Back to Journals » Nature and Science of Sleep » Volume 13
Novel Intraoral Negative Airway Pressure in Drug-Induced Sleep Endoscopy with Target-Controlled Infusion
Authors Kuo YH , Liu TJ, Chiu FH, Chang Y, Lin CM, Jacobowitz O , Hsu YS
Received 8 July 2021
Accepted for publication 21 October 2021
Published 24 November 2021 Volume 2021:13 Pages 2087—2099
DOI https://doi.org/10.2147/NSS.S327770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ahmed BaHammam
Video abstract presented by Ying-Shuo Hsu.
Views: 934
Yu-Hsuan Kuo,1,* Tien-Jen Liu,2,3,* Feng-Hsiang Chiu,4,5 Yi Chang,6 Chia-Mo Lin,7– 9 Ofer Jacobowitz,10 Ying-Shuo Hsu1,11
1Department of Otolaryngology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 2Department of Otolaryngology Head & Neck Surgery, MacKay Memorial Hospital, Taipei Branch, Taipei, Taiwan; 3School of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan; 4Department of Otolaryngology, Head and Neck Surgery, Tri-Service General Hospital, Taipei, Taiwan; 5School of Medicine, National Defense Medical Center, Taipei City, Taiwan; 6Department of Anesthesiology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei City, Taiwan; 7Division of Chest Medicine, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan; 8Department of Chemistry, Fu-Jen Catholic University, New Taipei City, Taiwan; 9Graduate Institute of Biomedical and Pharmaceutical Science, Fu Jen Catholic University, New Taipei City, Taiwan; 10ENT & Allergy Associates, New York, NY, USA; 11School of Medicine, Fu Jen Catholic University, New Taipei City, Taiwan
*These authors contributed equally to this work
Correspondence: Ying-Shuo Hsu
Department of Otolaryngology, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
Email [email protected]
Corrigendum for this paper has been published.
Background: In intermittent negative airway pressure (iNAP) therapy, soft tissues are reshaped into a forward-resting position, thus reducing airway obstruction during sleep. This study investigated the effect of iNAP therapy that was administered during drug-induced sleep endoscopy with target-controlled infusion (TCI-DISE) in patients with obstructive sleep apnea (OSA) intolerant of continuous positive airway pressure (CPAP) therapy.
Methods: This prospective case series study included 92 patients with polysomnography (PSG)-confirmed OSA who underwent TCI-DISE with iNAP from January 2018 to February 2020 at a tertiary referral hospital. Upper airway obstruction was evaluated and scored using the velum, oropharynx, tongue base, and epiglottis (VOTE) classification. Obstruction severity was assessed multiple times with the patient in the supine position with or without lateral rotation of the head and the application of iNAP therapy, respectively.
Results: After the application of iNAP therapy in the supine position, obstruction severity decreased significantly: from complete or partial obstruction to partial or no obstruction in 37, 12, and 36 patients (40.2%, 13%, and 39%, respectively) with velar obstruction, oropharyngeal, and tongue base obstruction, respectively. After simultaneously applying iNAP therapy with head rotation, obstruction severity decreased in 47, 43, and 19 patients (51%, 47%, and 21%, respectively) with velar, tongue base, and epiglottic obstruction, respectively.
Conclusion: In TCI-DISE, we found that iNAP therapy relieved velar, oropharyngeal, and tongue base obstruction in the supine position in some patients. Moreover, iNAP therapy can be combined with positional therapy to alleviate velar, tongue base, and epiglottic obstruction in some patients. TCI-DISE can also be used to screen the possible responders for iNAP therapy because it is less time consuming than PSG.
Keywords: OSA, target control infusion, drug induced sleep endoscopy, iNAP therapy, velar collapse, tongue collapse
Introduction
Obstructive sleep apnea (OSA) is highly prevalent worldwide, with an estimated 425 million adults having moderate-to-severe OSA.1 Common complications of OSA include sudden cardiac death,2 hypertension,3 cerebrovascular incidents,4 excessive daytime sleepiness,5 and type 2 diabetes.6 Although continuous positive airway pressure (CPAP) remains the gold standard and first-line treatment for OSA,7 alternative therapies are required because long-term adherence to CPAP therapy is often suboptimal.8
Oral pressure therapy (OPT) is an alternative treatment modality for patients with poor compliance to CPAP.9–13 Nigam et al10 reported that the OPT success rate (defined as a reduction of ≥50% from the baseline apnea–hypopnea index [AHI] and a post-OPT treatment residual AHI of ≤10) varies between 25% and 37%. However, the success rate does not correlate with OSA severity or the body mass index (BMI);10,12 therefore, identifying responders and nonresponders to OPT treatment necessitates the establishment and comprehensive examination of predictors.
Multiple factors may contribute to OSA including anatomical variations, body position, sleep stages, muscle tone, arousal threshold, and loop gain effect.14–17 Thus, upper airway obstruction has a varied and dynamic pattern, making it challenging to treat patients with OSA and predict patient response to each treatment. Therefore, identifying the sites of obstruction and the responses of patients to alternative OSA treatments, such as surgery, mandibular advancement device therapy, and OPT, is essential.18 However, polysomnography (PSG), the most common diagnostic tool for OSA,19 does not provide sufficient anatomical and real-time information of the upper airway.
Sleep endoscopy was first advocated by Croft and Pringle in the 1990s to target sites and structures involved in upper airway obstruction.20 Kezirian et al renamed this technique as drug-induced sleep endoscopy (DISE)21 and developed the velum, oropharyngeal lateral wall, tongue base, and epiglottis (VOTE) classification system.22 This procedure produces a dynamic image in real time, thus enabling the observation of the sites of upper airway obstruction in simulated sleep that is typically not possible during awake endoscopy, especially with regard to tongue base obstruction.23 Thus, DISE can potentially be used to identify responders and nonresponders to OPT treatment. Furthermore, DISE can help understand the pathophysiology of specific collapse patterns and determine the optimal treatment option accordingly.
Although multiple studies have validated DISE and reported its high interrater and test–retest reliability,24,25 polysomnographic outcomes after DISE-guided upper airway surgery are not always satisfactory.26–30 A possible reason for this finding may be the difficulty in controlling the sleep depth through the manual bolus injection of propofol. The bispectral index (BIS) and target-controlled infusion (TCI) were developed to monitor and control the sleep depth during the procedure.31 De Vito et al reported that DISE with TCI (TCI-DISE) may increase accuracy, stability, and safety.32 Our previous study reported that relatively poor outcomes were associated with preoperative and postoperative complete tongue base collapse and postoperative velar collapse identified in TCI-DISE.33 Thus, TCI-DISE results may be relatively reliable for predicting treatment outcomes including those of OPT therapy.
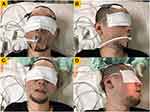
Intermittent negative airway pressure (iNAP) therapy (Figure 1), the latest form of OPT, is characterized by a lower oral tissue discomfort rate and possibly more favorable compliance.11–13 By providing intermittent negative pressure to the oral cavity, the iNAP device moves both the soft palate and tongue in an anterior direction, thereby increasing retropalatal and retroglossal areas and reducing airway obstruction during sleep.13 The device is battery powered, and the air flow is designed to stop when the targeted negative oral pressure is achieved and restart again only when air flow is required to prevent a decrease in target pressure.12 Through this design, iNAP enhances the patency of the pharyngeal upper airway without a mask and forced air, thereby preventing local tissue discomfort and systematic dryness of the mouth. In the first month of usage, the average sealing percentage is 60%–70%, which increases to 80%–90% after 2–3 months. The price of the device is approximately US$1370. The device may be indicated for patients with OSA who are intolerant to CPAP therapy. No definite contraindication has been indicated in the current literature.
 |
Figure 1 The intraoral negative airway pressure device. |
In the present study, we investigated the effect of iNAP therapy on patients with OSA intolerant to CPAP to determine the location of improvement of obstruction with iNAP therapy and improve the selection of patients for whom this therapy is suitable.
Methods
This study protocol was approved by the Institutional Review Board of Shin Kong Wu Ho-Su Memorial Hospital (IRB Number: 20180815R and 20190806D). This study was conducted in accordance with the Declaration of Helsinki. The informed consent to participate in the study have been obtained from the research subjects prior to study commencement. In addition, the participants gave consent to have their data published.
Study Patients
We performed a prospective case series study between January 2018 and February 2020 at Shin Kong Wu Ho-Su Memorial Hospital, a tertiary referral hospital in Taipei, Taiwan. We included patients with snoring who had a PSG-confirmed diagnosis of OSA (AHI > 5). All the included patients underwent TCI-DISE, the steps of which are presented as follows.
TCI-DISE System
TCI-DISE was performed in the bronchoscopy room of the outpatient clinic. The participants included in this procedure included one operator (a sleep surgeon), one anesthesiologist, and two technicians (as an assistant of the sleep surgeon and anesthesiologist, respectively). Cardiorespiratory activity (pulse oximetry, blood pressure monitoring, and electrocardiography) was monitored, and oxygen was administered if necessary. Patients were asked to fast for 8 hours before TCI-DISE to prevent regurgitation and aspiration. To control the depth of sleep during the procedure, a TCI system (Fresenius Kabi Injectomat TIVA Agilia, Bad Homburg, Germany) was used to maintain the BIS between 50 and 70.31,32 In brief, the Schnider model34 was used to administer propofol at a starting dose of 3.0 mcg/mL at increments of 0.1–0.3 mcg/mL subsequently until the target cerebral concentration was reached. When adequate sedation was achieved, a flexible endoscope was inserted into the nasal cavity and through the nasal passage for the examination of the nasopharynx, velum, oropharynx, tongue base, epiglottis, and larynx. The levels of snoring and obstruction were assessed.
iNAP in TCI-DISE
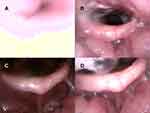
The TCI-DISE procedure commenced after the application of iNAP therapy; the patient was placed in the supine position (Figure 2A), and their head was rotated to the left (Figure 2B). Subsequently, the iNAP device was removed. DISE was repeated in the supine position (Figure 2C), with the patient’s head rotated again (Figure 2D). In addition, to prevent air leak from the mouth, iNAP was set to −150 mmHg during the whole procedure. To achieve a stable breathing pattern, heart rate, and SpO2 without major fluctuations, each maneuver was observed for 2 minutes. The whole DISE procedure for each patient lasted for approximately 20 minutes.
VOTE Classification System
All endoscopies were videotaped, and the degree of obstruction at each anatomical site was determined by experienced sleep specialists. Four levels of the collapsible segment of the upper airway were evaluated using the VOTE classification system,35 with the patient in the supine position with or without head rotation. The degree of collapse was reported as follows: no obstruction (collapse: <50%), partial obstruction (collapse: 50%–75%), or complete obstruction (collapse: >75%).
Statistical Analysis
The severity of obstruction (no, partial, or complete obstruction) before and after iNAP therapy at each VOTE site was compared using the McNemar–Bowker test. In addition, the proportion of complete relief in all the four sites of VOTE before and after iNAP therapy was examined using the McNemar–Bowker test. Patients with partial or complete obstruction before iNAP were classified as responders and nonresponders according to the improvement of obstruction severity after iNAP (ie, from complete obstruction to none or partial obstruction and from partial obstruction to none). Baseline characteristics, including PSG parameters before iNAP, between the responders and nonresponders were compared using the chi-square test for categorical variables (eg, sex) and the independent sample t test for continuous variables. The findings of the responders were compared with the nonresponders for each site of VOTE. Data analyses were conducted using SPSS 25 (IBM SPSS Inc., Chicago, Illinois). All the tests were two sided, and P values of <0.05 were considered statistically significant.
Results
Baseline Characteristics
We included consecutive patients who were intolerant of CPAP therapy and had undergone an attended full-night PSG (with an AHI > 5) and DISE. Patients who had undergone upper airway surgery except nasal or tonsillectomy were excluded. Finally, 92 patients (75 men, 81.5%) were included into the final analysis. The mean age of the patients was 47.1 ± 12.9 years, and the mean BMI was 26.3 ± 3.9 kg/m2. The average AHI was 34.2 ± 23.9 events/h. The average minimum arterial oxygen saturation (SaO2) was 78.4% ± 9.9% (Table 1).
 |
Table 1 Baseline Characteristics of the Participants (n = 92) |
Obstruction Severity at the Velum Before and After iNAP Therapy
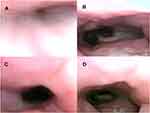
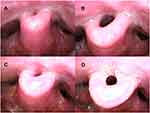
In the supine position, 83 patients exhibited complete velar obstruction before iNAP therapy (Figure 3A and B). After the application of iNAP therapy, the obstruction remained complete in 49 patients (59%; 49/83). A shift from complete to partial obstruction was observed in 16 patients (19.3%; 16/83), and the obstruction was completely resolved in 18 patients (21.7%; 18/83; Figure 3C and D; Supplementary Video). After iNAP therapy, the obstruction status of the three patients who exhibited no prior obstruction remained unchanged. Three patients with partial obstruction of the velum before iNAP exhibited no obstruction after iNAP therapy. Velar obstruction severity decreased in 40% of the patients (40.2%; 37/92) after the application of iNAP therapy in the supine position (P < 0.001; Table 2).
 |
Table 2 Changes in obstruction severity before and after intermittent negative iNAP therapy in the supine position with and without head rotation |
After iNAP therapy with head rotation, the obstruction remained complete in 40 patients (48.2%; 40/83). A shift from complete to partial obstruction was noted in 15 patients (18.1%; 15/83), and the obstruction was completely resolved in 28 patients (33.7%; 28/83; Figure 4C and D; Figure 4A and B showed head rotation without iNAP therapy). Four patients with partial obstruction of the velum before iNAP exhibited no obstruction after iNAP therapy with head rotation. After iNAP therapy was applied with head rotation, the velar obstruction severity decreased in half (51.1%; 47/92) of the patients (P < 0.001; Table 2). More detailed collapse directions, including the anterior–posterior, lateral, and concentric at the velum level, are described in Supplement Table 5.
Obstruction Severity at the Oropharynx Before and After iNAP Therapy
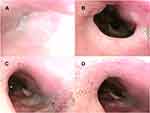
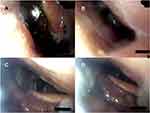
In the supine position, 20 patients exhibited complete oropharyngeal obstruction before iNAP therapy (Figure 5A and B). After iNAP therapy, the obstruction remained complete in 10 patients (50%; 10/20). A shift from complete to partial obstruction was noted in 7 patients (35%; 7/20), and the obstruction was completely resolved in 3 patients (15%; 3/20; Figure 5C and D; Supplementary Video 1). Two patients with partial obstruction of the oropharynx before iNAP exhibited no obstruction after iNAP therapy. However, one patient shifted from no obstruction to complete obstruction after iNAP. Overall, the severity of obstruction in the oropharynx decreased in 13% (12/92) of the patients after iNAP therapy (p = 0.019) in supine position (Table 2).
After iNAP therapy with head rotation, the obstruction remained complete in 14 patients (70%; 14/20). A shift from complete to partial obstruction was noted in 4 patients (20%; 4/20), and the obstruction was completely resolved in 2 patients (10%; 2/20). One patient with partial obstruction of the oropharynx before iNAP exhibited no obstruction after iNAP therapy. Of note, five patients shifted from nonobstruction to partial/complete obstruction after iNAP. Overall, after simultaneously applying iNAP therapy and head rotation position, the severity of obstruction in the oropharynx did not exhibit significant improvement (7.6%, 7/92, P = 0.209; Table 2).
Obstruction Severity at the Tongue Base Before and After iNAP Therapy
In the supine position, 26 patients exhibited complete obstruction of the tongue base before iNAP therapy (Figure 6A and B). Subsequently, the obstruction remained complete in only 9 (34.6%; 9/26) patients. A shift from complete to partial obstruction was noted in 7 patients (26.9%; 7/26), and the obstruction was completely resolved in 10 patients (38.5%; 10/26; Figure 6C and D; Supplementary Video). Among the 27 patients with partial tongue base obstruction before iNAP, the obstruction status remained unchanged in 7 patients (25.9%; 7/27), and the obstruction was completely resolved in 19 patients (70.4%; 19/27). However, one patient shifted from partial obstruction to complete obstruction after iNAP and two patients shifted from no obstruction to partial obstruction after INAP, respectively. Overall, the severity of obstruction in the tongue base decreased among the patients (39%; 36/92; P < 0.001; Table 2).
After iNAP therapy with head rotation, a shift from complete to partial obstruction was noted in 8 patients (30.8%; 8/26), and the obstruction was completely resolved in 13 patients (50%; 13/26; Figure 7C and D; Figure 7A and B showed head rotation without iNAP therapy). In the 27 patients with partial obstruction, 22 (81.5%; 22/27) exhibited complete resolution of the obstruction, whereas one of them (3.7%; 1/27) shifted to complete obstruction. In addition, four patients shifted from no obstruction to partial/complete obstruction after iNAP. Overall, after simultaneously applying iNAP therapy and head rotation position, the severity of obstruction in the tongue base showed significant improvement in 47% of the patients (43/92, P < 0.001; Table 2).
Obstruction Severity at the Epiglottis Before and After iNAP Therapy
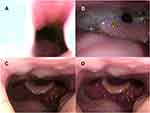
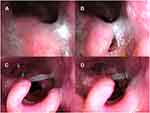
In the supine position, 5 (7.2%; 5/69) of the 69 patients without epiglottis obstruction before iNAP therapy experienced complete obstruction after iNAP therapy (Figure 8C and D; Supplementary Video) including anterior–posterior and lateral collapse. The obstruction status of 11 (64.7%; 11/17) of the 17 patients with complete obstruction before iNAP therapy (Figure 8A and B) remained unchanged after iNAP therapy. Thus, neither mitigation nor exacerbation of epiglottis obstruction was observed.
After iNAP therapy with head rotation, a shift from complete to partial obstruction was observed in 3 patients (17.6%; 3/17), and the obstruction was completely resolved in 11 patients (64.7%;11/17; Figure 9C and D; Figure 9A and B showed head rotation without iNAP therapy). Of the 6 patients with partial obstruction, 5 exhibited complete resolution, whereas one of them exhibited complete obstruction. In addition, two patients shifted from no obstruction to partial or complete obstruction after iNAP. Overall, after simultaneously applying iNAP therapy and head rotation position, the severity of obstruction in the epiglottis level significantly improved in 21% of the patients (19/92, P = 0.007; Table 2).
Complete Relief at All VOTE Sites After iNAP Therapy
The overall effect of iNAP therapy on alleviating the collapse of the entire airway, all four levels, is described in Table 3. Under the supine position, a shift from complete or partial obstruction to no obstruction was observed in 7 patients (7.7%) after iNAP therapy (P = 0.016). Under the supine position with head rotation, a shift from complete or partial obstruction to no obstruction was noted in 20 (22%) patients after iNAP therapy (P < 0.001).
 |
Table 3 Complete relief in all four sites of VOTE before and after intermittent negative iNAP therapy |
Baseline Characteristics Between the Responders and Nonresponders
We divided the patients into responders and nonresponders at each anatomical level. The responders at the velar level in the supine position had significantly lower BMIs, and those at the velar level on whom head rotation was performed were significantly older (Supplemental Table 1). However, other baseline characteristics including sex, age, AHI, and minimum SaO2, were not significantly different between the responders and nonresponders at any level, regardless of the patient position (Supplemental Tables 2–4).
Discussion
To the best of our knowledge, this is the first study to investigate the dynamic effects of iNAP therapy on upper airway obstruction in the supine position with head rotation and with TCI-DISE.
In the present study, after the application of iNAP therapy in the supine position, obstruction severity decreased significantly—from complete obstruction to partial or no obstruction in 34 of 83 (41%) patients with velar obstruction, 10 of 20 (50%) patients with oropharyngeal obstruction, and 17 of 26 (65.4%) patients with tongue base obstruction. Moreover, of the 27 patients with partial tongue base obstruction, no obstruction was noted in 19 (70.4%) patients after iNAP therapy. This finding is attributable to the fact that the intraoral and intermittent application of negative pressure reshapes soft tissues (ie, the tongue and soft palate) into a more forward-resting position.13 In one study, the iNAP device effectively improved the patency of the upper airway when the heads of patients with OSA syndrome were in the lateral position. The mechanism exhibited an increase in the distance between the tongue or soft palate and the back wall of the upper airway.12
Hung et al (2020) performed magnetic resonance imaging and reported that iNAP therapy could increase the volume of the upper airway and retropalatal region but did not exert a substantial effect on the retroglossal region.13 In other words, the effect of iNAP therapy on the hypopharyngeal airway was not notable; this explains why epiglottic collapse could not be adequately corrected by iNAP therapy alone in the present study. In addition, the pulling effect of the tongue after the application of iNAP therapy may increase the cross-sectional area of the lingual epiglottis, thereby exacerbating the anterior–posterior collapse. Nevertheless, this situation could be corrected through lateral head rotation. In the present study, applying iNAP therapy simultaneously with lateral head rotation significantly mitigated epiglottic obstruction. Thus, positional therapy accompanied with iNAP therapy may help alleviate epiglottic obstruction including floppy epiglottis.
After iNAP therapy, the number of patients with complete obstruction at the oropharyngeal level increased from 11 in the supine position to 17 in the head rotation position. This finding can be explained by the effect of positional therapy on oropharyngeal obstruction.36,37 Palatine tonsil collapse during the lateral rotation of the head resulted in a narrower lateral diameter at the oropharyngeal level. Lee et al (2015) concluded that the prevalence of lateral wall obstruction was not affected by positional changes.36 Beelen et al (2018) reported that when comparing the supine and lateral head rotation positions, the degree of obstruction at all levels except the oropharynx improved significantly.37
To investigate the overall effect of iNAP therapy on alleviating the collapse of the entire airway, we considered all the four obstruction levels together. We found that 7.7% of patients respond to iNAP therapy and were free of obstruction in the supine position. In addition, the overall obstruction appeared to be more remarkably alleviated while applying iNAP therapy and position therapy at the same time. Moreover, 22% of the patients were free of obstruction after iNAP therapy was applied together with head rotation. Both the aforementioned findings revealed significant differences (P = 0.016 and P < 0.001, respectively). However, the overall effect of iNAP therapy should be evaluated through PSG outcomes according to the OSA diagnostic standard. We focused more on the collapse pattern after iNAP therapy at each level of the upper airway in this study.
One study suggested the use of the iNAP device for the treatment of mild-to-moderate OSA.11 In an investigation of the therapeutic spectrum of the iNAP device, Hung et al (2020) reported that the best candidates for its use are individuals without obesity and with a baseline AHI between 5 and 50 events/h.12 The discrepancies in our study results indicated that neither the AHI nor BMI can be a favorable predictor.12 In the present study, the results of univariate analysis revealed no significant differences in baseline AHI. Regarding baseline characteristics, the velar-level respondents had significantly lower BMIs, and the velar-level respondents on whom head rotation was performed were significantly older. However, the validity of these results remains unclear. In sum, applying iNAP during TCI-DISE may be a new method for evaluating possible responders to this novel therapy. However, additional research is required for iNAP therapy on treating OSA.
Limitations
This study has some limitations. First, DISE itself has limitations. Its known limitations include the subjective scoring system; the various protocols involved may cause variability, and the degree of upper airway narrowing can be aggravated by the depth of sedation and can differ from that observable in natural sleep.31 To prevent this potential problem, we used the TCI system to control the depth of sleep. In addition, we did not evaluate the tone of upper airway muscles and the effect on the snoring level before or after iNAP therapy in the current study due to a lack of objective tool. According to a previous study conducted by Carlos O’Connor-Reina1et al, tongue peak pressure is a useful tool that helps in the identification of obstruction sites in patients with OSA/hypopnea syndrome.38 We would evaluate tongue peak pressure and snoring level in our ongoing study. Second, the examiner could not be blinded to the position of the patient or whether iNAP therapy was applied when assessing the DISE results. However, we invited two physicians (Dr. Hsu and Dr. Kuo) to review our DISE videos. Third, we used DISE findings as a proxy for treatment outcomes. Quantitative measures, such as the AHI, are preferred for their consistency and accuracy to OSA severity. Future research should examine the overall response of iNAP therapy and explore the potential of combining iNAP with positional therapy with prospective controlled trials.
Conclusions
In TCI-DISE, we found that iNAP therapy relieved velar, oropharyngeal, and tongue base obstruction in the supine position in some patients. Moreover, iNAP therapy can be combined with positional therapy to alleviate velar, tongue base, and epiglottic obstruction in some patients. TCI-DISE can also be used to screen the possible responders for iNAP therapy because it is less time consuming than PSG.
Acknowledgment
The authors thank the anonymous reviewers and editors for their comments.
Funding
This study was supported by a grant from Shin Kong Wu-Ho-Su memorial Hospital (2018SKHADR018). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Disclosure
Ofer Jacobowitz reports grants and personal fees from Livanova and personal fees from Nyxoah Medical, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi:10.1016/S2213-2600(19)30198-5
2. Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. doi:10.1016/j.jacc.2013.04.080
3. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/NEJM200005113421901
4. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N Engl J Med. 2005;353(19):2034–2041. doi:10.1056/NEJMoa043104
5. Engleman HM, Douglas NJ. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi:10.1136/thx.2003.015867
6. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi:10.1164/rccm.200504-637OC
7. Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi:10.1093/sleep/29.3.375
8. Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65(9):829–832. doi:10.1136/thx.2010.135848
9. Colrain IM, Black J, Siegel LC, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med. 2013;14(9):830–837. doi:10.1016/j.sleep.2013.05.009
10. Nigam G, Pathak C, Riaz M. Effectiveness of oral pressure therapy in obstructive sleep apnea: a systematic analysis. Sleep Breath. 2016;20(2):663–671. doi:10.1007/s11325-015-1270-3
11. Yamaguchi Y, Kato M. Pilot Study of Oral Negative Pressure Therapy for Obstructive Sleep Apnea-Hypopnea Syndrome. J Sleep Disord Ther. 2017;6(3). doi:10.4172/2167-0277.1000271
12. Hung T-C, Liu T-J, Hsieh W-Y, et al. A novel intermittent negative air pressure device ameliorates obstructive sleep apnea syndrome in adults. Sleep Breath. 2019;23(3):849–856. doi:10.1007/s11325-018-01778-z
13. Hung T-C, Liu T-J, Lu T-M, et al. Building a model to precisely target the responders of a novel intermittent negative air pressure device-with mechanism definition. Sleep Med. 2020;72:20–27. doi:10.1016/j.sleep.2020.03.014
14. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. doi:10.1164/rccm.201303-0448OC
15. Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper Airway Collapsibility (Pcrit) and Pharyngeal Dilator Muscle Activity are Sleep Stage Dependent. Sleep. 2016;39(3):511–521. doi:10.5665/sleep.5516
16. Lee CH, Kim DK, Kim SY, Rhee C-S, Won T-B. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: a DISE study. Laryngoscope. 2015;125(1):248–254. doi:10.1002/lary.24825
17. Sistla SK, Paramasivan VK, Agrawal V. Anatomic and Pathophysiologic Considerations in Surgical Treatment of Obstructive Sleep Apnea. Sleep Med Clin. 2019;14(1):21–31. doi:10.1016/j.jsmc.2018.11.003
18. Amos JM, Durr ML, Nardone HC, Baldassari CM, Duggins A, Ishman SL. Systematic Review of Drug-Induced Sleep Endoscopy Scoring Systems. Otolaryngol Head Neck Surg off J Am Acad Otolaryngol-Head Neck Surg. 2018;158(2):240–248. doi:10.1177/0194599817737966
19. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for Scoring Respiratory Events in Sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med JCSM off Publ Am Acad Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
20. Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16(5):504–509. doi:10.1111/j.1365-2273.1991.tb01050.x
21. Kezirian EJ. Drug-induced sleep endoscopy. Oper Tech Otolaryngol-Head Neck Surg. 2006;17(4):230–232. doi:10.1016/j.otot.2006.10.005
22. Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):1233–1236. doi:10.1007/s00405-011-1633-8
23. Lin H-Y, Lin Y-C, Hsu Y-S, et al. Comparison of Findings between Clinical Examinations and Drug-Induced Sleep Endoscopy in Patients with Obstructive Sleep Apnea Syndrome. Int J Environ Res Public Health. 2020;17:17. doi:10.3390/ijerph17176041
24. Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Neck Surg. 2010;136(4):393–397. doi:10.1001/archoto.2010.26
25. Rodriguez-Bruno K, Goldberg AN, McCulloch CE, Kezirian EJ. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg. 2009;140(5):646–651. doi:10.1016/j.otohns.2009.01.012
26. Blumen M, Bequignon E, Chabolle F. Drug-induced sleep endoscopy: a new gold standard for evaluating OSAS? Part I: technique. Eur Ann Otorhinolaryngol Head Neck Dis. 2017. doi:10.1016/j.anorl.2016.11.005
27. Blumen MB, Latournerie V, Bequignon E, Guillere L, Chabolle F. Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath. 2015;19(3):1021–1026. doi:10.1007/s11325-014-1107-5
28. Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope. 2012;122(2):473–479. doi:10.1002/lary.22474
29. Meraj TS, Muenz DG, Glazer TA, Harvey RS, Spector ME, Hoff PT. Does drug-induced sleep endoscopy predict surgical success in transoral robotic multilevel surgery in obstructive sleep apnea? Laryngoscope. 2017;127(4):971–976. doi:10.1002/lary.26255
30. Hsu YS, Jacobowitz O. Does Sleep Endoscopy Staging Pattern Correlate With Outcome of Advanced Palatopharyngoplasty for Moderate to Severe Obstructive Sleep Apnea? J Clin Sleep Med. 2017;13(10):1137–1144. doi:10.5664/jcsm.6756
31. De Vito A, Agnoletti V, Berrettini S, et al. Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques–a randomized controlled study. Eur Arch Oto-Rhino-Laryngol off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2011;268(3):457–462. doi:10.1007/s00405-010-1376-y
32. De Vito A, Agnoletti V, Zani G, et al. The importance of drug-induced sedation endoscopy (D.I.S.E.) techniques in surgical decision making: conventional versus target controlled infusion techniques—a prospective randomized controlled study and a retrospective surgical outcomes analysis. Eur Arch Otorhinolaryngol. 2017;274(5):2307–2317. doi:10.1007/s00405-016-4447-x
33. Chiu F-H, Chang Y, Liao -W-W, et al. Post-Operative Sleep Endoscopy with Target-Controlled Infusion After Palatopharyngoplasty for Obstructive Sleep Apnea: anatomical and Polysomnographic Outcomes. Nat Sci Sleep. 2021;13:1181–1193. doi:10.2147/NSS.S311702
34. Struys MM, Sahinovic M, Lichtenbelt BJ. Optimizing intravenous drug administration by applying pharmacokinetic/pharmacodynamic concepts | elsevier Enhanced Reader. Br J Anaesthesia. 2011;107:38. doi:10.1093/bja/aer108
35. Hohenhorst W, Ravesloot MJL, Kezirian EJ, de Vries N. Drug-induced sleep endoscopy in adults with sleep-disordered breathing: technique and the VOTE Classification system. Oper Tech Otolaryngol-Head Neck Surg. 2012;23(1):11–18. doi:10.1016/j.otot.2011.06.001
36. Lee CH, Kim DK, Kim SY, Rhee C-S, Won T-B. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: a DISE study. Laryngoscope. 2015;125(1):248. doi:10.1002/lary.24825
37. Beelen AMEH, Vonk PE, de Vries N. Drug-induced sleep endoscopy: the effect of different passive maneuvers on the distribution of collapse patterns of the upper airway in obstructive sleep apnea patients. Sleep Breath. 2018;22(4):909–917. doi:10.1007/s11325-018-1732-5
38. O’Connor-Reina C, Plaza G, Garcia-Iriarte MT, et al. Tongue peak pressure: a tool to aid in the identification of obstruction sites in patients with obstructive sleep apnea/hypopnea syndrome. Sleep Breath. 2020;24(1):281–286. doi:10.1007/s11325-019-01952-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.