megestrol acetate at a dose of 625 mg/5 mL to achieve an earlier body weight increase and appetite improvement while shortening the treatment duration when treating patients with cachexia or anorexia. Given that no serious or unexpected adverse events were observed with megestrol acetate at doses as high as 1,600 mg/day in patients with anorexia and cachexia,16 the proposed multiple daily regimen could be an effective and safe treatment option.
The present study was conducted in three parts using a two-way, two-period, two-treatment crossover design. This could be a limitation of this study because a four-way crossover may have been a more ideal design for evaluating the combined effects of food and formulation on the pharmacokinetics of megesterol acetate. However, a four-way crossover study takes longer to complete and more subjects may be needed, because of a greater dropout rate and a larger number of randomization sequences. For example, 16 randomization sequences and a total of at least 8 weeks would have been required to complete the study if it had been conducted using a four-way crossover design. The design adopted in the present study was sufficient to show the difference in the pharmacokinetics or lack thereof between the two formulations of megestrol acetate in the fasting and fed states.
Conclusion
Systemic exposure to megestrol acetate is less affected by lack of concomitant food intake when administered as the nanocrystal formulation. The nanocrystal formulation of megestrol acetate is well tolerated and could be a better treatment option than Megace® OS or other micronized formulations of megestrol acetate when treating patients with cachexia or anorexia.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published.
Disclosure
This study was sponsored by Boryung Pharmaceutical Co, Ltd, Seoul, Republic of Korea, but was designed and conducted by qualified investigators from the Department of Clinical Pharmacology and Therapeutics at Seoul National University College of Medicine and Hospital. The sponsor played a minor role in the study design and interpretation. Otherwise, none of the authors has any conflicts of interests to report regarding the content of this article.
References
Geller J, Albert J, Geller S, Lopez D, Cantor T, Yen S. Effect of megestrol acetate (Megace®) on steroid metabolism and steroid-protein binding in the human prostate. J Clin Endocrinol Metab. 1976;43(5):1000–1008. | |
Zhan P, Wang Q, Qian Q, Yu LK. Megestrol acetate in cancer patients with anorexia-cachexia syndrome: a meta-analysis. Transl Cancer Res. 2013;2(2):74–79. | |
Femia RA, Goyette RE. The science of megestrol acetate delivery: potential to improve outcomes in cachexia. BioDrugs. 2005;19(3):179–187. | |
Yeh SS, Lovitt S, Schuster MW. Pharmacological treatment of geriatric cachexia: evidence and safety in perspective. J Am Med Dir Assoc. 2007;8(6):363–377. | |
Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomedicine. 2009;4:185–192. | |
Yeh SS, Schuster MW. Megestrol acetate in cachexia and anorexia. Int J Nanomedicine. 2006;1(4):411–416. | |
Sylvestre JP, Tang MC, Furtos A, et al. Nanonization of megestrol acetate by laser fragmentation in aqueous milieu. J Control Release. 2011;149(3):273–280. | |
Food and Drug Administration, Center for Drug Evaluation and Research. Megace® ES (625 mg/5 mL). Clinical pharmacology and biopharmaceutics review. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021778s000_ClinPharmR.pdf. Accessed December 12, 2013. | |
Farinha A, Bica A, Tavares P. Improved bioavailability of a micronized megestrol acetate tablet formulation in humans. Drug Dev Ind Pharm. 2000;26(5):567–570. | |
Gaver RC, Movahhed HS, Farmen RH, Pittman KA. Liquid chromatographic procedure for the quantitative analysis of megestrol acetate in human plasma. J Pharm Sci. 1985;74(6):664–667. | |
Bosch HW. Pharmaceutical applications of finely dispersed systems. In: Stauffer DR, editor. High Speed Serdes Devices and Applications. New York, NY: Springer; 2008. | |
Huang Y, Luo X, You X, Xia Y, Song X, Yu L. The preparation and evaluation of water-soluble SKLB610 nanosuspensions with improved bioavailability. J Pharm Sci. 2013;14(3):1236–1243. | |
Wu Y, Loper A, Landis E, et al. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int J Pharm. 2004;285(1):135–146. | |
Mauludin R. Nanosuspensions of poorly soluble drugs for oral administration. Berlin, Germany: Freie Universitat Berlin; 2009. Available from: http://elib-a.ucl.ac.uk/full.php?id=596627. Published 2009. Accessed December 12, 2013. | |
Graham KK, Mikolich DJ, Fisher AE, Posner MR, Dudley MN. Pharmacologic evaluation of megestrol acetate oral suspension in cachectic AIDS patients. J Acquir Immune Defic Syndr. 1994;7(6):580–586. | |
Megace® oral suspension [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company; 2012. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.