Back to Journals » OncoTargets and Therapy » Volume 11
Novel EXO-T vaccine using polyclonal CD4+ T cells armed with HER2-specific exosomes for HER2-positive breast cancer
Authors Li R , Chibbar R, Xiang J
Received 22 August 2018
Accepted for publication 1 October 2018
Published 17 October 2018 Volume 2018:11 Pages 7089—7093
DOI https://doi.org/10.2147/OTT.S184898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Rong Li,1,2 Rajni Chibbar,3 Jim Xiang1,2
1Cancer Research Cluster, Saskatchewan Cancer Agency, Saskatoon, SK, Canada; 2Department of Oncology, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada; 3Department of Pathology, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Abstract: Breast cancer is the leading cause of death in women globally. The human epidermal growth factor receptor 2 (HER2)-positive breast cancer is often associated with poor prognosis and high mortality. Even though anti-HER2 monoclonal antibodies have improved the clinical outcome, resistance to the antibody therapy becomes a major obstacle in the treatment of HER2-positive breast cancer patients. Alternative approaches are therefore needed. HER2-specific vaccines have been developed to trigger patient’s immune system against HER2-positive breast cancer. This article describes the development of novel HER2-specific exosome (EXO)-T vaccine using polyclonal CD4+ T cells armed with HER2-specific dendritic cell-released EXO and demonstrates its therapeutic effect against HER2-positive tumor in double-transgenic HER2/HLA-A2 mice with HER2-specific self-immune tolerance. Therefore, our novel HER2-specific EXO-T vaccines are likely to assist in the treatment of HER2-positive breast cancer patients.
Keywords: EXO-T vaccine, polyclonal CD4+, T cell, HER2, exosome, breast cancer
Introduction
Breast cancer is the most commonly diagnosed cancer and the second most common cause of cancer death in women in the USA.1–3 The human epidermal growth factor receptor 2 (HER2) oncogene encodes for a 185 kD transmembrane glycoprotein receptor with intracellular tyrosine kinase activity.4 It belongs to the human EGFR family including HER1, HER2, HER3, and HER4 that control breast cancer cell proliferation, migration, and invasion.5 Amplification of HER2 is observed in approximately 20% of human breast cancers.6–8 HER2-positive breast cancer is associated with increased rates of metastasis, reduced time to relapse, poorer prognosis, and higher mortality.6,9 Development of HER2-targeted immunotherapeutics such as HER2-specific monoclonal antibodies trastuzumab and lapatinib has greatly improved therapeutic outcome.10 Trastuzumab is remarkably effective both as monotherapy and in combination with cytotoxic chemotherapy in patients with HER2-positive metastatic breast cancer. However, most patients sooner or later develop resistance to trastuzumab during trastuzumab treatment,11,12 warranting the development of other effective HER2-targeted therapies.
Three signals in CD8+ T cell response
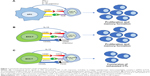
CD8+ cytotoxic T lymphocytes (CTLs) are responsible for adaptive immune responses against tumor. After antigen presentation to naïve CD8+ T cells by antigen-presenting cells, such as dendritic cells (DCs), CD8+ T cells start to proliferate and become cytotoxic effectors capable of inducing cancer cell death via secreting cytokines (tumor necrosis factor-α and interferon-γ [IFN-γ]) and cytolytic granzyme-B.13 There are three conventional signals participating in induction of CD8+ CTL immunity. The first signal is derived from the antigen peptide-presenting major histocompatibility complexes (pMHC-I) on DCs, which recognize the antigen-specific T-cell receptors (TCRs) on CD8+ T cells (Figure 1A). The second costimulatory signal is the interaction of DC’s costimulatory CD80 molecules with CD28 ligands on CD8+ T cells (Figure 1A). The third signal represents the innate inflammatory cytokines such as IL-12 and IFN-α-stimulating CD8+ T cells (Figure 1A). The first two signals are responsible for naïve CD8+ T-cell proliferation, while IL-12 and IFN-α are in charge of the development of CTL effector functions.13 Apart from those signals, IL-15 secreted by DCs induces T-cell memory formation.13
Exosome-targeted polyclonal CD4+ T cell vaccine
Some HER2-positive breast cancer patients have been found to develop spontaneous anti-HER2-specific immunity with both antibody and CD8+ T-cell responses,14,15 indicating that HER2 is an immunogenic target for the development of anti-HER2 vaccines to stimulate patient’s own immune system against breast cancer. HER2-specific vaccines using HER2-specific peptides, proteins, DNA, or DCs have been developed, but mostly showing relatively limited antitumor effects.16
Exosomes (EXOs) are small vesicles of 50–100 nm in diameter secreted by budding from the cellular membrane.17 DC-released EXOs are enriched in immunological molecules important for DC’s stimulatory machinery.17 Similar to the previous adoptive engineered CD8+ T-cell therapy using active polyclonal CD8+ T cells engineered to express tumor-specific TCR,18 we developed novel CD4+ T-cell-based (EXO-T) vaccines using active polyclonal CD4+ T cells armed with tumor-specific DC-released EXOs.19–25 In the former one, polyclonal CD8+ T cells are genetically engineered to express tumor-specific TCRs containing signaling domain of CD3 zeta-chain or to express chimeric antigen receptor containing single-chain Fv fused to signaling domain of T-cell costimulatory molecules such as 41BB leading to the currently well-known chimeric antigen receptor-T therapy.26 In the latter one, EXO-T vaccines prepared by simply incubation of ConA-stimulated polyclonal CD4+ T cells with antigen-specific DC-released EXOs. The polyclonal CD4+ T cells took up antigen-specific DC-released EXOs via interaction of exosomal CD54 with T cell lymphocyte function-associated antigen 1, leading to the expression of exosomal surface molecules (pMHC-I and CD80) on CD4+ T cells via vesicle internalization/recycling and direct membrane fusion.19 As a result, the polyclonal CD4+ T cells phenotypically armed with antigen-specific exosomal pMHC-I complexes and exosomal CD80 molecule became antigen-specific EXO-T vaccines.19–25
Compared with vaccination of DCs presenting the three conventional signals, EXO-T vaccines stimulate CD8+ T-cell responses via three distinct signals namely 1) acquired exosomal pMHC-I, 2) acquired exosomal CD80 and CD4+ T cell CD40L, and 3) CD4+ T cell IL-2 (Figure 1B). EXO-T vaccines have been found to stimulate potent CD4+ T-cell-independent CTL responses19–25 and to promote CTL memory via CD4+ T cell CD40L signaling.20
CD8+ CTL exhaustion with overexpression of inhibitory molecules such as PD-1, Tim-3, and LAG-3 and with functional deficiency in the production of effector cytokine IFN-γ and effector cytolytic granzyme-B is a state of dysfunction that commonly occurs during cancer and infection diseases, which leads to failure in reducing viral or tumor load.27 We demonstrated that EXO-T vaccine was able to convert CTL exhaustion in chronic infection via CD4+ T cell CD40L signaling-induced activation of mTORC1 pathway, leading to CTL proliferation, IFN-γ production, and rescuing CTL cytotoxic effect.21 Because tumor-specific effector CTLs that undergo tumor tolerogenic microenvironment also become terminally differentiated into exhausted CTLs without any antitumor properties,28 our novel EXO-T vaccine may thus be able to exert its conversional effect on exhausted CTLs within tumors (Figure 1C).
HER2-Texo vaccine
We have recently developed Neu-specific (the rat’s form of human HER2) or HER2-specific EXO-T vaccines (Neu-Texo and HER2-Texo) using active polyclonal CD4+ T cells with uptake of Neu- or HER2-specific DC-released EXOs.22 We demonstrated that Neu-specific EXO-T vaccine stimulated Neu-specific CTL responses against Neu-expressing breast cancer Tg1-1 in transgenic FVBneuN mice, while HER2-specific EXO-T vaccine stimulated HER2-specific immunity against HER2/HLA-A2-expressing BL6-10A2/HER2 melanoma in double transgenic HER2/HLA-A2 mice with HER2-specific self-immune tolerance.22 In addition, HER2-specific EXO-T-stimulated CTLs also showed potent therapeutic effect against both HER2-positive breast cancer T47D and trastuzumab-resistant HER2-positive breast cancer BT474 in athymic nude mice.22 Heterologous DNA vaccines composed of fused cDNA fragments encoding chimeric NH2-terminal human HER2 and COOH-terminal rat Neu sequences have been reported to stimulate stronger antibody responses and protective antitumor immunity than either HER2 or Neu DNA vaccine in transgenic mice with HER2-specific self-immune tolerance.29,30 These findings prompted us to have a hypothesis that heterologous HER2/Neu-specific T cell vaccine may induce more effective anti-HER2 CTL responses. To test this hypothesis, we construct an adenoviral vector (AdVHER2/Neu) expressing a fused cDNA fragment (Hu/Rt HER2/Neu) encoding chimeric NH2-terminal human (Hu) HER2 and COOH-terminal rat (Rt) Neu sequence by recombinant DNA technology.31 Based on AdVHER2/Neu, we further generated heterologous HuRt HER2/Neu-specific EXO-T vaccine (HuRt-Texo) using polyclonal CD4+ T cells with uptake of AdVHER2/Neu-transfected DC-release EXOs.31 We demonstrated that heterologous HuRt-Texo vaccine, in comparison with homologous HER2-Texo one, more strongly stimulated both HER-2-specific antibody and CTL responses leading to complete inhibition of growth of established lung metastasis of HER2-expressing 4T1HER2 breast cancer in BALB/c mice and complete protection of transgenic HLA-A2/HER2 mice from growth of HLA-A2/HER2-expressing BL6-10A2/HER2 melanoma in double transgenic HER2/HLA-A2 mice.31 In addition, HuRt-TEXO-stimulated CTLs are also able to eradicate established trastuzumab-resistant BT474 breast cancer in athymic nude mice.31
The long-term goal is to develop human therapeutic HER2/Neu-specific EXO-T vaccine using autologous polyclonal T cells with uptake of HER2/Neu-specific autologous DC (DCHER2/Neu)-released EXOs as a new novel personalized vaccine for breast cancer.32 The human autologous DCs derived from peripheral blood monocytes activated in culture medium by granulocyte-macrophage colony-stimulating factor, IL-4, and tumor necrosis factor-α33 followed by infection with HER2/Neu-specific adenoviral vector (AdVHER2/Neu) to form DCHER2/Neu.22
Conclusion
Taken together, our data indicate that HER2-specific EXO-T vaccine circumventing HER2 tolerance may provide a new therapeutic alternative for trastuzumab-resistant breast cancer patients with HER2-specific self-immune tolerance. Because many other human cancer antigens were also identified including α-fetal protein, carcinoembryonic antigen, CA125, CA19-9, and prostate-specific antigen in various types of cancer,34 novel EXO-T vaccines similarly generated by arming polyclonal CD4+ T cells with different tumor antigen-specific EXOs are thus likely to become a useful therapeutic strategy to assist in the treatment of various cancers.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. | ||
Pisani P, Parkin DM, Bray F, Ferlay J. Erratum: Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83(1):18–29. | ||
King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229(4717):974–976. | ||
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. | ||
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, Mcguire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. | ||
Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004;291(16):1972–1977. | ||
Owens MA, Horten BC, da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–69. | ||
Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–5706. | ||
Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. | ||
Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078–2099. | ||
Gampenrieder SP, Rinnerthaler G, Greil R. Neoadjuvant chemotherapy and targeted therapy in breast cancer: past, present, and future. J Oncol. 2013;2013:732047. | ||
Ramanathan S, Gagnon J, Dubois S, Forand-Boulerice M, Richter MV, Ilangumaran S. Cytokine synergy in antigen-independent activation and priming of naive CD8+ T lymphocytes. Crit Rev Immunol. 2009;29(3):219–239. | ||
Disis ML, Calenoff E, Mclaughlin G, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994;54(1):16–20. | ||
Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A. 1995;92(2):432–436. | ||
Vokes EE, Salgia R, Karrison TG. Evidence-based role of bevacizumab in non-small cell lung cancer. Ann Oncol. 2013;24(1):6–9. | ||
Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5(4):261–269. | ||
June CH, Maus MV, Plesa G, et al. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63(9):969–975. | ||
Hao S, Yuan J, Xiang J. Nonspecific CD4(+) T cells with uptake of antigen-specific dendritic cell-released exosomes stimulate antigen-specific CD8(+) CTL responses and long-term T cell memory. J Leukoc Biol. 2007;82(4):829–838. | ||
Xie Y, Wang L, Freywald A, Qureshi M, Chen Y, Xiang J. A novel T cell-based vaccine capable of stimulating long-term functional CTL memory against B16 melanoma via CD40L signaling. Cell Mol Immunol. 2013;10(1):72–77. | ||
Wang R, Xu A, Zhang X, et al. Novel exosome-targeted T-cell-based vaccine counteracts T-cell anergy and converts CTL exhaustion in chronic infection via CD40L signaling through the mTORC1 pathway. Cell Mol Immunol. 2017;14(6):529–545. | ||
Wang L, Xie Y, Ahmed KA, et al. Exosomal pMHC-I complex targets T cell-based vaccine to directly stimulate CTL responses leading to antitumor immunity in transgenic FVBneuN and HLA-A2/HER2 mice and eradicating trastuzumab-resistant tumor in athymic nude mice. Breast Cancer Res Treat. 2013;140(2):273–284. | ||
Nanjundappa RH, Wang R, Xie Y, et al. GP120-specific exosome-targeted T cell-based vaccine capable of stimulating DC- and CD4(+) T-independent CTL responses. Vaccine. 2011;29(19):3538–3547. | ||
Nanjundappa RH, Wang R, Xie Y, Umeshappa CS, Xiang J. Novel CD8+ T cell-based vaccine stimulates Gp120-specific CTL responses leading to therapeutic and long-term immunity in transgenic HLA-A2 mice. Vaccine. 2012;30(24):3519–3525. | ||
Wang R, Xie Y, Zhao T, Tan X, Xu J, Xiang J. HIV-1 Gag-specific exosome-targeted T cell-based vaccine stimulates effector CTL responses leading to therapeutic and long-term immunity against Gag/HLA-A2-expressing B16 melanoma in transgenic HLA-A2 mice. Trials Vaccinol. 2014;3:19–25. | ||
Moon EK, Wang LC, Dolfi DV, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20(16):4262–4273. | ||
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. | ||
Milad S-G, Hedayatollah S, Mahmoud R-K, Mahdi G-S, Mojtaba S. The role of inflammatory cytokines in creating T cell exhaustion in cancer. Cancer Biother Radiopharm. 2018;33:1–7. | ||
Quaglino E, Mastini C, Amici A, et al. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res. 2010;70(7):2604–2612. | ||
Jacob JB, Quaglino E, Radkevich-Brown O, et al. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer Res. 2010;70(1):119–128. | ||
Xie Y, Wu J, Xu A, et al. Heterologous human/rat HER2-specific exosome-targeted T cell vaccine stimulates potent humoral and CTL responses leading to enhanced circumvention of HER2 tolerance in double transgenic HLA-A2/HER2 mice. Vaccine. 2018;36(11):1414–1422. | ||
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. | ||
Ye Z, Chen Z, Sami A, El-Gayed A, Xiang J. Human dendritic cells engineered to express alpha tumor necrosis factor maintain cellular maturation and T-cell stimulation capacity. Cancer Biother Radiopharm. 2006;21(6):613–622. | ||
Vigneron N. Human tumor antigens and cancer immunotherapy. Biomed Res Int. 2015;2015:948501–948517. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.