Back to Journals » International Journal of Nanomedicine » Volume 15
Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications
Authors Rahman HS , Othman HH , Hammadi NI , Yeap SK, Amin KM , Abdul Samad N, Alitheen NB
Received 19 August 2019
Accepted for publication 10 October 2019
Published 15 April 2020 Volume 2020:15 Pages 2439—2483
DOI https://doi.org/10.2147/IJN.S227805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Heshu Sulaiman Rahman,1,2 Hemn Hassan Othman,3 Nahidah Ibrahim Hammadi,4 Swee Keong Yeap,5 Kawa Mohammad Amin,6 Nozlena Abdul Samad,7 Noorjahan Banu Alitheen8
1Department of Physiology, College of Medicine, University of Sulaimani, Sulaymaniyah 46001, Republic of Iraq; 2Department of Medical Laboratory Sciences, College of Health Sciences, Komar University of Science and Technology, Sulaymaniyah, Republic of Iraq; 3Department of Pharmacology and Toxicology, College of Pharmacy, University of Sulaimani, Sulaymaniyah 46001, Republic of Iraq; 4Department of Histology, College of Veterinary Medicine, University of Al-Anbar, Ramadi, Republic of Iraq; 5China-ASEAN College of Marine Sciences, Xiamen University Malaysia, Sepang, Malaysia; 6Department of Microbiology, College of Medicine, University of Sulaimani, Sulaymaniyah 46001, Republic of Iraq; 7Integrative Medicine Cluster, Institut Perubatan dan Pergigian Termaju (IPPT), Sains@BERTAM, Universiti Sains Malaysia, Kepala Batas 13200, Pulau Pinang, Malaysia; 8Department of Cell and Molecular Biology, Faculty of Biotechnology and Bio-Molecular Sciences, Universiti Putra Malaysia, Selangor, Malaysia
Correspondence: Noorjahan Banu Alitheen
Department of Cell and Molecular Biology, Faculty of Biotechnology and Bio-Molecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
Tel +60 126866947
Email [email protected]
Heshu Sulaiman Rahman
Department of Physiology, College of Medicine, University of Sulaimani, Sulaymaniyah 46001, Republic of Iraq
Tel +964 7726159598
Email [email protected]
Abstract: Many types of research have distinctly addressed the efficacy of natural plant metabolites used for human consumption both in cell culture and preclinical animal model systems. However, these in vitro and in vivo effects have not been able to be translated for clinical use because of several factors such as inefficient systemic delivery and bioavailability of promising agents that significantly contribute to this disconnection. Over the past decades, extraordinary advances have been made successfully on the development of novel drug delivery systems for encapsulation of plant active metabolites including organic, inorganic and hybrid nanoparticles. The advanced formulas are confirmed to have extraordinary benefits over conventional and previously used systems in the manner of solubility, bioavailability, toxicity, pharmacological activity, stability, distribution, sustained delivery, and both physical and chemical degradation. The current review highlights the development of novel nanocarrier for plant active compounds, their method of preparation, type of active ingredients, and their biomedical applications.
Keywords: nanomedicine, natural plant metabolite, biomedical application, carrier formulation, drug delivery
Introduction
Based on the recently reported data, more than 70% of new drugs formulated are showing poor water solubility, which becomes the limiting factor in the absorption drug after oral admission.1 The limitation in the bioavailability of natural products active components includes poor solubility of the ingredient, poor stability due to gastric and colonic acidity, poor metabolism by the effect of gut microflora, poor absorption across the intestinal wall, poor active efflux mechanism and first-pass metabolic effects are among the factors that make the failure of clinical trials.2,3
In this respect, developed novel drug delivery system and carriers for herbal drugs should ideally accomplish some prerequisites such as proper delivering of the drug at a rate oriented by the needs of the body, over the period of treatment and it should pass the active entity of herbal drug to the site of action.4 Many approaches have been adopted to increase drug solubility, sustainability, bioavailability and gastrointestinal permeability.5 Nanocarrier has gained tremendous attention in the development of new pharmaceutical carrier and delivery systems. One of the strategies to thwart this problem is to encapsulate natural plant metabolites into the biodegradable and biocompatible nanoparticle.6
Employment of innovative drug delivery systems including utilization of nanocarrier delivery to overcome the physicochemical and pharmacokinetic limitation of phytochemicals enhanced the controlled release and even efficacy of the bioactivities. This innovation shows the promising future of nanomedicine as a potential solution for impressive hindrance and handling of various chronic diseases.7
Additionally, altering the main features of nanocarriers such as their constituents (organic, inorganic or hybrid), sizes (small, medium or large), shapes (sphere, rod or cube) and surface properties (charge, functional groups, PEGylation or attachment of targeting moieties) are considered as a leading cause for tuning the physiochemical properties of nanocarriers. The overall aim of employing nanocarriers in drug delivery is to treat an unwellness effectively with the lowest side effects and potential outcomes.8
Nanomedicine has recently earned enhanced attraction for its ability to efficaciously diagnose and treat various ailments.9 Therefore, the aim of this review is to display the types of nanocarrier loaded natural plant products and focus on their role in various disease therapies with the promising use of nanomedicine.
Design of the Review
In this review, nanocarriers were being classified based on the types of nanocarrier, i.e. i) organic nanocarriers; ii) inorganic nanocarriers; iii) hybrid nanocarriers; and iv) biological nanocarriers. References were searched in Scopus data based using each class of nanocarriers as the keyword. Articles after the year 2010 were selected (unless the significant references for a particular type of nanocarrier, which were downloaded separately) and sorted based on the specific type of carrier for each of the above classes.
Nanocarrier
Nanocarrier is hopefully utilized to overcome the difficulty and issues related to conventional drug delivery systems such as their nonspecificity, side effects, burst release and detrimental destroying of large populations of the normal cells. Nanocarrier improves the bioavailability and therapeutic efficiency of drugs, as well as providing a preferential accumulation at the target site.10 Nowadays, a large number of nanocarriers have been produced but only some of them are clinically authorized for the delivery of materials because of their motivated actions at the targeted sites, especially antitumor agents.11
The particles of a nanocarrier vary in size, and those ranged from 10 to 100 nm give the most acceptable physicochemical characteristics. The main advantages of nanonization are improving solubility, reducing medicinal doses and side effects, and increasing the absorbency of medicinal herbs compared with the respective crude extract preparations.12
Types of Nanocarrier
Organic Nanocarrier
Lipid and Polymer-Based Nanocarrier
Lipids act as a suitable penetration enhancer of drugs in the digestive tract by supporting solubilization of the drug in the stomach surroundings and thereby reducing the first-pass metabolism by diffusion of the drug through a lymphatic to the circulatory system.10
SLN is a colloidal drug carrier that developed in the early 1990s in which the particle size ranges from 50 to 1000 nm (Figure 1A). SLN is processed by using emulsifier(s) to stabilize the dispersion that composed of melted solid lipid(s) in water.13 The high-pressure homogenization (HPH) technique and microemulsification are the most commonly used methods for preparing SLN.14
 |
Figure 1 A schematic illustration of nanostructured Lipid Carrier (NLC) on right and solid lipid nanoparticles (SLN) on left Notes: Reproduced from Hsu CY, Wang PW, Alalaiwe A, Lin ZC, Fang JY. Use of lipid Nanocarriers to improve Oral delivery of vitamins. Nutrients. 2019;11(1):68-97325 |
The main advantages of SLN are providing a highly lipophilic lipid matrix for drugs to be dispersed in,15 allowing the encapsulation, embedding with a wide range of molecules (such as drugs, antigens, proteins, and nucleotides) and also promoting the delivery of therapeutic loading into specific tissues and cells. Improving the in vitro and in vivo stability and reducing the adverse effects are also among the acceptable features of SLN.16 SLN is quite similar to nanoemulsions except that both solid and liquid lipids (oils) are used in the formulation of SLN whereas only liquid lipids are used in nanoemulsions.
The most extensively employed SLN is puerarin-loaded SLN in rats that characterized by rapid absorption, relatively improved bioavailability and increased tissue concentrations in targeted organs (heart and brain).15,17 Another group developed triptolide-loaded SLN as an antioxidant and anti-inflammatory product that showed a significant reduction in glutathione (GSH) and myeloperoxidase (MPO) activities. The aim of this development was to improve solubility, reduce toxicity, hyperemia, and irritation to the gastrointestinal tract (GIT)18 through minimizing direct contact with the mucosal surface, gradual drug-releasing, and avoiding high local drug concentrations. More examples in this respect are addressed in Table 1.15,17–21
It is considered as a second-generation lipid nanoparticle that contains a mixture of solid and liquid lipids (Figure 1B) and was originally developed from SLN but with more lipid matrix imperfections.22 A wide variety of solid lipids have been utilized such as hydrogenated palm oil (HPO), glyceryl monostearate, stearic acid, and cetyl alcohol whereas the most commonly used liquid lipids are olive oil, mustard oil, castor oil, and cod liver oil. The preferable stabilizer in this system is thimerosal.23
Generally, NLC preferred over the SLN because of better controlling of the drug release, more stability, enhanced drug-loading capacity, and minimized drug ejection during depository.24 Thus, various active ingredients have been incorporated into NLC in studies focused on modifying water solubility, enhancing gastrointestinal absorption and oral bioavailability, controlling release, lengthening circulation time by reducing identification by the reticuloendothelial system (RES), and co-delivery.12 Therefore, it is realized that NLC is a better carrier for oral delivery of several natural and chemically synthesized compounds.
In this respect, silymarin loaded NLC is the best example that has been used clinically to overcome many hepatic diseases as its low solubility, permeability, and bioavailability often occur with its therapy. NLC loaded tripterine, curcumin, and triptolide are also other successful examples of corroborated absorption enhancement by this system which may be due to their small particle size, lipid components, and surfactant contents.25
Cardomom essential oil (CEO) loaded NLCs have successfully been synthesized using food-grade lipids including cocoa butter and olive oil. The CEO loaded NLCs had a small size (90%), loading capacity (>25%) and provides good physical and chemical stability. This work overcame the limitation of applying the CEO to aqueous-based foods.26 Currently, various novel and innovative NLC have been produced as a carrier to target anticancer functions such as zerumbone,27 thymoquinone28,29 and citral30 and as a worthy drug observably increased antitumor activity in leukemia and breast tumor cells in vitro and in vivo. More examples of compounds loaded NLC are presented in Table 1.31–37
 |  |  |  |  |  |  |  |  |  |  |  |  |
Table 1 Nanocarrier Encapsulated Herbal Formulations |
It refers to an optically single isotropic and thermodynamic stable transparent (translucent) nonhomogeneous colloidal dispersion system (Figure 2) with a droplet size of less than 100 nm. Generally, the NE is composed of stabilized oil and water with the aid of surfactant and cosurfactant an interfacial film molecule.9
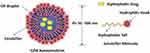 |
Figure 2 A schematic illustration of oil (O) in water (W) nanoemulsion. Notes: Reproduced from Agnihotri N, Soni GC, Chanchal DK, Tiwari S. A Scientific Review On Nanoemulsion For Targeting Drug Delivery System. Int J Life Sci Rev. 2019;5(2):16-29326 |
After the lipophilic drugs loaded into either oil/water or oil/water/oil suspension, the oil driblets are engulfed by the macrophage and find in a high concentration in the spleen, liver, and kidneys since the quantity of the liquefied medicate is too big. Whereas the hydrophilic drug is encapsulated into water/oil or water/oil/water nanoemulsion, it can be well condensed in the lymphatic system through subcutaneous or intramuscular insertion due to the higher internal membrane permeability.9,38
This system is characterized by targeted sustained release, the stability of the solubilized components, enhanced the permeability of materials to the mucous and skin, solubilized components of varied lipophilicity, improved drug absorption, lowered viscosity with inducing less pain or allergic reactions and simpleness of production and decontamination as well.39 Moreover, the intestinal absorption of NE is attributed to the lymphatic conveyance processes that ameliorate the oral bioavailability of encapsulated materials.40,41
NE serves as an attractive vehicle for the delivery of drugs and essential oils (especially as repellent and antimicrobial agents, nucleic acids as well as imaging agents).39,42,43 In the last few years ago, a modern system has upgraded the transdermal remedial use of NE, such as Transcutol®P, phospholipid, alkyl polyglycosides, PEGylated fatty acid ester, and fatty alcohol.44–46
Herbal drugs including camptothecin, rutin, genistein, resveratrol, and oils of Brucea javanica, coixenolide, and zedoary have been loaded into NE for various applications.47,48 With great application prospects of NE, Syagrus romanzoffiana fruit pulp extracts were incorporated into O/W NE using the phase inversion method to evaluate antioxidant activity.49 More examples of herbal loaded NE are presented in Table 1.51–53
It is a nanovesicular colloidal dispersion system (Figure 3) that exhibits a typical core-shell structure in which the drug is confined to a reservoir or within a cavity surrounded by a polymer membrane or coating.52 The cavity can contain the active substance in liquid (an oily or an aqueous core) or solid form or as a molecular in which the core-shell structure and composition are the main features of NC especially controlling the drug release.54 Likewise, this formula can be lipophilic or hydrophobic according to the preparation method and raw materials used. The main aim in developing this formula is to alter the oral bioavailability of ailing hydrophilic active components.55
 |
Figure 3 A schematic illustration of silver-loaded titanium dioxide nanocapsule. Notes: Adapted from Hérault N, Wagner J, Abram SL, et al. Silver-Containing Titanium Dioxide Nanocapsules for Combating Multidrug-Resistant Bacteria. Int J Nanomed. 2020;15:1267-1281327 |
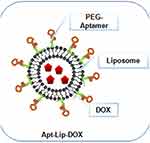 |
Figure 4 A schematic illustration of Polyethylene glycate (PEG)-aptamer-liposome-doxorubicin (DOX); a type of lipid drug-conjugate. Notes: Reproduced from Dou XQ, Wang H, Zhang J, et al. Aptamer–drug conjugate: targeted delivery of doxorubicin in a HER3 aptamer-functionalized liposomal delivery system reduces cardiotoxicity. Int J Nanomed. 2018;13:763-776328 |
Additionally, as asserted by different authors, other advantages of NC as a carrier system include high drug encapsulation efficiency due to optimized drug solubility in the core, low polymer content compared to other systems, drug polymeric shell protection against degradation factors like pH and light and the reduction of tissue irritation due to the polymeric shell.56 Recently, a ligand-modified or multifunctional NC that carries the active substance on their surfaces or imbibed in the polymeric membrane has been developed to attain higher delivery of therapies to the targeted site more actively.54 Different preparation methods such as nanoprecipitation, emulsion–diffusion, double emulsification, emulsion-coacervation, polymer-coating, and layer-by-layer were employed to develop various types of this carrier.55
In this area, the well-known anticancer natural herbal product, artemisinin (ART) (from Artemisia annua) crystals were encapsulated with polyelectrolytes (chitosan, gelatin, and alginate) for the purpose of controlled release through self-assembly of polyelectrolytes on drug crystals, and improved hydrophilicity of the crystal using the layer-by-layer technique.54 More examples of herbal loaded NE are presented in Table 1.57–62
The union of agents with polymers is a new approach to modify drug property and its pharmacokinetics. LDCs are lipidic drugs that covalently or noncovalently coupled to a lipid moiety, such as diglyceride, phosphoglyceride and fatty acid (Figure 4).63 In several instances, LDC may also be known as Pharmacosomes especially when the drug is conjugated with a phospholipid. LDC is the most accepted lipid-based nanoparticle, especially when considered drug is hydrophilic in nature in which it is converted into water-insoluble lipid-drug conjugate by conjugating it with a lipid component.64
LDC is characterized by possessing controlled drug release, drug targeting, an increase in gastrointestinal permeability, an increment in bioavailability.65 Additionally, adding targeted motifs to the polymer to produce functionalized polymer–drug conjugate can also be constructed. One of the appreciable natural products with high edible polyphenolic content is resveratrol that is widely known to be used for improving age-related diseases such as cancers of various organs and Alzheimer’s disease. Resveratrol efficacy was halted significantly due to its instability, and solubility especially in vivo model. Thus, resveratrol conjugated transferrin (Tf)-modified polyethylene glycol-polylactic acid (PEG-PLA) nanoparticle (Tf-PEG-PLA-RSV) was developed to target transferrin receptor overexpression in C6 glioma cells in vitro and to inhibit tumor maturation in rats induced with C6 glioma.66 More examples of herbal loaded NE are presented in Table 1.67–71
A liposome is a spherical shaped polar lipid nanoparticle that encapsulates an aqueous core by single or multiple natural or synthetic lipid bilayer membranes, in which it freely diffuses into its interior (Figure 5A).72 A liposome is known to have both hydrophilic and lipophilic groups on the same molecules and thus it can load both hydrophilic and lipophilic materials and can have single or multiple homocentric membranes as well.73
The pharmacokinetic profiles of drugs, herbs, vitamins, and enzymes can be modified extraordinarily by encapsulating them with liposomes for the purpose of preparing vaccines, cosmetics, and nutraceuticals.74 Because of liposome’s unique feature of having phospholipid bilayers as well as accommodating both water-soluble and lipid-soluble agents, it is able to enhance the solubility, bioavailability, delivery, intracellular uptake and biodistribution performance of the products both in vitro and in vivo.75,76 Additionally, defending of active drug from environmental factors, overwhelming primal destruction of the loaded material, less costly and prompt treatment with minimum systemic morbidness that has magnified their use in biomedicine formulations.4
The most commonly used polymers to elongate their half-life, as well as stability, are PEG and poly(lactic-co-glycolic acid) (PLGA). On the other hand, antibodies or ligands can be conjugated to liposome in order to enhance their target specificity, such as incorporating of curcumin into liposomes coated with PSMA antibodies by Thangapazham et al to enhance targeted delivery of curcumin for prostate cancer. They used LNCaP and C4-2B human prostate cancer cell lines in their study and realized that treatment of cells with liposomal curcumin leading to at least 70–80% inhibition of cellular proliferation without affecting their viability, with a 10-fold dose advantage over free curcumin.77 More examples of herbal loaded NE are presented in Table 1.78–83
The conventional liposomes do not deeply penetrate the skin and remain confined to the outer layer of stratum corneum. Thus, new classes of lipid vesicles such as transferosomes have been developed as an enhanced type of liposomes,11 which is an ultra-flexible lipid-based elastic vehicle with highly deformable membranes that enhance the sending of materials to deeper skin tissues through a nonoccluded method which penetrates the intercellular lipid lamellar regions of stratum corneum due to the hydration or osmotic force of the skin.84
It is composed of phospholipid and a single chain surfactant that provides elasticity and deformability to the vesicles (Figure 5B) that can be used topically for the purpose of supplying the nutrients locally to maintain the skin.85 This unique infrastructure endows transfer some to entrap hydrophilic, lipophilic, and amphiphilic drugs and thereof it can be utilized as drug carriers for small molecules, peptides, proteins, and herbal components as well as it can accommodate drug molecules with a wide range of solubility.9 On the other hand, chemically, transfersomes are not stable because of their predisposition to oxidative degradation and the purity of natural phospholipids is another criterion militating against the adoption of transfersomes as drug delivery vehicles.86
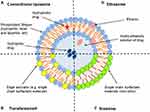 |
Figure 5 A schematic illustration of liposome (A), transferosome (B), niosome (C) and ethosome (D).Notes: Adapted with permission from Frontier in Pharmacology. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286.324 |
Additionally, transferosomes are not difficult to scale up, as the process is simple, easy to scale up without using pharmaceutically unsatisfactory additives.87 In this respect, ginsenoside Rh1 from Red ginseng (the steamed root of Panax ginseng C. A. Mayer) transferosome has been developed for skin maintenance that provided significantly higher skin penetration and higher topical absorption in comparison to ethosome and conventional liposome using rat dorsal skin in vitro.88 More examples of herbal loaded transferosomes are presented in Table 1.89–93
Niosome is a nonionic nanosphere vesicle with a diameter of 100 nm to 2 um, in which its center is watery that surrounded by layers of nonionic amphiphilic lipids in lamellar phase (Figure 5C).94 It is prepared by thin-film hydration method, sonication, microfluidization, multiple membrane extrusion, reverse phase evaporation technique, remote loading, bubble method and proniosome pre-formulation technique.95
Niosome is almost similar to liposome in structure but with more penetrating capability, more stability and therapeutic index of a drug, and less toxicity, thus it could offer more advantages over liposome.96 The advantages of niosome include cost-effectiveness, high solubility and flexibility and controlled release of its content. Therefore, they have been utilized widely as a targeting vehicle for neoplasia or as peptide carrier, hemoglobin carrier, and transdermal delivery.97
In tropical application, niosomes were also showed prolonged circulation, sustained release and retention in the skin and facilitated the permeation of the drug into the skin.98 Niosomes were reported to be more stable without significant toxicity than liposomes especially when used topically for treatment of skin diseases. In this regard, niosome loaded resveratrol for topical treatment of skin cancers is one of the potential candidate.99,100 Similarly, the topical gel from Zingiber cassumunar Roxb. extract loaded niosome for anti-inflammatory activity-enhanced skin permeation and stability of compound D was developed using croton oil-induced ear edema model in male ICR mice.101 More examples of herbal loaded niosome are shown in Table 1.102–106
It is a novel liposome that defined as a soft, non–invasive lipid-based elastic vesicles (Figure 5D) developed for topical, transdermal and systemic applications with the high efficient ability of both hydrophilic and lipophilic drugs and active ingredient delivery to deeper skin layers and blood circulation.8,10
Ethosome is composed of water, certain phospholipids (phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, and phosphatidylglycerol), and a relatively high concentration of alcohol (30–45%) (ethanol and isopropyl alcohol).109,110 This composition provides higher deformability and entrapment efficiency to ethosome that enhances topical drug delivery of highly concentrated active ingredients and transdermal transport efficiency and prolongs the physical stability of ethosomes via flexibility of the lecithin bilayer when compared to liposome.109
The disadvantage of ethosome is size growing from tens nanometers to micrometers due to its poor stability that caused by alcohol evaporation and then loaded compounds leaks out after a while. To control this shortcoming, alcohol can be situated with a combination of trehalose and propylene glycol.12
In this connection, curcumin-encapsulated PEGlycated and traditional liposomes and ethosomes were developed and tested for their potency as a transporter for the carrying of products to the skin. PEGlycated liposomes presented the most accepted ex vivo transdermal drug delivery system in rat skin and showed a higher suppression of paw edema in the rat model of induced inflammation.110 More examples of herbal loaded NE are presented in Table 1.88,111,112
A dendrimer is a tree-like synthesized polymer that was characterized as having a single central core that gives frequent branches of variously armed macromolecules (external capping and multifunctional groups) (Figure 6) to achieve better targeting to specific sites. Generally, they are made up of natural or synthetic components such as sugars, nucleotides and amino acids.113,114
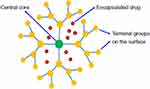 |
Figure 6 A schematic illustration of dendrimer. Notes: Reproduced from ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291-73098 |
The unique feature of this polymer is that its structure and hydrophilicity are easily controllable during formation to get higher solubility, permeability, biocompatibility, biodistribution, clearance and consequently reducing side effects.115
Instantly, the polyamidoamine (PAMAM) dendrimers have recently been studied as carriers as they can be developed in various shapes, sizes and surfaces, in order to get functionalized nanoscale formulas.116 Thus, this dendrimer can offer to target ligands to promote particular binding to cellular receptors.117 Additionally, the small size of this dendrimer renders it to be promptly cleared from the body through the renal and escape from the reticuloendothelial system.118 Furthermore, broad internal cavities of PAMAM dendrimers allow them to complex hydrophobic drugs either by a covalent or non-covalent conjunction.119,120
In this regard, a group of researchers investigated the effectiveness of quercetin-loaded PAMAM dendrimers after oral administration as a Biopharmaceutical Classification System (BCS) class II molecule. They assessed the water solubility of quercetin in 4 generated dendrimers with 5 different concentrations. Consequently, they found that all generations with respective concentrations of PAMAM dendrimers showed potential positive effects on solubility enhancement and in vitro quercetin dual releasing pattern of an initial quicker release then sustained release. Furthermore, the efficacy of this dendrimer on a carrageenan-induced paw edema model to evaluate the acute activity of this nanocarrier in response to inflammation was also evaluated.121 More examples of herbal loaded NE are presented in Table 1.122–128 However, many other dendrimers such as polyamidoamine organosilicon (PAMAMOS), polypropyleneimine (PPI), and glycodendrimers have been developed and studied but with less common use.120
It is a nanosized (10–100 nm) polymer particles or colloidal dispersion (Figure 7) that consists of a single core-shell with narrow and small-sized self-assembly of synthetic amphiphilic di- or tri-block copolymers with both hydrophobic and hydrophilic segments in aqueous media.129,130 Solubilization enhancement, intracellular drug accumulation, and protection against degradation are provided by the inner hydrophobic core, while the hydrophilic layer providing improved biocompatibility and active site-specific cell targeting, as well as thermal, pH, and photosensitivity properties.131
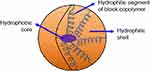 |
Figure 7 A schematic illustration of micelle.Notes: Reproduced from ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291-73098 |
In order to achieve a higher accumulation of drugs at the tumor site and achieve a prolonged circulation in blood, micelles must maintain good stability in the body. This proper stability can be achieved in two paths that are dynamic stability in which the micelle being decomposed into the single polymer chain, and the other is thermodynamic stability in which depends on the anti-dilution capacity of polymeric micelles.131
The best-studied block copolymers for use in micelle construction are polyethylene glycol (PEG)-block-poly3-caprolactone (PCL), PEG-b-polylactide-coglycolide, and PEG-b-poly-c-benzyl L-glutamate. Among them, PEG-PCL is the most preferable one due to its acceptable features such as biodegradability, safety, and high loading for lipid-soluble biomaterials.132
In this respect, artemisinin encapsulated PEG-PCL micelle introduced with LyP-1 (cyclizine-amino acid peptide) has been developed that recognizes and binds to the p32/gC1qR receptor and consequently expressed highly in specific cancer cells of tissues and lymph vessels. This polymeric micelle modification enhances the artemisinin delivery to extremely metastasized mammary adenocarcinoma and its surrounding lymphatic tissues both in vitro and in vivo successfully.133
Generally, polymeric micelles are penetrating the tumors via active or passive targeting mechanisms in which the latter enhanced permeability and retention effects produced after intravenous administration of particles, while active targeting depends on the basic receptor-mediated interaction between the ligand-modified on the surface of micelles and the molecular markers specifically over-expressed in the cancer cells, such as folate receptors, integrins, and epidermal growth factor receptors.134,135 More examples of herbal loaded NE are presented in Table 1.136–140
Nanosphere is a colloidal aqueous solution with amorphous or crystalline nature having a size range between 10 and 200 nm (Figure 8) that composed of a polymeric core encapsulating active ingredients and/or adsorbing them onto the nanoparticles.141,142
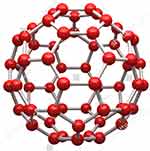 |
Figure 8 A schematic illustration of nanosphere.Notes: Reproduced from Harper 3D.142 |
The main virtue of this system includes delayed drug release, regular plasma drug concentrations, more stability in biological fluids, high protection from enzymatic and chemical degradation, improved bioavailability, potential antitumor efficiency, enhance complete entrapment of the drug, and reduced toxicity.143 These most outstanding features of NS are directly due to hydrophobic surfaces of these particles that are highly susceptible to opsonization and clearance by the reticuloendothelial system.12
Biodegradable NS includes albumin NS, modified starch NS, gelatin NS, polypropylene dextran NS and polylactic acid NS. In addition, there are 2 more types of NS, immune NS and magnetic NS. Immunomagnetic NS can be prepared by combining the above two kinds of NS, which could significantly improve its targeting.144
Most nanospheres are prepared with biodegradable, biocompatible, and synthetic polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and their co-polymer polylactide-coglycolide (PLGA) using emulsion evaporation technique.144 Moreover, NS can be also prepared using pre-formed polymers by nanoprecipitation (omitting the oil in the formulation) or interfacial deposition of polymer (containing the oil). The type of polymer is also important for evaluating the rate of release by NS. Because of the small size of NS, they can be administered orally, locally, and systemically.12,145
Interestingly, oridonin (ORI)-loaded poly(D, L-lactic acid) (PLA) modified with a functionalized polymer [RGD (Arg-Gly-Asp peptides)] to improve antitumor activity is generated that comes with tissue targeting, and better in vivo tumor inhibitory effects than oridonin alone or ORI loaded PLA nanoparticles.146 More examples of herbal loaded NE are presented in Table 1.142,143,147–149
They are pure drug crystals with nanosized particles (Figure 9) in which toxic side effects resulting from the encapsulating/solubilizing excipients may be eliminated.150 The best known features of nanocrystals are high drug-loading capacity and platform stability that render them to be widely used to deliver poorly hydrophilic materials in the form of a colloidal dispersion.151 Cells of the mononuclear phagocytic system can recognize the nanocrystal particles in the bloodstream as an exogenous material which results in passive accumulation in liver, spleen, and lung.61,152
 |
Figure 9 A schematic illustration of fentofibrate nanocrystals (FNB-NCs). Notes: Reproduced from Kevadiya BD, Chen L, Zhang L, Thomas MB, Davé RN. Fenofibrate Nanocrystal Composite Microparticles for Intestine-Specific Oral Drug Delivery System. Pharmaceuticals. 2019;12(3):109-124329 |
Nanocrystals are generally developed by the “bottom-up” (such as Nanomorph™, Soliqs/Abbott), “top-down” technologies (such as Dissocubes®, SkyePharma) or combination technologies (such as NANOEDGE, Baxter). However, the top-down technique is the procedure of choice for nanocrystals in products developed in the pharmaceutical, cosmetic or clinical trials mainly due to the simple and easy scale-up which better serves the industry.61
The nanocrystals are made by wet milling methods, such as bead milling or high-pressure homogenization technique that improves the oral bioavailability and enhanced the transdermal efficacy of poorly soluble drugs.153 Physically, the biodistribution of nanocrystals is affected by particle size, morphology and surface modification. Additionally, in order to target nanocrystals to specific pathogenic sites, ligand conjugation and stimuli-responsive polymers can be used.154
In 2011, a group of researchers prepared a natural product derived nanocrystal focused on Camptothecin (CPT) active compound that was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used as a cancer treatment in Traditional Chinese Medicine.155 In their study, they examined the particle characteristics, cellular cytotoxicity, and animal anticancer effect. Finally, they realized that CPT nanocrystals were more potent to MCF-7 human breast cancer cells than CPT alone in vitro. Additionally, CPT nanocrystals exhibited significant suppression of tumor growth in MCF-7 xenografted BALB/c mice model and the drug concentration in the tumor site was 5 times more at 24 hrs by using the nanocrystal treatment than by using the CPT solution. Storage stability study indicated that the nanocrystals were stable for at least 6 months.155 More examples of herbal loaded NE are presented in Table 1.156–163
Water-soluble phytochemicals such as flavonoids and polyphenols are poorly absorbed in the body due to their large molecular size which did not allow them to be absorbed by passive diffusion, as well as their poor lipid solubility makes a serious limiting to their pass across the lipid-rich biological membranes, subsequent poor bioavailability.164
Phytosome is a patented formula developed to incorporate medicinal plant active ingredients and water-soluble phytochemicals into phospholipids to create lipid-compatible molecular complexes in order to immensely modify their absorption and bioavailability.165 The novelty of phytosome formulation is that there is a molecular complex and chemical bonding between phosphatidylcholine and the plant materials at a ratio of either 1:1 or a 2:1 relying on the substance(s) conjugated, whereas no chemical bonds are formed in liposome and thousands of phosphatidylcholine molecules enclosing the water-soluble compound can be observed freely.166
Phytolipid delivery system is a specifically modified phytosome for delivering of herbal drugs that made by incorporation of standardized plant extracts or water-soluble phytochemicals into phospholipids to produce lipid-compatible complexes to enhance better absorption and bioavailability without resorting the pharmacological or structural changes of the ingredients.167
The phytosomes are small-sized particles (Figure 10) that produce better transiting from a water-soluble condition into the lipid-soluble condition of the enterocyte cell membrane and then into the cell, lastly arriving the blood and protecting the valuable ingredients of the herbal drug from gastric enzyme destruction and gut bacteria.168
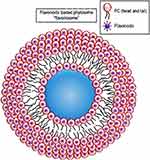 |
Figure 10 A schematic illustration of phytosome. Notes: Reproduced Karthivashan G, Masarudin MJ, Kura AU, Abas F, Fakurazi S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int J Nanomed. 2016;11:3417-3434169 |
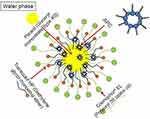 |
Figure 11 A schematic illustration of APC-SNEDDS dissolved in distilled water. APC: Akebia saponin D-phospholipid complex. Notes: Reproduced from Shen J, Bi J, Tian H, et al. Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with akebia saponin D–phospholipid complex. Int J Nanomed. 2016;11:4919-4929183 |
The recently produced phytosome-loaded herbal content is optimizing a sequential technique by a group of researchers from Malaysia to encapsulate several flavonoids in a single phytosome that named flavonosome. Three widely constituted and therapeutically valuable flavonoids named quercetin (Q), kaempferol (K), and apigenin (A) were tested in the ethyl acetate fraction of Moringa oleifera leaf extract and encapsulated in a single flavonosome (QKA–phosphatidylcholine) via 4 various techniques. After checking for many physicochemical properties, they suggested that this three-in-one flavonosome with sustained activity is a good candidate as an antioxidant, hepatoprotective, and heat supplement agent.169 More examples of herbal loaded NE are presented in Table 1.170–175
SNEDDS is a lipid-based anhydrous isotropic mixture of oil, surfactant(s) and cosurfactant(s) with a particle size of 20–200 nm (Figure 11).9,176 It produces fine oil-in-water nanoemulsions upon gentle agitation after dilution in aqueous media, such as gastrointestinal fluids; thus, it can be given orally in soft or hard gelatin capsules. This leads to in situ solubilization of drug that can subsequently be absorbed by lymphatic pathways, bypassing the hepatic first-pass effect.177 On the other hand, efforts were made to overcome the limited aqueous solubility, low ocular bioavailability and short pre-ocular retention and absorption of drugs by introducing SNEDD in the form of eye drop.178
Physically stability upon storage, easy to produce, improved dissolution rates and absorption that results in more reproducible blood–time profiles are among the most accepted features of SNEDDS. Additional advantages of SNEDDS over conventional emulsions and other lipid carriers are the significantly reduced energy requirement for their preparation and easier to manufacture in a large scale.179
Among the successful example of previously prepared crude plant extract-loaded SNEDDS is a persimmon (Diospyros kaki) leaf extract (PLE) loaded SNEDDS that was characterized to compare its in vitro dissolution and relative bioavailability with a commercially available agent (Naoxinqing tablets). They indicated that this developed formula shows better stability, solubility and sustained release than the commercial drug, as well as it is a promising drug delivery system for increasing the oral bioavailability following oral administration in fasting beagle dogs.180 Recently, several novel herbal loaded SNEDDSs with desirable properties have been reported and are presented in Table 1.181–185
SMEDDS is a lipid-based nanoparticle that is composed of oil, surfactant (Cremophor RH40, Cremophor EL, or Polysorbate 80) and co-surfactant (Figure 12). The role of surfactant in SMEDDS is to improve intestinal permeability by lowering surface tension and hence facilitating touch with gastrointestinal mucosa and additionally inhibiting drug efflux by P-glycoprotein.186,187
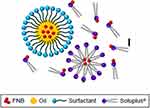 |
Figure 12 A schematic illustration of SMEDDS. Notes: Adapted from Quan G, Niu B, Singh V, et al. Supersaturable solid self-microemulsifying drug delivery system: precipitation inhibition and bioavailability enhancement. Int J Nanomed. 2017;12:8801-8811330 |
Among the most preferred property of SMEDDS is bioavailability improvement due to its small particles and wide surface area, which ameliorate absorption, solubilization, and releasing capacity. Additionally, SMEDDS decreases the first-pass metabolism by facilitating drug absorption via the lymphatic system of the intestine, and thus it provides a promising way to raise bioavailability for poorly hydrophilic products. Moreover, SMEDDS is very stable, easy to administer, and easy to construct at industrial scale especially solid SMEDDS.188
SMEDDS can produce microemulsions, nanoemulsions, or emulsions followed by injection into aqueous media with mild agitation that may develop drug precipitation. In order to overcome this phenomenon, a super-saturable self-micro emulsifying drug delivery system (S-SMEDDS) is developed that contains precipitation inhibitor with good crystallization-inhibiting capacity such as Polyvinylpyrrolidone (PVP) K17.189 Instantly, ligand-modified SMEDDS was reported with targeted delivery of active compounds to specific absorption sites such as targeting of folate receptor overexpression in colorectal carcinoma by producing folate-modified SMEDDS.190
In this connection, resveratrol, a poorly hydrophilic component isolated from some commonly desired fruits loaded SMEDDS is generated for oral delivery. This novel SMEDDS is also characterized by maintaining high drug solubilization for long period to improve drug absorption, improved bioavailability and provided more stability due to its small particle size (approximately 50 nm) and high zeta potential in a neutral environment. The antioxidant capacity and cytotoxicity of the formulation were also detected using DCFH-DA and CCK-8 assays. The formulation exhibited a greater antioxidant capacity with less toxicity than a free compound.191 More examples of herbal loaded NE are presented in Table 1.192–196
It is composed of solid polymer fibers with diameters of 10–1000 nm that have a large surface area with a small pore size (Figure 13) and is prepared by the electrospinning method.12 This novel nanocarrier has a limited role in delivering active components but with potential improvements in the therapeutic treatments and support the using of active compounds in several biomedical areas such as tissue regeneration.197,198
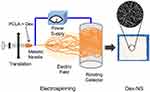 |
Figure 13 A schematic illustration of dexamethasone loaded nanofibers (Dex-NS). Notes: Adapted from Lee JW, Lee HY, Park SH, et al. Preparation and evaluation of dexamethasone-loaded electrospun nanofiber sheets as a sustained drug delivery system. Materials. 2016;9(3):175-186331 |
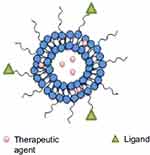 |
Figure 14 A schematic illustration of polymerosome. Notes: Adapted from Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomed. 2015;10:1001-101884 |
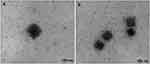 |
Figure 15 Transmission electron micrographs of 20(S)-protopanaxadiol cubosome with (A) and without (B) Pierine. Notes: Reproduced from Jin X, Zhang ZH, Sun E, et al. Enhanced oral absorption of 20 (S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: in vitro and in vivo studies. Int J Nanomed. 2013;8:641-652332 |
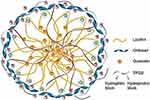 |
Figure 16 A schematic illustration of chitosan nanoparticle.Notes: Reproduced from Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomed. 2011;6:16211630.233 |
The nanofibers are most likely carbon-based as they are extracted from various plants and thus they can be generated from different polymers and hence have different physical properties and application potentials.199 Among the potential benefits of the nanofibers is to modify wound healing and preventing infection. Also, it is suggested that nanofibers have very strong adhesive features such as that is found with a gecko that allows it to easily climb surfaces using bundles of nanofibers on the surface of its feet. Moreover, the scaffolding of nanofibers to initiate the repair of damaged tissue is among the pronounced features.200
Regarding the anticancer potential of nanofiber, curcumin-loaded self-assembling peptide nanofiber as a novel tumor-targeting carrier was developed that showed high cellular uptake in αvβ3 integrin-positive HepG2 liver carcinoma cells, thereby leading to significantly higher cytotoxicity than nonloaded one. Additionally, ex vivo studies further demonstrated that curcumin could accumulate markedly in mouse tumors after administration via the tail vein.201,202 More examples of herbal loaded nanofibers are displayed in Table 1.203–207
Polymersome is a self-assembled polymeric nanosphere vesicle that may have relatively thick membranes (up to 40 nm), which are formed by synthetic amphiphilic block copolymers (Figure 14).208 They are able of incorporating hydrophilic and nonhydrophilic drugs, proteins, peptides, DNA and RNA fragments in their membrane which acts as a barrier to protect them from the biological environment. Additionally, the membrane flexibility of PS makes them applicable in targeting and control release.209 Polymersomes have some similarities to liposomes especially in the structure but are more stable and less permeable than liposomes. The PS is capable to bind with biologically active ligands, antibodies and biotinylated conjugation to their surface which enhances targeted therapy and imaging strategy.210
It was documented that PS was used as anti-tumor agents for several drugs due to its controlled release, high permeation, retention and loading capacity of drugs into PS than liposome membrane.211,212 In this respect, biotin functionalized leuko-polymersome to proctor and treat inflammation, cancer, and cardiovascular disease and Tat-loaded PS as a fantabulous agent for cellular tracking were also investigated as a promising tumor-fighting agent.213 More examples of herbal loaded PS are found in Table 1.208,214–217
It is a viscous isotropic vesicle that made mainly of unsaturated monoglycerides (monoolein-water) binary system with thermodynamically steric stable bi-continuous cubic liquid crystalline phase (poloxamers) (Figure 15).218,219
Features such as high internal surface area per unit volume (approximately 400 m2/g) and a 3D structure with hydrophilic and hydrophobic domains make them entrap water-soluble and nonsoluble and amphiphilic materials successfully. Its large interfacial area can provide a complex diffusion pathway for sustained release of entrapped drug molecules, whereas lipid constituents are biocompatible, bio-adhesive, and digestible.220 They are usually constructed via dispersion or fragmentation of the cubic phases of gels in a liquid condition.221 Previous works on somatostatin, insulin, indomethacin, and rifampicin drug encapsulation within cubosomes have been done intensively. Additionally, various pharmaceutical applications of cubosomes have also been investigated such as peptides, enzymes, antimuscarinic drugs, antibiotics, and analgesic delivery.221,222
Cubosomes easily evacuate their contents to the epidermis as they have an almost same structure to that of the stratum corneum, as well as the properties of adhesion and penetration enhancement of cubosomes suggest their potential utility in skin cancer (melanoma) treatment.223 On the highlight of this, very recently, a report of biocompatible polymer-free cubosomes for potential application in both photodynamic therapy and bioimaging of skin malignant melanoma has been published with very low cytotoxicity to the cutaneous formulation.224 More examples of herbal loaded cubosome are shown in Table 1.225–229
Biopolymer-Based Nanocarrier (BBN)
They have derived from proteins (such as gelatin, albumin, and milk proteins), polysaccharides (such as chitosan, hyaluronan, dextran, cyclodextrins, pectin, guar gum, cellulose, sodium alginate, and starch), and/or their modified versions, derivatives or their combinations. The most interesting features of these materials that render them to be used for BBN production are a biological realization, bioactivity, biodegradability, less toxicity, easy modification, and simplicity of producing gels from them.230 This type of nanocarrier is well established to have plausive water solubility, stability, degradation, and biocompatibility for a wide range of utilization that earned from their variable charges, molecular weights, and compositions.9,12 Various approaches for fabrication of BBN delivery systems are well addressed which including coacervation, spray drying, electrospinning, electrospray, supercritical fluid, emulsion–diffusion, reverse micelle, emulsion-droplet coalescence, emulsification/solvent evaporation, salting-out, ultrasonication and high-pressure homogenization.231
Chitosan, a cationic biocompatible and biodegradable linear polysaccharide, containing d-glucosamine and N-acetyl glucosamine units, which is extracted from the exoskeleton of crustacean arthropods such as insects, crabs, lobster and shrimps232 reported to be the best example of natural pure biopolymer to deliver plant components such as curcumin, quercetin,233 and trans-resveratrol with better mucoadhesion, solubility, dissolution rate, and specific targeting.234,235 Additionally, chitosan has been utilized for various biomedical applications such as in tissue engineering in the form of scaffolds, drug delivery carriers, fabricating surgical thread, bone healing, and as a wound dressing substance. On the other hand, modified chitosan molecules such as dextran sulfate-conjugated chitosan, biotinylated and galactosylated chitosan are well developed with advanced properties such as altering the surface charge, providing pH-sensitive swelling, more stability, enhancing bioactivity by modifying targeting to the specific site of action (Figure 16).236 More examples on this nanoparticle are available in Table 1.237–241
Hydrogels are cross-linked polymeric networks with hydrophilic functionalities that supply spaces for homing aqueous biological fluids (Figure 17) and have is known as a promising bio-compatible material in numerous therapeutic applications.12,242 This formula is characterized by adorable biocompatibility, high porosity, hydrophilicity that results in controlled drug release. Naturally available biopolymers such as chitosan, alginate, hyaluronic acid (HA), collagen, and gelatin are used to construct inherently biodegradable BBH that frequently pre-functionalized to integrin binding sites permitting for adherence and integrated cellular responses.9 However, the application of these substances is somewhat restricted because significant batch-to-batch variability and potential immunogenicity within foreign models are obtained.243
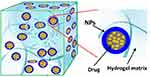 |
Figure 17 A schematic illustration of biopolymeric hydrogel. Notes: Reproduced with permission from MDPI. Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J. Composites of 2075 polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials. 2015;5(4):2054–2130.242 |
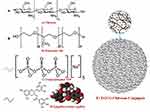 |
Figure 18 A schematic illustration of biopolymeric drug conjugate. Notes: Reproduced from Safer AM, Leporatti S, Jose J, Soliman MS. Conjugation Of EGCG And Chitosan NPs As A Novel Nano-Drug Delivery System. Int J Nanomed. 2019;14:8033-8046.333 |
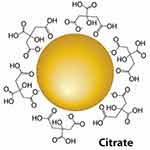 |
Figure 19 A schematic illustration of gold nanoparticle.Notes: Reproduced with permission from Luna Nanotech.260 |
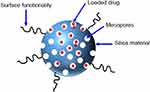 |
Figure 20 A schematic illustration of silica nanoparticle. Notes: Reproduced from ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291-7309.8 |
 |
Figure 21 A schematic illustration of magnetic nanoparticle.Notes: Adapted with permission from Frontier in Microbiology. Souza AC, Amaral AC. Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol. 2017;8(336):1–13. 280 |
 |
Figure 22 A schematic illustration of veteran cockle shell-derived calcium carbonate nanoparticles. Notes: Reproduced from Muhammad Mailafiya M, Abubakar K, Danmaigoro A, et al. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Appl Sci. 2019 ;9(14):2897-2922.334 |
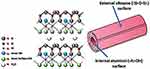 |
Figure 23 A schematic illustration of halloysite clay nanotubes. Notes: Reproduced with permission from Kamal N, Kochkodan V, Zekri A, Ahzi S. Polysulfone Membranes Embedded with Halloysites Nanotubes: Preparation and Properties. Membranes. 2020;10(1):2-29.335 |
 |
Figure 24 A schematic illustration of single walled carbon nanotube (A) and double walled carbon nanotube (B). Notes: Reproduced from ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291-7309.8 |
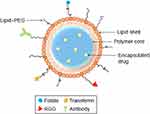 |
Figure 25 Types and structures of hybrid nanocarrier. Notes: Adapted from Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomed. 2015;10:1001-1018.84 |
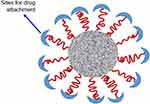 |
Figure 26 A schematic illustration of biological nanocarrier. Notes: Reproduced from ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291-7309.8 |
In this connection, biopolymer-based pH-sensitive hydrogels were prepared using chitosan with PEG of different molecular weights in the presence of silane crosslinker. The incorporated components remain undissolved in different swelling media as they are connected by siloxane linkage which was confirmed by FTIR spectroscopy. The swelling in water was enhanced by the addition of higher molecular weight PEG. The swelling behavior of the hydrogels against pH showed high swelling in acidic and basic pH, whereas, the low swelling was examined at pH 6 and 7. This characteristic pH-responsive behavior at neutral pH made them suitable for injectable controlled drug delivery.244 More examples on this nanoparticle are available in Table 1.245–249
Thermally sensitive biopolymer with the potential ability to quickly form insoluble viscous co-acervate at body temperature can be used for this purpose (Figure 18).250,251 Although some of the biomedical polymer–drug conjugates are approved for clinical trials, they lack photothermal properties and multi-imaging capabilities, impeding them from imaging-guided precision cancer therapy and total cancer arrested development.252 Thus, researchers introduced a novel all-in-one biopolymer–drug conjugate nanotheranostics, such as that of intracellular pH-sensitive polydopamine–doxorubicin conjugate nanoparticles under a mild situation that are characterized by excellent photothermal attribute, dual stimuli-triggered drug release activity, and about elongated blood circulation time than nonconjugated doxorubicin.253 More examples on this nanoparticle are available in Table 1.254–259
Inorganic Nanocarrier
Recently, different kinds of inorganic nanocarriers have been developed and investigated for their potential delivery of plant active ingredients.
Metal Nanoparticle (MN)
These are nanoparticles such as silver nanoparticles (AgNPs), gold nanoparticles (AuNPs) (Figure 19),260 copper nanoparticles (CuNPs), zinc oxide (ZnONPs) nanoparticles, quantum dots, cerium oxide (CeO2) nanoparticles, iron oxide nanoparticles (Fe3O4), yttrium oxide (Y2O3) nanoparticles and titanium dioxide nanoparticles (TiO2) possess special benefits in biomedical application due to their contents of essential mineral elements that have strong activity for human body.261 These nanoparticles gained their noncovalent interaction or covalent conjugation drug-loading capacity due to their surface plasmon resonance (SPR) ability, structural diversity, poor toxicity, and high biocompatibility. Thus, they can be utilized for achieving intracellular drug delivery and controlled release through a photothermal route.262
Moreover, a novel metal nanoparticle with multi-functional groups is also investigated by developing much active component-loaded complex metal nanoparticle integrated multifunctional liposomes to improve intracellular drug delivery, overwhelm multi-drug resistant (MDR), fasten anti-tumor activity, and lower side effects.263 Very recently, it has proven that biopolymers complexed with bioactive nanoparticles endowing antimicrobial and anti-inflammatory properties have a fantastic effect in wound care to prompt the healing mechanism of wound infections caused by hyperglycemia.
In this regard, a combination of antibacterial nanoparticles such as silver, gold, or copper nanoparticles with polymeric matrix could potentially suppress bacterial propagation and similarly fastens the healing process of a wound and mitigate the diabetes mellitus-based foot ulcer.264 More examples on this nanoparticle are available in Table 1.265–269
Mesoporous Silica Nanoparticle (MSN)
MSN is the most recent promising carrier for drug storage and delivery that has large surface area with high loading capacity for therapeutic agents, high pore volume and porosity (honeycomb-like architecture), adjustable pore diameter, modifiable surface potential, selective surface functionality, morphology control, adorable biocompatibility and controlled release properties (Figure 20).270,271
Moreover, MSN has been applied in pharmaceutics to improve drug bioavailability, reduce drug toxicity, and deliver with cellular targetability. Particularly, the exciting progress in the development of MSN-based effective delivery systems for poorly soluble drugs, anticancer agents, and therapeutic genes.272
In general, MSNs are synthesized by using a silica precursor (tetraethylorthosilicate or sodium silicate) in an alcoholic solution under basic conditions and incorporating a surfactant. On the other hand, the synthesis of mesoporous silica particles in the nonalcoholic medium was conducted but the formation of spherical particles is limited by the amount of the surfactant.273
The developed formula in this area is silybin from the seed of the milk thistle (Silybum marianum)-meglumine encapsulated MSN with high drug-loading capability, in vitro, sustained release, and in vivo high absorption ability.274 More examples on this nanoparticle are available in Table 1.275–279
Magnetic Nanoparticle (MNP)
Attributing with the use of developed magnetic nanoparticle (especially iron oxide) owing to receive the highest target positioning and best trigger drug release, biocompatibility, and nontoxicity in a magnetic field (Figure 21).280 Magnetic nanoparticles with appropriate surface coatings are used clinically for various biomedical applications, such as magnetic resonance imaging, hyperthermia, drug delivery, tissue repair, cell, and tissue targeting and transfection.281 Other benefits of using magnetic nanocarriers are referred to be more rapid and effective for curing diseases even if a small amount of drug is consumed; thus, it can reduce the concentration of the drug in healthy tissues, and consequently diminished side effects. Moreover, MNP small size renders them to gain more bioaccessibility to deserted tissues and bio interact with them at molecular and cellular levels, also binding to particular tumor-suppressor antibodies and conveying these adsorbed anti-tumor materials to the site of the tumor.282 In this respect, gambogic acid (from the brownish or orange resin from Garcinia hanburyi)-loaded magnetic iron oxide (Fe3O4) nanoparticles were produced and investigated for its improvement in the water solubility of gambogic acid and halting the proliferation and migration of Panc-1 pancreatic cells by inactivating transcription factor ETS1 in vitro using MTT and scratch assays, respectively.283 More examples on this nanoparticle are available in Table 1.284–288
Calcium Carbonate (CaCO3) Nanoparticle
Aragonite CaCO3 nanoparticle is less stress to achieve, environmentally pleasant, and less costly process that involved a simply automated stirring of cockle shell powder in the occurrence of BS-12 as a biomineralization catalyst (Figure 22).289,290 CaCO3 is utilized as therapeutic agents with outstanding efficacy due to its biocompatibility, nontoxicity, changeable surface chemistry, excellent physicochemical property, simple preparatory methods in a bulk scale, controlling release, slow biodegradability, pH-sensitivity, and porous nature.291
CaCO3 potential to be functionalized with targeting agents gives it the distinctive property that can be used in targeted delivery systems for anticancer drugs, in addition to slow CaCO3 matrix degradation, constant and organized discharge property, controlled release, at the targeted location.292
The best example in this area is doxorubicin-loaded CaCO3-NPs for cancer therapy. Generally, the physiological pH of blood and the extracellular spaces around tumors is about 6.8–7.2, while the pH of endolysosomes of cancer cells is highly acidic (pH <6). Then, CaCO3 nanoparticles holding DOX are swiftly unprotected to the acidic environments of endosomes, loss its stability, and is believed to discharge drugs at lesser pH, which results in a rise in the cellular uptakes of drugs.293 Unfortunately, until this moment plant metabolite loaded CaCO3 nanoparticles are not available in the research area.
Nanotube
Halloysite Clay Nanotubes (HNT)
They are aluminosilicate clay that constructed from 2 different dimensional structures (tetrahedral and octahedral) through surface weathering of aluminosilicate and composed of aluminum, silicon, oxygen, and hydrogen (Figure 23).294 These hollow tubes with several nanocavities are best known for having the high surface area, biocompatibility and loading capacity.295 Halloysite nanotubes are natural green cylindrical clays that are not costly, not difficult to collect, and possessing chemical composition similar to that of kaolin. Features such as proper lumens, high aspect length–diameter ratio and low hydroxyl density on their surface make them be more adjustable to be used for many projects.296 Continuing in this area, resveratrol (from berry family)-loaded halloysite nanotube coated layer-by-layer with polyelectrolytes in order to control its drug release ability and to reduce its toxicity is developed successfully. Additionally, the system showed enhanced resveratrol cytotoxicity to MCF-7, breast cancer cells and produced pronounced apoptosis.297 More examples on this nanoparticle are available in Table 1.298–302
Carbon Nanotubes (CNT)
CNTs are allotropes of carbon with a cylindrical nanostructure that have unusual properties, which are valuable for nanotechnology, electronics, and optics with some remarkable properties such as excellent thermal conductivity, mechanical strength, and electrical conductivity (Figure 24).303,304
One of the most recently developed nanodevices for biomarker detection is CNT in which a single-walled CNT as a high-resolution atomic force microscopy (AFM) for the selective detection of specific sequences of kilobase-size DNA from single-base mismatch sequences was used.305 The principle of this technique is hybridizing of targeted DNA fragments with labeled oligonucleotides to be easily detected by AFM. This method enabled the straight detection of genetic disorders causing cancers that encoded by specific haplotypes. Additionally, this model can be utilized as nanoscale carriers for bioimaging, drug delivery and used for photothermal destruction of cancer cells.306
In this connection, freshly prepared Ocimum tenuiflorum (tulsi extract) mediated photosynthesized AgNP loaded into emulsified multiwalled carbon nanotube (MWCNT) was developed that characterized by a spherical shape, 5–40 nm size and surface plasmonic resonance at 430 nm. Their targetability to the intracellular part of the sperm cell (without disrupting the sperm cell membrane) for its further application in biosensing-based infertility diagnosis was also investigated in detail and confirmed that AgNP-MWCNT composite is suitable in fertility diagnosis and reproductive health care.307
Hybrid Nanocarrier (HNC)
This type of nanocarrier is composed either from the combination of 2 different organic materials or a combination of an organic with an inorganic material to emerge improved drug delivery performance. Many HNC possesses a core-shell structure that composed of different types of biomaterials (Figure 25).308,309 This composition endows the HNC with many desirable properties, such as high encapsulation and loading ability, the betterment of stability, sustained release, improvement of intracellular drug delivery, and the enhancement of conjugating with targeting ligands.295 On the other hand, specific functionalities of organic materials at the surface of inorganic materials can be promoted to improve the selectivity and efficiency of therapy especially those utilized as anticancer agents.310 In this respect, hybrid nanocarrier conjugated folic acid for targeted letrozole (LTZ) delivery for breast cancer treatment was produced in which physicochemical properties, in vitro in vitro drug release, cytotoxicity and ex vivo work of the formula were studied intensively. As a result, the system could overwhelm the restrictions related to the LTZ as a potent nonsteroidal drug. Finally, it was concluded that both the entrapment and therapeutic efficiency of LTZ in the amphiphilic carrier were enhanced using the lipid nanoparticles and the surface modification, respectively.311 More example of this nanoparticle is available in Table 1.312
Biological Nanocarriers (BNC)
These are naturally occurring highly diverse nanoparticles that are shared a common structure of a shell composed of capsid proteins surrounding the DNA or RNA viral genome. They have variable sizes (within the nanometer range) and morphologies from simple spheres to rods to icosahedrons (Figure 26).313,314 In this respect, viruses have been dedicated to targeting the most pronounced organisms and tissues. Most applications use virus nanoparticle (VNP) and virus-like particles (VLP) are native viral capsid proteins without nucleic acid in order not to cause infection.299 VNP and VLP are nanosized (approximately 100 nm), self-assembled robust protein net that possessing uniform nanostructures and distinct geometry. Recently, this system is used for many purposes such as drug delivery and gene therapy in the form of nanoreactors, filamentous or spherical scaffolds.12
Modified VNP and VLP are also utilized in vivo for vaccination either to induce immunity against the parent virus or to modify other diseases. Additionally, VLP conjugated to appropriate epitopes have been used for anti-tumor vaccines and for vaccines against chronic diseases such as hypertension.315 Regarding their use in bioimaging system, VLP has also been adapted for use as contrast agents in MRI and PET. Furthermore, the natural affinity of VLP for defined cell types allows targeted delivery, as well as the VLP, can be modified by conjugation to targeting molecules, such as folic acid, for cell-specific delivery.316 More examples of herbal loaded BNC are presented in Table 1.317–321
Conclusion
Poor solubility in water and bioavailability have limited the therapeutic efficacy of naturally available potential natural plant products. Currently, recent studies have attempted to address these problems using nanocarriers and studies have anticipated that nanomedicine as a plausible approach for diagnosis, imaging, and therapeutics for a variety of disease treatment and management including cancer, diabetes, hyperglycemia, hypertension, and anemia. Currently, several nanocarrier-encapsulated natural plant extract formulations are in clinical or preclinical development and some of them are already approved by the Food and Drug Administration (FDA) to be used safely in human especially those that are already confirmed that they do not have potential long-term toxicity, degradation, and incomplete metabolism after mitigated by the concept of modifications and piolet study that could be developed as an inexpensive, safe, tolerable, and an appropriate approach for disease control and management.
Acknowledgement
The authors appreciate a grant that has been funded by a Research University Grant Scheme (RUGS) (Project No. UPM/700-2/1/GPBI/2017/9554100) provided by Universiti Putra Malaysia (UPM), Malaysia.
Disclosure
The authors declared that there is no conflict of interest in this current review article.
References
1. Krishnaiah YS. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J Bioequivalence Bioavailab. 2010;2(2):28–36. doi:10.4172/jbb.1000027
2. Teeranachaideekul V, Müller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC) effects of formulation parameters on physicochemical stability. Int J Pharm. 2007;340(1–2):198–206. doi:10.1016/j.ijpharm.2007.03.022
3. Siddiqui IA, Sanna V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol Nutr Food Res. 2016;60(6):1330–1341. doi:10.1002/mnfr.201600035
4. Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–141. doi:10.1016/j.canlet.2013.02.032
5. Adhami VM, Mukhtar H. Human cancer chemoprevention: hurdles and challenges. Top Curr Chem. 2013;329:203–220.
6. Bharali DJ, Siddiqui IA, Adhami VM, et al. Nanoparticle delivery of natural products in the prevention and treatment of cancers: current status and future prospects. Cancers. 2011;3(4):4024–4045. doi:10.3390/cancers3044024
7. Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25:363–376. doi:10.1016/j.jnutbio.2013.10.002
8. ud Din F, Aman W, Ullah I, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–7309. doi:10.2147/IJN.S146315
9. Saraf A. Applications of novel drug delivery system for herbal formulations. Fitoterapia. 2010;81(7):680–689. doi:10.1016/j.fitote.2010.05.001
10. Ni S. Nanoparticles carrying natural product for drug delivery. J Drug Delivery Ther. 2017;7(3):73–75. doi:10.22270/jddt.v7i3.1425
11. Nagalingam A. Drug delivery aspects of herbal medicines. Jpn Kampo Med Treat Common Dis Focus Inflammation. 2017;17:143.
12. Liu Y, Feng N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv Colloid Interface Sci. 2015;221:60–76. doi:10.1016/j.cis.2015.04.006
13. Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25(2):219–234. doi:10.1016/j.jfda.2017.02.001
14. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68(3):701–787.
15. Luo CF, Yuan M, Chen MS, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410:138–144. doi:10.1016/j.ijpharm.2011.02.064
16. Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JE, Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;18:56–61. doi:10.1016/j.chemphyslip.2014.03.006
17. Luo CF, Hou N, Tian J, et al. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int J Nanomed. 2013;8:933–940. doi:10.2147/IJN.S39349
18. Zhang C, Gu C, Peng F, et al. Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules. 2013;18(18):13340–13356. doi:10.3390/molecules181113340
19. Dang YJ, Zhu CY. Oral bioavailability of cantharidin-loaded solid lipid nanoparticles. Chin Med. 2013;8:1–6. doi:10.1186/1749-8546-8-1
20. Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomedicine. 2013;9(4):492–503. doi:10.1016/j.nano.2012.10.003
21. Li J, Guo X, Liu Z, et al. Preparation and evaluation of charged solid lipid nanoparticles of tetrandrine for ocular drug delivery system: pharmacokinetics, cytotoxicity and cellular uptake studies. Drug Dev Ind Pharm. 2014;40:980–987. doi:10.3109/03639045.2013.795582
22. Rahman HS, Rasedee A, How CW, et al. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomed. 2013;8:2769–2781. doi:10.2147/IJN.S45313
23. Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–151. doi:10.1016/j.ejps.2012.05.010
24. Muhammad HS. Anti-Leukemic Effects of Zerumbone Nanoparticle on Human Jurkat T Lymphoblastoid Cell Lines In vitro and Murine Leukemic WEHI-3B Model In vivo [Doctoral dissertation]. Universiti Putra Malaysia; 2014.
25. Shangguan M, Feng Q, Zhao J, et al. Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. Food Chem Toxicol. 2012;50:1460–1467.
26. Nahr FK, Ghanbarzadeh B, Hamishehkar H, Kafil HS. Food grade nanostructured lipid carrier for cardamom essential oil: preparation, characterization and antimicrobial activity. J Funct Foods. 2018;40:1–8. doi:10.1016/j.jff.2017.09.028
27. Rahman HS, Rasedee A, Abdul AB, et al. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int J Nanomed. 2014;9:527–538. doi:10.2147/IJN.S54346
28. Ng WK, Saiful Yazan L, Yap LH, et al. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Biomed Res Int. 2015;2015:1–10.
29. Ong YS, Saiful Yazan L, Ng WK, et al. Thymoquinone loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine. 2018;13(13):1567–1582. doi:10.2217/nnm-2017-0322
30. Nordin N, Yeap SK, Zamberi NR, et al. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. Peer J. 2018;6:e3916. doi:10.7717/peerj.3916
31. Mohamad NE, Abu N, Rahman HS, et al. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of zerumbone in 4T1 challenged mice. RSC Adv. 2015;5(28):22066–22074. doi:10.1039/C5RA00144G
32. Hosseinpour M, Abdul AB, Rahman HS, et al. Comparison of apoptotic inducing effect of zerumbone and zerumbone-loaded nanostructured lipid carrier on human mammary adenocarcinoma MDA-MB-231 cell line. J Nanomater. 2014;2014:1–10. doi:10.1155/2014/742738
33. Rahman HS, Rasedee A, How CW, et al. Antileukemic effect of zerumbone-loaded nanostructured lipid carrier in WEHI-3B cell-induced murine leukemia model. Int J Nanomed. 2015;10:1649–1666. doi:10.2147/IJN.S67113
34. Rahman HS, Rasedee A, Othman HH, et al. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. Biomed Res Int. 2014;2014:1–15.
35. Jia Ning F, Gayathri TS, Rahman HS, et al. Zerumbone-loaded nanostructured lipid carrier induces apoptosis of canine mammary adenocarcinoma cells. Biomed Res Int. 2018;2018:1–18.
36. Nathaniel C, Elaine-Lee YL, Yee BC, et al. Zerumbone-loaded nanostructured lipid carrier induces apoptosis in human colorectal adenocarcinoma (Caco-2) cell line. Nanosci Nanotechnol Lett. 2016;8(4):294–302. doi:10.1166/nnl.2016.2136
37. Shi F, Yang G, Ren J, Guo T, Du Y, Feng NP. Formulation design, preparation, and in vitro and in vivo characterizations of β-elemene-loaded nanostructured lipid carriers. Int J Nanomed. 2013;8:2533–2541. doi:10.2147/IJN.S46578
38. Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. doi:10.1007/s13205-014-0214-0
39. Mahato R. Nanoemulsion as targeted drug delivery system for cancer therapeutics. J Pharm Sci Pharmacol. 2017;3(2):83–97. doi:10.1166/jpsp.2017.1082
40. Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(05):626–639. doi:10.4236/jbnb.2011.225075
41. Chhabra G, Chuttani K, Mishra AK, Pathak K. Design and development of nanoemulsion drug delivery system of amlodipine besilate for improvement of oral bioavailability. Drug Dev Ind Pharm. 2011;37(8):907–916. doi:10.3109/03639045.2010.550050
42. Topuz OK, Özvural EB, Zhao Q, Huang Q, Chikindas M, Gölükçü M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of food-borne pathogens. Food Chem. 2016;203:117–123. doi:10.1016/j.foodchem.2016.02.051
43. Zhang S, Zhang M, Fang Z, Liu Y. Preparation and characterization of blended cloves/cinnamon essential oil nanoemulsions. LWT-Food Sci Tech. 2017;75:316–322. doi:10.1016/j.lwt.2016.08.046
44. Kotta S, Khan AW, Pramod K, Ansari SH, Sharma RK, Ali J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2012;9(5):585–598. doi:10.1517/17425247.2012.668523
45. Mahajan HS, Mahajan MS, Nerkar PP, Agrawal A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014;21(2):148–154. doi:10.3109/10717544.2013.838014
46. Khani S, Keyhanfar F, Amani A. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv. 2016;23(6):2035–2043. doi:10.3109/10717544.2015.1088597
47. Patel RP, Joshi JR. An overview on nanoemulsion: a novel approach. Int J Pharm Sci Res. 2012;3(12):4640–4650.
48. Wang X, Wang YW, Huang Q Enhancing stability and oral bioavailability of polyphenols using nanoemulsions.
49. Mezadri H. Development of Nanoemulsions Containing Extracts of Fruits of Syagrus Romanzoffiana (Cham.) Glassman and Phytochemical Study of These Extracts [Dissertation] Ouro Preto, Brazil: Faculdade de Ciências Farmacêuticas, UFOP; 2010. Portuguese.
50. Zeng Z, Zhou G, Wang X, et al. Preparation, characterization and relative bioavailability of oral elemene o/w microemulsion. Int J Nanomed. 2010;7:567–572. doi:10.2147/IJN.S12485
51. Ghosh V, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason Sonochem. 2013;20:338e344. doi:10.1016/j.ultsonch.2012.08.010
52. Bonifácio BV, Da Silva PB, dos Santos Ramos MA, Negri KM, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomed. 2014;9:1–15.
53. Censi R, Martena V, Hoti E, Malaj L, Di Martino P. Permeation and skin retention of quercetin from microemulsions containing Transcutol® P. Drug Dev Ind Pharm. 2012;38(9):1128–1133. doi:10.3109/03639045.2011.641564
54. Chen Y, Lin X, Park H, Greever R. Study of artemisin in nanocapsules as anticancer drug delivery system. Nanomed. 2009;5(3):316–322. doi:10.1016/j.nano.2008.12.005
55. Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385(1–2):113–142. doi:10.1016/j.ijpharm.2009.10.018
56. Abellan-Pose R, Teijeiro-Valiño C, Santander-Ortega MJ, et al. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: effect of the particle size. Int J Pharm. 2016;509(1–2):107–117. doi:10.1016/j.ijpharm.2016.05.034
57. Zhao YQ, Wang LP, Ma C, Zhao K, Liu Y, Feng NP. Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. Int J Nanomed. 2013;8:4169–4181. doi:10.2147/IJN.S50557
58. Alshamsan A. Nanoprecipitation is more efficient than emulsion solvent evaporation method to encapsulate cucurbitacin I in PLGA nanoparticles. Saudi Pharm J. 2014;22(3):219–222. doi:10.1016/j.jsps.2013.12.002
59. Christofoli M, Costa EC, Bicalho KU, et al. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in bemisia tabaci populations. Ind Crops Prod. 2015;70:301–308. doi:10.1016/j.indcrop.2015.03.025
60. Souza CF, Baldissera MD, Cossetin LF, Dalla Lana DF, Monteiro SG. Achyrocline satureioides essential oil loaded in nanocapsules ameliorate the antioxidant/oxidant status in heart of rats infected with Trypanosoma evansi. Microb Pathog. 2017;105:30–36. doi:10.1016/j.micpath.2017.02.005
61. Rivas CJ, Tarhini M, Badri W, et al. Nanoprecipitation process: from encapsulation to drug delivery. Int J Pharm. 2017;532(1):66–81. doi:10.1016/j.ijpharm.2017.08.064
62. Sneha S, Swarnlata S, Chanchal DK, Shailendra S. Biocompatible nanoparticles for sustained topical delivery of anticancer phytoconstituent quercetin. Pak J Biol Sci. 2013;16(13):601–609. doi:10.3923/pjbs.2013.601.609
63. Adhikari P, Pal P, Das AK, Ray S, Bhattacharjee A, Mazumder B. Nano lipid-drug conjugate: an integrated review. Int J Pharm. 2017;529(1–2):629–641. doi:10.1016/j.ijpharm.2017.07.039
64. Saracibar BL, Mendoza AEH, Guada M, Vieitez CD, Prieto MJB. Lipid nanoparticles for cancer therapy: state of the art and future prospects. Expert Opin Drug Deliv. 2012;9(10):1245–1261. doi:10.1517/17425247.2012.717928
65. Neupane YB, Sabir MD, Ahmad N, Ali M, Kohli K. Lipid drug conjugate nanoparticle as a novel lipid nanocarrier for the oral delivery of decitabine: ex vivo gut permeation studies. Nanotechnology. 2013;24(41):1–11. doi:10.1088/0957-4484/24/41/415102
66. Guo W, Li A, Jia Z, Yuan Y, Dai H, Li H. Transferrin modied PEG-PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. Eur J Pharmacol. 2013;718:41–47. doi:10.1016/j.ejphar.2013.09.034
67. Shen J, Zhang D, Zhao Z, et al. Synthesis, characterization, in vitro and in vivo evaluation of PEGylated oridonin conjugates. Int J Pharm. 2013;456:80–86. doi:10.1016/j.ijpharm.2013.08.014
68. Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–163. doi:10.1016/j.ijpharm.2006.09.025
69. Singh M, Bhatnagar P, Mishra S, et al. PLGA encapsulated tea polyphenols enhance the chemotherapeutic efficacy of cisplatin against human cancer cells and mice bearing ehrlich ascites carcinoma. Int J Nanomed. 2015;10:6789–6809. doi:10.2147/IJN.S79489
70. Sanna V, Siddiqui IA, Sechi M, Mukhtar H. Resveratrol-loaded nanoparticles based on poly(epsiloncaprolactone) and poly(D,L-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol Pharm. 2013;10:3871–3881. doi:10.1021/mp400342f
71. Pan M, Li W, Yang J, et al. Plumbagin-loaded aptamer-targeted poly D, L-lactic-co-glycolic acid-b-polyethylene glycol nanoparticles for prostate cancer therapy. Medicine. 2017;96(30):e7405. doi:10.1097/MD.0000000000007405
72. Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 2017;6:37–56. doi:10.1016/j.scp.2017.07.002
73. Robson AL, Dastoor PC, Flynn J, et al. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front Pharmacol. 2018;9(80):1–8. doi:10.3389/fphar.2018.00080
74. Lee WH, Loo CY, Young PM, Traini D, Rohanizadeh R. The development and achievement of polymeric nanoparticles for cancer drug treatment. Part Technol Delivery Ther. 2017;25–82.
75. Song Z, Lin Y, Zhang X, et al. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: enhanced cellular uptake and improved therapeutic effects. Int J Nanomed. 2017;12:1941–1958. doi:10.2147/IJN.S125573
76. Hong SS, Kim SH, Lim SJ. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int J Pharm. 2015;483(1–2):142–150. doi:10.1016/j.ijpharm.2015.02.013
77. Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32:1119–1123.
78. Hong SS, Choi JY, Kim JO, Lee MK, Kim SH, Lim SJ. Development of paclitaxel-loaded liposomal nanocarrier stabilized by triglyceride incorporation. Int J Nanomed. 2016;11:4465–4477. doi:10.2147/IJN.S113723
79. Kannan V, Balabathula P, Divi MK, Thoma LA, Wood GC. Optimization of drug loading to improve physical stability of paclitaxel-loaded long-circulating liposomes. J Liposome Res. 2015;25(4):308–315. doi:10.3109/08982104.2014.995671
80. Li N, Feng L, Tan Y, Xiang Y, Zhang R, Yang M. Preparation, characterization, pharmacokinetics and biodistribution of baicalin-loaded liposome on cerebral ischemia-reperfusion after iv administration in rats. Molecules. 2018;23(7):1747–1761. doi:10.3390/molecules23071747
81. Wang X, Guan Q, Chen W, Hu X, Li L. Novel nanoliposomal delivery system for polydatin: preparation, characterization, and in vivo evaluation. Drug Des Devel Ther. 2015;9:1805–1813. doi:10.2147/DDDT.S77615
82. Yi C, Fu M, Cao X, et al. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, flammulina velutipes sterols, through liposomal encapsulation. J Agric Food Chem. 2013;61(25):5961–5971. doi:10.1021/jf3055278
83. Wang Y, Wang S, Firempong CK, et al. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: preparation and in vitro and in vivo evaluations. AAPS PharmSciTech. 2017;18(3):586–594. doi:10.1208/s12249-016-0537-8
84. Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomed. 2015;10:1001–1018. doi:10.2147/IJN.S56932
85. Chauhan P, Tyagi BK. Herbal novel drug delivery systems and transferosomes. J Drug Delivery Ther. 2018;8(3):162–168. doi:10.22270/jddt.v8i3.1772
86. Rai S, Pandey V, Rai G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev Exp. 2017;8(1):1–18. doi:10.1080/20022727.2017.1325708
87. El-Refaie WM, Elnaggar YS, El-Massik MA, Abdallah OY. Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: development, in-vitro appraisal and in-vivo studies. Int J Pharm. 2015;486(1–2):88–98. doi:10.1016/j.ijpharm.2015.03.052
88. Choi JH, Cho SH, Yun JJ, Yu YB, Cho CW. Ethosomes and transfersomes for topical delivery of ginsenoside Rh1 from red ginseng: characterization and in vitro evaluation. J Nanosci Nanotechnol. 2015;15(8):5660–5662. doi:10.1166/jnn.2015.10462
89. Ma H, Guo D, Fan Y, Wang J, Cheng J, Zhang X. Paeonol-loaded ethosomes as transdermal delivery carriers: design, preparation and evaluation. Molecules. 2018;23(7):1756–1771. doi:10.3390/molecules23071756
90. Sarwa KK, Mazumder B, Rudrapal M, Verma VK. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2015;22(5):638–646. doi:10.3109/10717544.2013.871601
91. Jangdey MS, Gupta A, Saraf S, Saraf S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: in vitro evaluation. Artif Cells Nanomed Biotechnol. 2017;45(7):1452–1462. doi:10.1080/21691401.2016.1247850
92. Avadhani KS, Manikkath J, Tiwari M, et al. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24(1):61–74. doi:10.1080/10717544.2016.1228718
93. Lu K, Xie S, Han S, et al. Preparation of a nano emodin transfersome and study on its anti-obesity mechanism in adipose tissue of diet-induced obese rats. J Transl Med. 2014;12(1):72–86. doi:10.1186/1479-5876-12-72
94. Nagalakshmi S, Krishnaraj K, Jothy AM, Chaudhari PS, Pushpalatha HB, Shanmuganthan S. Fabrication and characterization of herbal drug-loaded nonionic surfactant based niosomal topical gel. J Pharm Sci Res. 2016;8(11):1271–1278.
95. Un RN, Barlas FB, Yavuz M, et al. Phyto-niosomes: in vitro assessment of the novel nanovesicles containing marigold extract. Int J Polym Mater Polym Bio Mater. 2015;64(17):927–937. doi:10.1080/00914037.2015.1030663
96. Rohilla R, Garg T, Goyal AK, Rath G. Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. 2016;23(5):1645–1661. doi:10.3109/10717544.2014.945018
97. Thakkar M, Brijesh S. Physicochemical investigation and in vivo activity of anti-malarial drugs co-loaded in tween 80 niosomes. J Liposome Res. 2017;28:1–7.
98. Ambwani S, Tandon R, Ambwani TK, Malik YS. Current knowledge on nanodelivery systems and their beneficial applications in enhancing the efficacy of herbal drugs. J Exp Biol Agric Sci. 2018;6(1):87–107. doi:10.18006/2018.6(1).87.107
99. Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M. Proliferation and in vitro wound healing effects of the microniosomes containing Narcissus tazetta L. bulb extract on primary human fibroblasts (HDFs). DARU. 2018;26(1):31–42. doi:10.1007/s40199-018-0211-7
100. Pando D, Matos M, Gutiérrez G, Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf B Biointerfaces. 2015;128:398–404. doi:10.1016/j.colsurfb.2015.02.037
101. Priprem A, Janpim K, Nualkaew S, Mahakunakorn P. Topical niosome gel of Zingiber cassumunar Roxb. extract for anti-inflammatory activity enhanced skin permeation and stability of compound D. AAPS PharmSciTech. 2016;17(3):631–639. doi:10.1208/s12249-015-0376-z
102. Barani M, Mirzaei M, Torkzadeh-Mahani M, Nematollahi MH. Lawsone-loaded niosome and its antitumor activity in MCF-7 breast cancer cell line: a nano-herbal treatment for cancer. DARU. 2018;26(1):11–27. doi:10.1007/s40199-018-0207-3
103. Anghore D, Kulkarni GT. Development of novel nano niosomes as drug delivery system of spermacoce hispida extract and in vitro antituberculosis activity. Curr Nanomat. 2017;2(1):17–23. doi:10.2174/2405461502666170314151949
104. Alam MS, Ahad A, Abidin L, Aqil M, Mir SR, Mujeeb M. Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in wistar rats. Biomed Pharmacother. 2018;97:1514–1520. doi:10.1016/j.biopha.2017.11.073
105. Gunes A, Guler E, Un RN, et al. Niosomes of nerium oleander extracts: in vitro assessment of bioactive nanovesicular structures. J Drug Deliv Sci Technol. 2017;37:158–165. doi:10.1016/j.jddst.2016.12.013
106. Budhiraja A, Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015;22(6):723–730. doi:10.3109/10717544.2014.903010
107. Scognamiglio I, De Stefano D, Campani V, et al. Nanocarriers for topical administration of resveratrol: a comparative study. Int J Pharm. 2013;440:179–187. doi:10.1016/j.ijpharm.2012.08.009
108. Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014;460:280–288. doi:10.1016/j.ijpharm.2013.11.017
109. Abdulbaqi IM, Darwis Y, Assi RA, Khan NA. Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug Des Devel Ther. 2018;12:795–813. doi:10.2147/DDDT.S158018
110. Zhao YZ, Lu CT, Zhang Y, et al. Selection of high efficient transdermal lipid vesicle for curcumin skin delivery. Int J Pharm. 2013;454:302–309. doi:10.1016/j.ijpharm.2013.06.052
111. Yu Z, Lv H, Han G, Ma K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLoS One. 2016;11(7):e0159967. doi:10.1371/journal.pone.0159967
112. Fatima Z. Formulation and performance evaluation of berberis aristata extract loaded ethosomal gel. Asian J Pharm. 2017;11(03):176–183.
113. Liu F, Sun Y, Kang C, Zhu H. Pegylated drug delivery systems: from design to biomedical applications. Nano Life. 2016;6:1642002. doi:10.1142/S1793984416420022
114. Tolia GT, Choi HH. The role of dendrimers in topical drug delivery. Pharm Technol. 2008;32(11):88–98.
115. Estanqueiro M, Amaral MH, Conceicao J, SousaLobo JM. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–648. doi:10.1016/j.colsurfb.2014.12.041
116. Iacobazzi RM, Porcelli L, Lopedota AA, et al. Targeting human liver cancer cells with lactobionic acid-G (4)-PAMAM-FITC sorafenib loaded dendrimers. Int J Pharm. 2017;528(1–2):485–497. doi:10.1016/j.ijpharm.2017.06.049
117. Chittasupho C, Anuchapreeda S, Sarisuta N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur J Pharm Biopharm. 2017;119:310–321. doi:10.1016/j.ejpb.2017.07.003
118. Luong D, Kesharwani P, Deshmukh R, et al. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016;43:14–29. doi:10.1016/j.actbio.2016.07.015
119. Ertürk AS, Gürbüz MU, Tülü M. The effect of PAMAM dendrimer concentration, generation size and surface functional group on the aqueous solubility of Candesartan cilexetil. Pharm Dev Technol. 2017;22(1):111–121. doi:10.1080/10837450.2016.1219372
120. Abderrezak A, Bourassa P, Mandeville JS, Sedaghat-Herati R, Tajmir-Riahi HA. Dendrimers bind antioxidant polyphenols and cisplatin drug. PLoS One. 2012;7:e33102. doi:10.1371/journal.pone.0033102
121. Madaan K, Lather V, Pandita D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv. 2016;23(1):254–262. doi:10.3109/10717544.2014.910564
122. Wang L, Xu X, Zhang Y, et al. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. J Mater Sci Mater Med. 2013;24:2137–2144. doi:10.1007/s10856-013-4969-3
123. Falconieri MC, Adamo M, Monasterolo C, Bergonzi MC, Coronnello M, Bilia AR. New dendrimer-based nanoparticles enhance curcumin solubility. Planta Med. 2017;83(5):420–425. doi:10.1055/s-0042-103161
124. Gu L, Wu ZH, Qi X, et al. Polyamidomine dendrimers: an excellent drug carrier for improving the solubility and bioavailability of puerarin. Pharm Dev Technol. 2013;18(5):1051–1057. doi:10.3109/10837450.2011.653822
125. Kambhampati SP, Kannan RM. Dendrimer nanoparticles for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29(2):151–165. doi:10.1089/jop.2012.0232
126. Diaz C, Guzmán J, Jiménez VA, Alderete JB. Partially PEGylated PAMAM dendrimers as solubility enhancers of silybin. Pharm Dev Technol. 2018;23(7):689–696. doi:10.1080/10837450.2017.1315134
127. Yesil Celiktas O, Pala C, Cetin Uyanikgil EO, Sevimli Gur C. Synthesis of silica-PAMAM dendrimer nanoparticles as promising carriers in neuro blastoma cells. Anal Biochem. 2017;519:1–7. doi:10.1016/j.ab.2016.12.004
128. Qu WJ, Li HF, Su YY, Dong ZQ, Ge YR. Absorption enhancing effects and safety of PAMAM dendrimers on liquiritin. China J Chi Mater Med. 2017;42(9):1766–1771. doi:10.19540/j.cnki.cjcmm.2017.0070
129. Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci. 2016;83:184–202. doi:10.1016/j.ejps.2015.12.031
130. Boutet E. Scheme of a micelle formed by phospholipids in an aqueous solution. Available from: https://commons.wikimedia.org/wiki/File:Micelle_scheme-en.svg. Accessed December 5, 2019.
131. Zou F, Wei K, Peng X. Thermodynamics of micellization and sustained release of folate targeted capecitabine loaded nanomicelles. Nanosci Nanotechnol. 2016;16:8519–8527. doi:10.1166/jnn.2016.12710
132. Han R, Sun Y, Kang C, Sun H, Wei W. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J Drug Target. 2017;25:140–148. doi:10.1080/1061186X.2016.1207649
133. Wang Z, Yu Y, Ma J, et al. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol Pharm. 2012;9:2646–2657. doi:10.1021/mp3002107
134. Ren J, Fang Z, Yao L, et al. A micelle-like structure of poloxamer–methotrexate conjugates as nanocarrier for methotrexate delivery. Int J Pharm. 2015;487(1–2):177–186. doi:10.1016/j.ijpharm.2015.04.014
135. Zhang Y, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials. 2012;33(2):679−691.
136. Gou M, Men K, Shi H, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3(4):1558–1567. doi:10.1039/c0nr00758g
137. Abdelmoneem MA, Mahmoud M, Zaky A. et al. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J Control Release;2018. 78–93. doi:10.1016/j.jconrel.2018.08.026
138. Wu H, Yu T, Tian Y, Wang Y, Zhao R, Mao S. Enhanced liver-targeting via coadministration of 10-hydroxycamptothecin polymeric micelles with vinegar baked radix bupleuri. Phytomedicine. 2018;44:1–8. doi:10.1016/j.phymed.2018.04.022
139. Su Y, Huang N, Chen D, et al. Successful in vivo hyperthermal therapy toward breast cancer by Chinese medicine shikonin-loaded thermosensitive micelle. Int J Nanomed. 2017;12:4019. doi:10.2147/IJN.S132639
140. Anantaworasakul P, Okonogi S. Encapsulation of sesbania grandiflora extract in polymeric micelles to enhance its solubility, stability, and antibacterial activity. J Microencapsul. 2017;34(1):73–81. doi:10.1080/02652048.2017.1284277
141. Baldissera MD, Souza CF, Boligon AA, et al. Solving the challenge of the blood–brain barrier to treat infections caused by Trypanosoma evansi: evaluation of nerolidol-loaded nanospheres in mice. Parasitology. 2017;144(11):1543–1550. doi:10.1017/S003118201700110X
142. Harper 3D. Nano sphere from carbon atoms isolated on white background. Available from: https://www.shutterstock.com/image-illustration/nano-sphere-carbon-atoms-isolated-on-50117683.
143. Wang T, Wu C, Fan G, Li T, Gong H, Cao F. Ginkgo biloba extracts-loaded starch nano-spheres: preparation, characterization, and in vitro release kinetics. Int J Biol Macromol. 2018;106:148–157. doi:10.1016/j.ijbiomac.2017.08.012
144. Pirouzmand H, Khameneh B, Tafaghodi M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm Biol. 2017;55(1):212–227. doi:10.1080/13880209.2016.1257032
145. Elzoghby A. Polymeric nanocarriers as robust platforms for cancer therapy. Curr Pharm Des. 2017;23(35):5211–5212. doi:10.2174/1381612823999171106125801
146. Li C, Zhang D, Guo H, et al. Preparation and characterization of galactosylated bovine serum albumin nanoparticles for liver-targeted delivery of oridonin. Int J Pharm. 2013;448:79–86. doi:10.1016/j.ijpharm.2013.03.019
147. Snima KS, Arunkumar P, Jayakumar R, Lakshmanan VK. Silymarin encapsulated poly(D, L-lactic-co-glycolic acid) nanoparticles: a prospective candidate for prostate cancer therapy. J Biomed Nanotechnol. 2014;10:559–570. doi:10.1166/jbn.2014.1735
148. Pereira K, Quintela E, Da Silva D, et al. Characterization of nanospheres containing Zanthoxylum riedelianum Fruit essential oil and their insecticidal and deterrent activities against Bemisia tabaci (hemiptera: aleyrodidae). Molecules. 2018;23(8):2052–2070. doi:10.3390/molecules23082052
149. Holz JP, Bottene MK, Jahno VD, Einloft S, Ligabue R. Menthol-loaded PLGA micro and nanospheres: synthesis, characterization and degradation in artificial saliva. Mater Res. 2018;21(2):1–9. doi:10.1590/1980-5373-mr-2017-0488
150. Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66. doi:10.1016/j.jconrel.2014.03.030
151. Lu Y, Qi J, Dong X, Zhao W, Wu W. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22(4):744–750. doi:10.1016/j.drudis.2017.01.003
152. Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6(2):106–113. doi:10.1016/j.apsb.2015.11.005
153. Sharma OP, Patel V, Mehta T, et al. Nanocrystal for ocular drug delivery: hope or hype. Drug Deliv Transl Res. 2016;6:399–413. doi:10.1007/s13346-016-0292-0
154. Choi JS, Park JS. Development of docetaxel nanocrystals surface modified with transferrin for tumor targeting. Drug Des Devel Ther. 2017;11:17–26. doi:10.2147/DDDT.S122984
155. Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415(1–2):293–300. doi:10.1016/j.ijpharm.2011.05.075
156. de Waard H, Frijlink HW, Hinrichs WL. Bottom up preparation techniques for nanocrystals of lipophilic drugs. Pharmtech Res. 2011;28:1220e1223.
157. Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(1):13–23. doi:10.1016/j.ajps.2014.08.005
158. Malamatari M, Taylor KM, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23:534–547. doi:10.1016/j.drudis.2018.01.016
159. Al Shaal L, Shegokar R, Müller RH. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int J Pharm. 2011;420(1):133–140. doi:10.1016/j.ijpharm.2011.08.018
160. Srivalli KM, Mishra B. Drug nanocrystals: a way toward scale-up. Saudi Pharm J. 2016;24(4):386–404. doi:10.1016/j.jsps.2014.04.007
161. Vidlarova L, Romero GB, Hanuš J, et al. Nanocrystals for dermal penetration enhancement – effect of concentration and underlying mechanisms using curcumin as model. Eur J Pharm Biopharm. 2016;104:216–225. doi:10.1016/j.ejpb.2016.05.004
162. Pi J, Liu Z, Wang H, et al. Ursolic acid nanocrystals for dissolution rate and bioavailability enhancement: influence of different particle size. Curr Drug Deliv. 2016;13(8):1358–1366. doi:10.2174/1567201813666160307142757
163. Sahoo NG, Kakran M, Shaal LA, et al. Preparation and characterization of quercetin nanocrystals. J Pharm Sci. 2011;100(6):2379–2390. doi:10.1002/jps.22446
164. Sathishkumar P, Gu FL, Zhan Q, Palvannan T, Yusoff AR. Flavonoids mediated ‘green' nanomaterials: a novel nanomedicine system to treat various diseases–current trends and future perspective. Mater Lett. 2018;210:26–30. doi:10.1016/j.matlet.2017.08.078
165. Mir M, Ishtiaq S, Rabia S, et al. Nanotechnology: from in vivo imaging system to controlled drug delivery. Nanoscale Res Lett. 2017;12(1):500–515. doi:10.1186/s11671-017-2249-8
166. Singh RP, Gangadharappa HV, Mruthunjaya K. Phytosome loaded novel herbal drug delivery system: a review. Int Res J Pharm. 2016;7(6):15–21. doi:10.7897/2230-8407
167. Rasaie S, Ghanbarzadeh S, Mohammadi M, Hamishehkar H. Nano phytosomes of quercetin: a promising formulation for fortification of food products with antioxidants. Pharm Sci. 2014;20(3):96–101.
168. Onoue S, Yamada S, Chan HK. Nanodrugs: pharmacokinetics and safety. Int J Nanomed. 2014;9:1025–1037. doi:10.2147/IJN
169. Karthivashan G, Masarudin MJ, Kura AU, Abas F, Fakurazi S. Optimization, formulation, and characterization of multiflavonoids-loaded flavanosome by bulk or sequential technique. Int J Nanomed. 2016;11:3417–3434. doi:10.2147/IJN.S112045
170. Anwar E, Farhana N. Formulation and evaluation of phytosome-loaded maltodextrin-gum Arabic microsphere system for delivery of Camellia sinensis extract. J Young Pharm. 2018;10(2s):S56. doi:10.5530/jyp
171. El-Menshawe SF, Ali AA, Rabeh MA, Khalil NM. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: an approach for acceptable level of evidence of an effective novel herbal weight loss product. Int J Nanomed. 2018;13:307–318. doi:10.2147/IJN.S153429
172. Hooresfand Z, Ghanbarzadeh S, Hamishehkar H. Preparation and characterization of rutin-loaded nanophytosomes. Pharm Sci. 2015;21(3):145–151. doi:10.15171/PS.2015.29
173. Vu HT, Hook SM, Siqueira SD, Müllertz A, Rades T, McDowell A. Are phytosomes a superior nanodelivery system for the antioxidant rutin? Int J Pharm. 2018;122:214–229.
174. Gahandule MB, Jadhav SJ, Gadhave MV, Gaikwad DD. Formulation and development of hepato-protective butea monosperma-phytosome. Int J Res Pharm Pharm Sci. 2016;1(4):21–27.
175. Singh RP, Gangadharappa HV, Mruthunjaya K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: in vitro and in vivo evaluation. Eur J Pharm Sci. 2018;122:214–229. doi:10.1016/j.ejps.2018.06.028
176. Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro and in vivo evaluation. J Mol Liq. 2016;218:219–232. doi:10.1016/j.molliq.2016.02.081
177. Senapati PC, Sahoo SK, Sahu AN. Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed Pharmacother. 2016;80:42–51. doi:10.1016/j.biopha.2016.02.039
178. Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23(9):3639–3652. doi:10.1080/10717544.2016.1214990
179. Agrawal AG, Kumar A, Gide PS. Formulation of solid self-nanoemulsifying drug delivery systems using N-methyl pyrrolidone as cosolvent. Drug Dev Ind Pharm. 2015;41:594–604. doi:10.3109/03639045.2014.886695
180. Li W, Yi S, Wang Z, et al. Self-nanoemulsifying drug delivery system of persimmon leaf extract: optimization and bioavailability studies. Int J Pharm. 2011;420(1):161–171. doi:10.1016/j.ijpharm.2011.08.024
181. Avachat AM, Patel VG. Self-nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm J. 2015;23(3):276–289. doi:10.1016/j.jsps.2014.11.001
182. Tran TH, Guo Y, Song D, Bruno RS, Lu X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J Pharm Sci. 2014;103(3):840–852. doi:10.1002/jps.23858
183. Shen J, Bi J, Tian H, et al. Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with akebia saponin D–phospholipid complex. Int J Nanomed. 2016;11:4919–4929. doi:10.2147/IJN.S108765
184. Shukla M, Jaiswal S, Sharma A, et al. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Drug Dev Ind Pharm. 2017;43(5):847–861. doi:10.1080/03639045.2016.1239732
185. Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–561. doi:10.3109/10717544.2013.878003
186. Yeom DW, Chae BR, Son HY, et al. Enhanced oral bioavailability of valsartan using a polymer-based supersaturable self-microemulsifying drug delivery system. Int J Nanomed. 2017;12:3533–3545. doi:10.2147/IJN.S136599
187. Yan B, Wang Y, Ma Y, Zhao J, Liu Y, Wang L. In vitro and in vivo evaluation of poly (acrylic acid) modified mesoporous silica nanoparticles as pH response carrier for β-elemene self-micro emulsifying. International Journal of Pharmaceutics. 2019;572:118768- 18778.
188. Allen JL. Basics of compounding-nonsterile: compounding self-emulsifying drug delivery systems and other self-emulsifying lipid formulations, part 1. Int J Pharm Compd. 2018;22(3):220–228.
189. Jakab G, Fülöp V, Santha K, Szeröczei D, Balogh E, Antal L. Formulation possibilities of self-emulsifying drug delivery systems, microemulsions and nanoemulsions. Acta Pharm Hung. 2017;87(1):27–34.
190. Zhang L, Zhu W, Yang C, et al. A novel folate-modified self-microemulsifying drug delivery system of curcumin for colon targeting. Int J Nanomed. 2012;7:151–162. doi:10.2147/IJN.S27639
191. Chen Y, Zhang H, Yang J, Sun H. Improved antioxidant capacity of optimization of a self-microemulsifying drug delivery system for resveratrol. Molecules. 2015;20:21167–21177. doi:10.3390/molecules201219750
192. Jaisamut P, Wiwattanawongsa K, Graidist P, Sangsen Y, Wiwattanapatapee R. Enhanced oral bioavailability of curcumin using a supersaturatable self-microemulsifying system incorporating a hydrophilic polymer; in vitro and in vivo investigations. AAPS PharmSciTech. 2018;19(2):730–740. doi:10.1208/s12249-017-0857-3
193. Qiao J, Ji D, Sun S, et al. Oral bioavailability and lymphatic transport of pueraria flavone-loaded self-emulsifying drug-delivery systems containing sodium taurocholate in rats. Pharmaceutics. 2018;10(3):147–160. doi:10.3390/pharmaceutics10030147
194. Sornsuvit C, Hongwiset D, Yotsawimonwat S, Toonkum M, Thongsawat S, Taesotikul W. The bioavailability and pharmacokinetics of silymarin SMEDDS formulation study in healthy thai volunteers. Evid Based Complement Altern Med. 2018;1:1–7. doi:10.1155/2018/1507834
195. Dhumal DM, Akamanchi KG. Self-microemulsifying drug delivery system for camptothecin using new bicephalous heterolipid with tertiary-amine as branching element. Int J Pharm. 2018;541(1–2):48–55. doi:10.1016/j.ijpharm.2018.02.030
196. Sato Y, Joumura T, Nashimoto S, et al. Enhancement of lymphatic transport of lutein by oral administration of a solid dispersion and a self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2018;127:171–176. doi:10.1016/j.ejpb.2018.02.013
197. Karami Z, Rezaeian I, Zahedi P, Abdollahi M. Preparation and performance evaluations of electrospun poly (ε‐caprolactone), poly (lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J Appl Polym Sci. 2013;129(2):756–766. doi:10.1002/app.38683
198. Available from: http://science.sciencemag.org/content/294/5547/1684/F1 .
199. Wang H, Wei J, Yang C, et al. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials. 2012;33(24):5848–5853. doi:10.1016/j.biomaterials.2012.04.047
200. Wagh A, Singh J, Qian S, Law B. A short circulating peptide nanofiber as a carrier for tumoral delivery. Nanomedicine. 2013;9(4):449–457. doi:10.1016/j.nano.2012.10.009
201. Liu J, Xu H, Zhang Y, et al. Novel tumor-targeting, selfassembling peptide nanofiber as a carrier for effective curcumin delivery. Int J Nanomed. 2014;9:197–207. doi:10.2147/IJN.S55875
202. Ranjbar MM, Bahrami SH. Electrospun curcumin loaded poly (ε-caprolactone)/gum tragacanth nanofibers for biomedical application. Int J Biol Macromol. 2016;84:448–456. doi:10.1016/j.ijbiomac.2015.12.024
203. Choi J, Yang BJ, Bae GN, Jung JH. Herbal extract incorporated nanofiber fabricated by an electrospinning technique and its application to antimicrobial air filtration. J Ocul Pharmacol Ther. 2015;7(45):25313–25320. doi:10.1021/acsami.5b07441
204. Bonan RF, Bonan PR, Batista AU, et al. In vitro antimicrobial activity of solution blow spun poly (lactic acid)/polyvinylpyrrolidone nanofibers loaded with copaiba (Copaifera sp.) oil. Mater Sci Eng C. 2015;48:372–377. doi:10.1016/j.msec.2014.12.021
205. Jouybar A, Seyedjafari E, Ardeshirylajimi A, et al. Enhanced skin regeneration by herbal extract‐coated poly‐L‐lactic acid nanofibrous scaffold. Artif Organs. 2017;41(11):E296–E307. doi:10.1111/aor.12926
206. Parvathi K, Krishnan AG, Anitha A, Jayakumar R, Nair MB. Poly (L-lactic acid) nanofibers containing cissus quadrangularis induced osteogenic differentiation in vitro. Int J Biol Macromol. 2018;110:514–521. doi:10.1016/j.ijbiomac.2017.11.094
207. Wang J, Tian L, He L, et al. Lycium barbarum polysaccharide encapsulated poly lactic-co-glycolic acid nanofibers: cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci Rep. 2018;8(1):8669–8683. doi:10.1038/s41598-018-26837-z
208. Lee JS, Feijen J. Biodegradable polymersomes as carriers and release systems for paclitaxel using oregon green® 488 labeled paclitaxel as a model compound. J Control Release. 2012;158(2):312–318. doi:10.1016/j.jconrel.2011.10.025
209. Pijpers IA, Abdelmohsen LK, Xia Y, et al. Adaptive polymersome and micelle morphologies in anticancer nanomedicine: from design rationale to fabrication and proof‐of‐concept studies. Adv Ther. 2018;1:1800068–1800081. doi:10.1002/adtp.201800068
210. Gupta PK, Jaiswal AK, Asthana S, Dube A, Mishra PR. Antigen presenting cells targeting and stimulation potential of lipoteichoic acid functionalized lipo-polymerosome: a chemo-immunotherapeutic approach against intracellular infectious disease. Biomacromolecules. 2015;16(4):1073–1087. doi:10.1021/bm5015156
211. Gupta PK, Asthana S, Jaiswal AK, et al. Exploitation of lectinized lipo-polymerosome encapsulated amphotericin B to target macrophages for effective chemotherapy of visceral leishmaniasis. Bioconjug Chem. 2014;25(6):1091–1102. doi:10.1021/bc500087h
212. Gupta PK, Jaiswal AK, Kumar V, et al. Covalent functionalized self-assembled lipo-polymerosome bearing amphotericin B for better management of leishmaniasis and its toxicity evaluation. Mol Pharm. 2014;11(3):951–963. doi:10.1021/mp400603t
213. Hammer DA, Robbins GP, Haun JB, et al. Leukopolymersomes. Faraday Discuss. 2008;139:129–141. doi:10.1039/b717821b
214. Xu J, Zhao Q, Jin Y, Qiu L. High loading of hydrophilic/hydrophobic doxorubicin into polyphosphazene polymersome for breast cancer therapy. Nanomedicine. 2014;10(2):349–358. doi:10.1016/j.nano.2013.08.004
215. Yang J, Dai G, Hou Y, et al. Quantification of oxymatrine in rat plasma by UPLC-MS/MS to support the pharmacokinetic analyses of oxymatrine-loaded polymersomes. Anal Methods. 2014;6(6):1811–1817. doi:10.1039/C3AY42294A
216. Goyal K, Konar A, Kumar BH, Koul V. Lactoferrin-conjugated pH and redox-sensitive polymersomes based on PEG-SS-PLA-PCL-OH boost delivery of bacosides to the brain. Nanoscale. 2018;10(37):17781–17798. doi:10.1039/C8NR03828G
217. Pang Z, Feng L, Hua R, et al. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm. 2010;7(6):1995–2005. doi:10.1021/mp100277h
218. Tu YS, Fu JW, Sun DM, et al. Preparation, characterisation and evaluation of curcumin with piperine-loaded cubosome nanoparticles. J Microencapsul. 2014;31(6):551–559. doi:10.3109/02652048.2014.885607
219. Rizwan SB, Boyd BJ. Cubosomes: structure, preparation and use as an antigen delivery system. In: Subunit Vaccine Delivery. New York, NY, USA: Springer; 2015:125–140.
220. Tian Y, Li JC, Zhu JX, et al. Folic acid-targeted etoposide cubosomes for theranostic application of cancer cell imaging and therapy. Med Sci Monit. 2017;23:2426–2435. doi:10.12659/MSM.904683
221. Azmi ID, Moghimi SM, Yaghmur A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther Deliv. 2015;6(12):1347–1364. doi:10.4155/tde.15.81
222. Saraf S, Gupta A, Alexander A, Khan J, Jangde M, Saraf S. Advancements and avenues in nanophytomedicines for better pharmacological responses. J Nanosci Nanotechnol. 2015;15(6):4070–4079. doi:10.1166/jnn.2015.10333
223. Nithya R, Jerold P, Siram K. Cubosomes of dapsone enhanced permeation across the skin. J Drug Deliv Sci Technol. 2018;48:75–81. doi:10.1016/j.jddst.2018.09.002
224. Bazylińska U, Kulbacka J, Schmidt J, Talmon Y, Murgia S. Polymer-free cubosomes for simultaneous bioimaging and photodynamic action of photosensitizers in melanoma skin cancer cells. J Colloid Interface Sci. 2018;522:163–173. doi:10.1016/j.jcis.2018.03.063
225. Elnaggar YS, Etman SM, Abdelmonsif DA, Abdallah OY. Novel piperine-loaded tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in alzheimer’s disease: pharmaceutical, biological, and toxicological studies. Int J Nanomed. 2015;10:5459–5473. doi:10.2147/IJN.S87336
226. Herman A, Herman AP. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia. 2016;114:18–25. doi:10.1016/j.fitote.2016.08.008
227. Archana A, Vijayasri K, Madhurim M, Kumar CA. Curcumin loaded nano cubosomal hydrogel: preparation, in vitro characterization and antibacterial activity. Chem Sci Trans. 2014;4(1):75–80.
228. Ou N, Sun Y, Zhou S, et al. Evaluation of optimum conditions for achyranthes bidentata polysaccharides encapsulated in cubosomes and immunological activity in vitro. Int J Biol Macromol. 2018;109:748–760. doi:10.1016/j.ijbiomac.2017.11.064
229. Matloub AA, AbouSamra MM, Salama AH, Rizk MZ, Aly HF, Fouad GI. Cubic liquid crystalline nanoparticles containing a polysaccharide from ulva fasciata with potent antihyperlipidaemic activity. Saudi Pharm J. 2018;26(2):224–231. doi:10.1016/j.jsps.2017.12.007
230. Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14(1):1629–1654. doi:10.3390/ijms14011629
231. Fathi M, Martin A, McClements DJ. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci Tech. 2014;39(1):18–39. doi:10.1016/j.tifs.2014.06.007
232. Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar-Jain P, Adivarekar RV. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int J Biol Macromol. 2018;121:936–946. doi:10.1016/j.ijbiomac.2018.10.125
233. Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomed. 2011;6:16211630. doi:10.2147/IJN.S25646
234. Bu L, Gan LC, Guo XQ, et al. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. Int J Pharm. 2013;452(1–2):355–362. doi:10.1016/j.ijpharm.2013.05.007
235. Samrot AV, Burman U, Philip SA, Shobana N, Chandrasekaran K. Synthesis of curcumin loaded polymeric nanoparticles from crab shell derived chitosan for drug delivery. Inf Med Unlocked. 2018;10:159–182. doi:10.1016/j.imu.2017.12.010
236. Esfanjani AF, Jafari SM. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf B Biointerfaces. 2016;146:532–543. doi:10.1016/j.colsurfb.2016.06.053
237. Khan N, Bharali DJ, Adhami VM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35:415–423. doi:10.1093/carcin/bgt321
238. Loch-Neckel G, Santos-Bubniak L, Mazzarino L, et al. Orally administered chitosan-coated polycaprolactone nanoparticles containing curcumin attenuate metastatic melanoma in the lungs. J Pharm Sci. 2015;104:3524–3534. doi:10.1002/jps.24548
239. Karri VV, Kuppusamy G, Talluri SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol. 2016;93:1519–1529. doi:10.1016/j.ijbiomac.2016.05.038
240. Feng B, Ashraf MA, Peng L. Characterization of particle shape, zeta potential, loading efficiency and outdoor stability for chitosan-ricinoleic acid loaded with rotenone. Open Life Sci. 2016;11(1):380–386. doi:10.1515/biol-2016-0050
241. Suksaeree J, Monton C, Madaka F, et al. Formulation, physicochemical characterization, and in vitro study of chitosan/HPMC blends-based herbal blended patches. AAPS PharmSciTech. 2015;16(1):171–181. doi:10.1208/s12249-014-0216-6
242. Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials. 2015;5(4):2054–2130. doi:10.3390/nano5042054
243. Dimatteo R, Darling NJ, Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev. 2018;127:167–184. doi:10.1016/j.addr.2018.03.007
244. Atta S, Khaliq S, Islam A, et al. Injectable biopolymer based hydrogels for drug delivery applications. Int J Biol Macromol. 2015;80:240–245. doi:10.1016/j.ijbiomac.2015.06.044
245. Córdoba AL, Deladino L, Martino M. Effect of starch filler on calcium alginate hydrogels loaded with yerba mate antioxidants. Carbohydr Polym. 2013;95:315–323. doi:10.1016/j.carbpol.2013.03.019
246. Mun S, Kim YR, McClements DJ. Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015;173:454–461. doi:10.1016/j.foodchem.2014.10.053
247. Balestrin LA, Bidone J, Bortolin RC, Moresco K, Moreira JC, Teixeira HF. Protective effect of a hydrogel containing Achyrocline satureioides extract loaded nanoemulsion against UV-induced skin damage. J Photochem Photobiol B. 2016;163:269–276. doi:10.1016/j.jphotobiol.2016.08.039
248. Lustosa AK, de Jesus Oliveira AC, Quelemes PV, et al. In situ synthesis of silver nanoparticles in a hydrogel of carboxymethyl cellulose with phthalated-cashew gum as a promising antibacterial and healing agent. Int J Mol Sci. 2017;18(11):2399–2413. doi:10.3390/ijms18112399
249. Qureshi MA, Khatoon F, Rizvi MA, Zafaryab M. Ethyl acetate Salix alba leaves extract-loaded chitosan-based hydrogel film for wound dressing applications. J Biomater Sci Polym Ed. 2015;26(18):1452–1464. doi:10.1080/09205063.2015.1100843
250. Muhsin MD, George G, Beagley K, Ferro V, Armitage C, Islam N. Synthesis and toxicological evaluation of a chitosan-L-leucine conjugate for pulmonary drug delivery applications. Biomacromolecules. 2014;15(10):3596–3607. doi:10.1021/bm5008635
251. Available from: https://www.labsexplorer.com/service/polymer-drug-conjugation-service_525.
252. Shohani S, Mondanizadeh M, Abdoli A, et al. Trimethyl chitosan improves anti-HIV effects of atripla as a new nanoformulated drug. Curr HIV Res. 2017;15(1):56–65. doi:10.2174/1570162X14666161216142806
253. Du C, Qian J, Zhou L, Su Y, Zhang R, Dong CM. Biopolymer–drug conjugate nanotheranostics for multimodal imaging-guided synergistic cancer photothermal–chemotherapy. J Ocul Pharmacol Ther. 2017;9(37):31576–31588. doi:10.1021/acsami.7b10163
254. Yi J, Liu Y, Zhang Y, Gao L. Fabrication of resveratrol-loaded whey protein–dextran colloidal complex for the stabilization and delivery of β-carotene emulsions. J Agric Food Chem. 2018;66(36):9481–9489. doi:10.1021/acs.jafc.8b02973
255. Singh A, Dutta PK, Kumar H, Kureel AK, Rai AK. Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr Polym. 2018;193:99–107. doi:10.1016/j.carbpol.2018.03.092
256. Singh A, Kureel AK, Dutta PK, Kumar S, Rai AK. Curcumin loaded chitin-glucan quercetin conjugate: synthesis, characterization, antioxidant, in vitro release study, and anticancer activity. Int J Biol Macromol. 2018;110:234–244. doi:10.1016/j.ijbiomac.2017.11.002
257. Chen J, Qin X, Zhong S, Chen S, Su W, Liu Y. Characterization of curcumin/cyclodextrin polymer inclusion complex and investigation on its antioxidant and antiproliferative activities. Molecules. 2018;23(5):1179–1191. doi:10.3390/molecules23051179
258. Zhang H, Xu W, Omari-Siaw E, et al. Redox-responsive PEGylated self-assembled prodrug-nanoparticles formed by single disulfide bond bridge periplocymarin-vitamin E conjugate for liver cancer chemotherapy. Drug Deliv. 2017;24(1):1170–1178. doi:10.1080/10717544.2017.1365393
259. Kang C, Sun Y, Wang M, Cheng X. Nanosized camptothecin conjugates for single and combined drug delivery. Eur J BioMed Res. 2016;2:8–14. doi:10.18088/ejbmr.2.1.2016.pp8-14
260. Luna Nanotech. Gold Nanoparticles - Citrate. Available from: https://www.lunanano.com/product-page/gold-nanoparticles-citrate-coated.
261. Poudel BK, Soe ZC, Ruttala HB, et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int J Pharm. 2018;548(1):92–103. doi:10.1016/j.ijpharm.2018.06.056
262. Vio V, Jose Marchant M, Araya E, Kogan JM. Metal nanoparticles for the treatment and diagnosis of neurodegenerative brain diseases. Curr Pharm Des. 2017;23(13):1916–1926. doi:10.2174/1381612823666170105152948
263. Vodyanoy V, Daniels Y, Pustovyy O, MacCrehan WA, Muramoto S, Stan G. Engineered metal nanoparticles in the sub-nanomolar levels kill cancer cells. Int J Nanomed. 2016;11:1567–1576. doi:10.2147/IJN
264. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2018;122:137–148. doi:10.1016/j.ijbiomac.2018.10.120
265. Sun YW, Wang LH, Meng DL, Che X. A green and facile preparation approach, licochalcone A capped on hollow gold nanoparticles, for improving the solubility and dissolution of anticancer natural product. Oncotarget. 2017;8(62):105673–105681. doi:10.18632/oncotarget.22387
266. Azeez L, Lateef A, Adebisi SA. Silver nanoparticles (AgNPs) biosynthesized using pod extract of Cola nitida enhances antioxidant activity and phytochemical composition of Amaranthus caudatus Linn. Appl Nanosci. 2017;7(1–2):59–66. doi:10.1007/s13204-017-0546-2
267. Namvar F, Rahman HS, Mohamad R, et al. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. Evid Based Complement Altern Med. 2015;2015:1–11. doi:10.1155/2015/593014
268. Danafar H, Sharafi A, Kheiri S. Co-delivery of sulforaphane and curcumin with PEGylated iron oxide-gold core shell nanoparticles for delivery to breast cancer cell line. Iran J Pharm Res. 2018;17(2):480–494.
269. Azizi S, Shahri MM, Rahman HS, Rahim RA, Rasedee A, Mohamad R. Green synthesis palladium nanoparticles mediated by white tea (Camellia sinensis) extract with antioxidant, antibacterial, and antiproliferative activities toward the human leukemia (MOLT-4) cell line. Int J Nanomed. 2017;12:8841–8853. doi:10.2147/IJN.S149371
270. Poonia N, Lather V, Pandita D. Mesoporous silica nanoparticles: a smart nanosystem for management of breast cancer. Drug Discov Today. 2017;23(2):315–332. doi:10.1016/j.drudis.2017.10.022
271. Al-Asmar A, Giosafatto CV, Sabbah M, Sanchez A, Villalonga Santana R, Mariniello L. Effect of Mesoporous Silica Nanoparticles on The Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials. 2020;10(1):52–70.
272. Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8(2):165–177. doi:10.1016/j.apsb.2018.01.007
273. Desai D, Zhang J, Sandholm J, et al. Lipid bilayer-gated mesoporous silica nanocarriers for tumor-targeted delivery of zoledronic acid in vivo. Mol Pharmacol. 2017;14(9):3218–3227. doi:10.1021/acs.molpharmaceut.7b00519
274. Cao X, Deng WW, Fu M, et al. In vitro release and in vitro–in vivo correlation for silybin meglumine incorporated into hollow-type mesoporous silica nanoparticles. Int J Nanomed. 2012;7:753–762. doi:10.2147/IJN.S28348
275. AbouAitah K, Swiderska-Sroda A, Farghali AA, et al. Folic acid–conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget. 2018;9(41):26466–26490. doi:10.18632/oncotarget.25470
276. Lin J, Cai Q, Tang Y, et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: design, characterization and its cytotoxic effect. Int J Pharm. 2018;536(1):272–282. doi:10.1016/j.ijpharm.2017.10.043
277. Li T, Chen X, Liu Y, et al. pH-sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur J Pharm Sci. 2017;96:456–463. doi:10.1016/j.ejps.2016.10.019
278. Kumar B, Kulanthaivel S, Mondal A, et al. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf B Biointerfaces. 2017;150:352–361. doi:10.1016/j.colsurfb.2016.10.049
279. Choi JY, Ramasamy T, Kim SY, et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016;39:94–105. doi:10.1016/j.actbio.2016.05.012
280. Souza AC, Amaral AC. Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol. 2017;8(336):1–13. doi:10.3389/fmicb.2017.00336
281. García Rubia G, Peigneux A, Jabalera Y, et al. pH-dependent adsorption release of doxorubicin on mamc-biomimetic magnetite nanoparticles. Langmuir. 2018;34(45):13713–13724. doi:10.1021/acs.langmuir.8b03109
282. Lungu II, Radulescu M, Mogosanu GD, Grumezescu AM. pH sensitive core-shell magnetic nanoparticles for targeted drug delivery in cancer therapy. Rom J Morphol Embryol. 2016;57(1):23–32.
283. Wang C, Zhang H, Chen Y, Shi F, Chen B. Gambogic acid-loaded magnetic Fe3O4 nanoparticles inhibit panc-1 pancreatic cancer cell proliferation and migration by inactivating transcription factor ETS1. Int J Nanomed. 2012;7:781–787. doi:10.2147/IJN.S28509
284. Rayegan A, Allafchian A, Sarsari IA, Kameli P. Synthesis and characterization of basil seed mucilage coated Fe3O4 magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int J Biol Macromol. 2018;113:317–328. doi:10.1016/j.ijbiomac.2018.02.134
285. Arokiyaraj S, Saravanan M, Prakash NU, Arasu MV, Vijayakumar B, Vincent S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: an in vitro study. Mater Res Bull. 2013;48(9):3323–3327. doi:10.1016/j.materresbull.2013.05.059
286. Namvar F, Rahman HS, Mohamad R, et al. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomed. 2014;9:2479–2488. doi:10.2147/IJN.S59661
287. Allafchian A, Jalali SA, Hosseini F, Massoud M. Ocimum basilicum mucilage as a new green polymer support for silver in magnetic nanocomposites: production and characterization. J Environ Chem Eng. 2017;5(6):5912–5920. doi:10.1016/j.jece.2017.11.023
288. Dorniani D, Hussein MZ, Kura AU, Fakurazi S, Shaari AH, Ahmad Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int J Nanomed. 2012;7:5745–5756. doi:10.2147/IJN.S35746
289. Wang C, Liu X, Chen S, Hu FQ, Sun J, Yuan H. Facile preparation of phospholipid-amorphous calcium carbonate hybrid nanoparticles: toward controllable burst drug release and enhanced tumor penetration. Chem Commun. 2018;54(93):13080–13083. doi:10.1039/C8CC07694D
290. Zhao L, Zhang Y, Miao Y, Nie L. Controlled synthesis, characterization and application of hydrophobic calcium carbonate nanoparticles in PVC. Powder Technol. 2016;288:184–190. doi:10.1016/j.powtec.2015.11.001
291. Näkki S, Wang JT, Wu J, et al. Designed inorganic porous nanovector with controlled release and MRI features for safe administration of doxorubicin. Int J Pharm. 2019;554:327–336. doi:10.1016/j.ijpharm.2018.10.074
292. Hammadi NI, Abba Y, Hezmee MNM, et al. Formulation of a sustained release docetaxel loaded cockle shell-derived calcium carbonate nanoparticles against breast cancer. Pharm Res. 2017;34:1193–1203. doi:10.1007/s11095-017-2135-1
293. Shi H, Li L, Zhang L, et al. Designed preparation of polyacrylic acid/calcium carbonate nanoparticles with high doxorubicin payload for liver cancer chemotherapy. Cryst Eng Comm. 2015;17:4768–4773. doi:10.1039/C5CE00708A
294. Tully J, Fakhrullin R, Lvov Y. Halloysite clay nanotube composites with sustained release of chemicals. In: Nanomaterials and Nanoarchitectures. Springer, Dordrecht; 2015:87–118.
295. Lazzara G, Cavallaro G, Panchal A, et al. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr Opin Colloid Interface Sci. 2018;35:42–50. doi:10.1016/j.cocis.2018.01.002
296. Tully J, Yendluri R, Lvov Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules. 2016;17(2):615–621. doi:10.1021/acs.biomac.5b01542
297. Vergaro V, Lvov YM, Leporatti S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol Biosci. 2012;12(9):1265–1271. doi:10.1002/mabi.v12.9
298. Lee MH, Seo HS, Park HJ. Thyme oil encapsulated in halloysite nanotubes for antimicrobial packaging system. J Food Sci. 2017;82(4):922–932. doi:10.1111/1750-3841.13675
299. Cavallaro G, Lazzara G, Massaro M, et al. Biocompatible poly (N-isopropylacrylamide)-halloysite nanotubes for thermo responsive curcumin release. J Phys Chem C. 2015;119(16):8944–8951. doi:10.1021/acs.jpcc.5b00991
300. Massaro M, Piana S, Colletti CG, et al. Multicavity halloysite–amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J Mater Chem B. 2015;3(19):4074–4081. doi:10.1039/C5TB00564G
301. Biddeci G, Cavallaro G, Di Blasi F, et al. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr Polym. 2016;152:548–557. doi:10.1016/j.carbpol.2016.07.041
302. Gorrasi G. Dispersion of halloysite loaded with natural antimicrobials into pectins: characterization and controlled release analysis. Carbohydr Polym. 2015;127:47–53. doi:10.1016/j.carbpol.2015.03.050
303. Chamorro-Garcia A, Merkoçi A. Nanobiosensors in diagnostics. Nanobiomedicine. 2016;3:1849543516663574. doi:10.1177/1849543516663574
304. Iannazzo D, Piperno A, Pistone A, Grassi G, Galvagno S. Recent advances in carbon nanotubes as delivery systems for anticancer drugs. Curr Med Chem. 2013;20(11):1333–1354. doi:10.2174/0929867311320110001
305. Ng CM, Loh H-S, Muthoosamy K, Sridewi N, Manickam S. Conjugation of insulin onto the sidewalls of single-walled carbon nanotubes through functionalization and diimide-activated amidation. Int J Nanomed. 2016;11:1607–1614. doi:10.2147/IJN.S98726
306. Floyd EL, Sapag K, Oh J, Lungu CT. Photothermal desorption of single-walled carbon nanotubes and coconut shell-activated carbons using a continuous light source for application in air sampling. Ann Occup Hyg. 2014;58(7):877–888. doi:10.1093/annhyg/meu043
307. Jha PK, Jha RK, Rout D, Gnanasekar S, Rana SV, Hossain M. Potential targetability of multi-walled carbon nanotube loaded with silver nanoparticles photosynthesized from Ocimum tenuiflorum (tulsi extract) in fertility diagnosis. J Drug Target. 2017;25(7):616–625. doi:10.1080/1061186X.2017.1306534
308. Azandaryani AH, Kashanian S, Derakhshandeh K. Folate Conjugated hybrid nanocarrier for targeted letrozole delivery in breast cancer treatment. Pharm Res. 2017;34(12):2798–2808. doi:10.1007/s11095-017-2260-x
309. Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J Control Release. 2017;271:60–73. doi:10.1016/j.jconrel.2017.12.016
310. Sedki M, Khalil IA, El-Sherbiny IM. Hybrid nanocarrier system for guiding and augmenting simvastatin cytotoxic activity against prostate cancer. Artif Cells Nanomed Biotechnol. 2018;46:1–10.
311. Lim WQ, Phua SZ, Xu HV, Sreejith S, Zhao Y. Recent advances in multifunctional silica-based hybrid nanocarriers for bioimaging and cancer therapy. Nanoscale. 2016;8(25):12510–12519. doi:10.1039/C5NR07853A
312. Zhang J, Han X, Li X, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomed. 2012;7:4299–4310. doi:10.2147/IJN.S32385
313. Stanley S. Biological nanoparticles and their influence on organisms. Curr Opin Biotechnol. 2014;28:69–74. doi:10.1016/j.copbio.2013.11.014
314. Loredo-Tovias M, Duran-Meza AL, Villagrana-Escareño MV, et al. Encapsidated ultrasmall nanolipospheres as novel nanocarriers for highly hydrophobic anticancer drugs. Nanoscale. 2017;9(32):11625–11631. doi:10.1039/C7NR02118F
315. Singh P, Kim YJ, Zhang D, Yang DC. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599. doi:10.1016/j.tibtech.2016.02.006
316. Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. 2013;3(13):1–13.
317. Shukla S, Wen AM, Ayat NR, et al. Biodistribution and clearance of a filamentous plant virus in healthy and tumor-bearing mice. Nanomedicine. 2014;9(2):221–235. doi:10.2217/nnm.13.75
318. Wen AM, Lee KL, Yildiz I, Bruckman MA, Shukla S, Steinmetz NF. Viral nanoparticles for in vivo tumor imaging. J Vis Exp. 2012;69:1–12.
319. Leong HS, Steinmetz NF, Ablack A, et al. Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc. 2010;5(8):1406–1417. doi:10.1038/nprot.2010.103
320. Lee KL, Murray AA, Le DH, et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 2017;17(7):4019–4028. doi:10.1021/acs.nanolett.7b00107
321. Badri Narayanan K, Soo Han S. Genetic modifications of icosahedral plant virus-based nanoparticles for vaccine and immunotherapy applications. Curr Protein Pept Sci. 2017;18(11):1141–1151. doi:10.2174/1389203718666170424153109
322. Qi J, Zhuang J, Wu W, et al. Enhanced effect and mechanism of water-in-oil microemulsion as an oral delivery system of hydroxysafflor yellow A. Int J Nanomed. 2011;6:985–991. doi:10.2147/IJN.S18821
323. Bhargava K, Conti DS, da Rocha SR, Zhang Y. Application of an oregano oil nanoemulsion to the control of food-borne bacteria on fresh lettuce. Food Microbiol. 2015;47:69–73. doi:10.1016/j.fm.2014.11.007
324. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
325. Hsu CY, Wang PW, Alalaiwe A, Lin ZC, Fang JY, Use of lipid Nanocarriers to improve Oral delivery of vitamins. Nutrients 2019;11(1):68–97.
326. Agnihotri N, Soni GC, Chanchal DK, Tiwari S, Fang JY, A Scientific Review On Nanoemulsion For Targeting Drug Delivery System. Int J Life Sci Rev. 2019;5(2):16–29.
327. Hérault N, Wagner J, Abram SL, et al. Silver-Containing Titanium Dioxide Nanocapsules for Combating Multidrug-Resistant Bacteria. Int J Nanomed. 2020;15:1267–1281.
328. Dou XQ, Wang H, Zhang J, Aptamer–drug conjugate: targeted delivery of doxorubicin in a HER3 aptamer-functionalized liposomal delivery system reduces cardiotoxicity. Int J Nanomed. 2018;13:763–776.
329. Kevadiya BD, Chen L, Zhang L, Thomas MB, Davé RN, et al. Fenofibrate Nanocrystal Composite Microparticles for Intestine-Specific Oral Drug Delivery System. Pharmaceuticals. 2019;12(3):109–124.
330. Quan G, Niu B, Singh V, et al. Supersaturable solid self-microemulsifying drug delivery system: precipitation inhibition and bioavailability enhancement. Int J Nanomed. 2017;12:8801–8811.
331. Lee JW, Lee HY, Park SH, et al. Preparation and evaluation of dexamethasone-loaded electrospun nanofiber sheets as a sustained drug delivery system. Materials. 2016;9(3):175–186.
332. Jin X, Zhang ZH, Sun E, et al. Enhanced oral absorption of 20 (S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: in vitro and in vivo studies. Int J Nanomed. 2013;8:641–652.
333. Safer AM, Leporatti S, Jose J, Soliman MS, et al. Conjugation Of EGCG And Chitosan NPs As A Novel Nano-Drug Delivery System. Int J Nanomed. 2019;14:8033–8046.
334. Muhammad Mailafiya M, Abubakar K, Danmaigoro A, et al. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Appl Sci. 2019;9(14):2897–2922.
335. Kamal N, Kochkodan V, Zekri A, Ahzi S et al. Polysulfone Membranes Embedded with Halloysites Nanotubes: Preparation and Properties. Membranes 2020;10(1):2–29.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
