Back to Journals » OncoTargets and Therapy » Volume 12
Notch pathway is involved in the suppression of colorectal cancer by embryonic stem cell microenvironment
Authors Lan G, Lin Z, Zhang J, Liu L, Zhang J, Zheng L, Luo Q
Received 20 December 2018
Accepted for publication 13 March 2019
Published 16 April 2019 Volume 2019:12 Pages 2869—2878
DOI https://doi.org/10.2147/OTT.S199046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Guanghui Lan,1 Zongwei Lin,1 Jinhui Zhang,1 Li Liu,2 Jianjun Zhang,2 Lei Zheng,3 Qiong Luo4
1Shenzhen Hospital, Southern Medical University, Shenzhen 518101, People’s Republic of China; 2GI Surgery, The People’s Hospital of Nanshan District, Shenzhen, 518067, People’s Republic of China; 3Central Laboratory, Harrison International Peace Hospital, Hengshui 053000, People’s Republic of China; 4Affiliated Hengyang Hospital, Southern Medical University (Hengyang Central Hospital), Hengyang 421000, People’s Republic of China
Objectives: Recently, embryonic microenvironment is being known for its non-permissive property for tumor growth. However, the regulatory mechanism to maintain the balance between differentiation and tumorigenicity of cancer cell in microenvironment is not well understood.
Materials and Methods: qRT-PCR was performed to detect the levels of gene expression in HT29, LoVo and Caco-2 colorectal cancer cells, and Western blot was used to measure the protein levels. Cell migration and apoptosis were measured by Transwell and flow cytometry assays. Cancer cell markers were detected using immunohistochemical staining. In vivo tumor formation assay was conducted by subcutaneous injection of embryonic microenvironment-treated cancer cells.
Results: Colorectal cancer cell lines were treated with human embryonic stem cell conditioned culture and then collected for in vivo tumor formation assay and in vitro assays assessing the aggressive properties. We found exposure of cancer cells in human ES cultures resulted in inhibition of growth, migration of tumor cells. Moreover, we found that manipulation of Notch pathway in the ES cells microenvironment could influence the stemness of tumor. We specifically discovered that some factor in the embryonic microenvironment could suppress Notch1 pathway in the cancer cells, leading to a reduction in tumorigenesis and invasiveness.
Conclusions: This study may provide another evidence to understand the crosstalk between tumor cells and embryonic environment and may offer new therapeutic strategies to inhibit colorectal cancer progression.
Keywords: embryonic stem cell microenvironment, colorectal cancer, tumorigenicity, Notch pathway
Introduction
Globally, colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second in females, with 1.65 million new cases and almost 835,000 deaths, according to the data in 2015.1 Although there emerge new therapies for CRC treatment, including immunotherapy like PD-1/PD-L1, due to the heterogeneousness of the cancer itself and inter-individual variability, there is still huge unmet need in this area.2–6 Thus, a thorough understanding of the CRC pathogenesis is urged to develop innovative therapy for better clinical outcome.
Cancer cells were known to possess some similar phenotype to stem cells, such as high proliferation, signaling transduction and self-renewal.7–9 Moreover, there was an impressive finding in the gene signature of melanoma cell lines which revealed that aggressive tumor cells express genes associated with embryonic stem cells, suggesting that aggressive melanoma cells acquire a multipotent phenotype.10 This unexpected finding leads to the thinking in this field that how to target tumor cells with stem cell properties.
The embryonic microenvironment (EM) of the stem cells plays a critical role in stem cell differentiation into various lineages, which prompted some pioneering working using cultures from embryonic stem cells to reprogram the malignant phenotype of aggressive cells.11–15 Hendrix’s lab successfully reversed the tumorigenicity of melanoma cell lines by exposure to human embryonic stem cells.16 Following this, Prostovit et al and He et al treated breast cancer cells with conditioned medium from human or mouse stem cell and found that tumor growth was significantly inhibited and the stemness of cancer cells was weakened.14,17
To elucidate the molecular mechanisms by which EM reprogram cancer cells towards a benign phenotype, so that may help discover new therapies for tumor, we developed an in vitro model to investigate the effect of EM on colorectal cancers. Notch pathway is a conserved pathway known for determining the cell fate and controlling the differentiation of embryonic cells. Inhibition of Notch drives the pluripotent stem cell to a differentiated phenotype,18 whereas activation of Notch maintains the stemness. Here, we propose whether modulation of Notch pathway in embryonic stem cells could alter the capacity of the embryonic environment in controlling the tumor growth. In the current study, we have discovered that hESC microenvironment suppresses the tumorigenic phenotype of human colorectal cancer cells. Mechanistically, manipulation of Notch pathway in the stem cells could somehow alter the EM which further regulates the malignancy of tumor cells possibly by changing the Notch signaling pathway in the tumor cells, suggesting there might exist a cross-talk and feedback loop between microenvironment and tumor cells.
Materials and methods
Cell lines and cell culture
Human colorectal cancer cell lines HT29 (TChu 103), LoVo (TChu 82), Caco-2 (TChu146) were purchased from cell bank of China academic institute, cultured in DMEM-F12 (Hyclone) with 10% FBS (Hyclone) and passaged every 2–3 days. Human stem cell line H1 (SCSP-301) was purchased from stem cell bank of China academic institute, cultured in mTeSR™1 stem cell culturing medium (Stemcell Technologies) growing on pre-made plate covered with Matrigel matrix (BD) and passaged with ratio of 1:6 to 1:10 every 3–4 days.
Human stem cell 3D embryonic microenvironment construction
The embryonic microenvironment condition was constructed according to previous study.19 The H1 hESC lines were maintained at a passage of 5×105 in gelatin-coated 6-well dishes on feeder layers of irradiated mouse embryonic fibroblasts plated at 200,000 cells per well. The cells were cultured in 2.5 ml of stem cell medium (SM) consisting of 80% Dulbecco’s modified Eagle’s medium–F12, 20% knockout serum replacer, 1% nonessential amino acids, 1 mM L-Glutamine and 4 ng/ml basic fibroblast growth factor (Invitrogen) and were incubated at 37°C in 5% CO2. We have added this part in the materials and methods. Cell-free 3D microenvironment was obtained by adding 20 mM NH4OH and PBS wash. Cancer cell lines at logarithmic growth phase were seeded at a density of 2.5×105 per well on the pre-made 6-well microenvironment mentioned above and incubated for 4 days.
Colony formation assay
The colony formation assay was aiming to determine the proliferation of colorectal cancer cells. Colorectal cancer cells were seeded at a density of 100 cells/well in 96 well plate for 7 days. At the end point, medium was discarded, and crystal violet was added. After 20 mins incubation, the number of colonies was counted using microscope and ELISPOT AID iSpot system.
Transwell migration assay
In vitro cell migration assays were performed as described previously using Trans-well chambers (8 μm pore size; Costar). EM-treated cancer cells, when reaching subconfluency (∼75–80%), were trypsinized and resuspended in serum-free medium and added to the upper chamber in a density of 1×106 cells/ml, while complete medium was added to the bottom wells. 24 hrs later, cells that had not migrated were removed from the upper surface using cotton swabs. Cancer cells that had migrated were fixed with 4% PFA and stained with 0.25% Trypan Blue to determine the number of migratory cells. The mean of triplicate assays for each experimental condition was used.
In vivo tumor formation assay
All animal studies were approved by Animal Care and Use Committee at the Affiliated Hengyang Hospital, Southern Medical University (Hengyang Central Hospital). National Institutes of Health guide for the care and use of laboratory animals was strictly followed by us during animal experiments. The BALB/c nude mice at the age of 8–12 weeks were purchased from Vital River (Beijing, China) and were housed under specific pathogen-free conditions according to the experimental guidelines for more than one week to acclimate. The nude mice (n=3/group) were injected with 0.1 ml cell suspension with a density of 5×107/ml cancer cell lines after embryonic microenvironment treatment. Tumor volume was measured every 5 days, using the formula of V=0.5×length×width2.
Immunochemistry
Tumor samples collected from nude mice were isolated and fixed in 4% paraformaldehyde and embedded in paraffin in a 5-µm thickness. Slides were then dehydrated in gradient solutions and stained with specific antibodies. After washed with PBS for three times and incubated with secondary antibodies protected with light for around 1 hr, images were captured by a Zeiss Axioplan fluorescence microscope for at least 3 fields per condition.
Real-time qPCR assay
For quantitative reverse transcription-polymerase chain reaction (qRT-PCR), total mRNA was extracted from cultured cells using RNA purification kits (Invitrogen). The quality of RNA was determined by running in 1% agarose gel with separation of bands of 5s rRNA, 18s rRNA and 28s rRNA. Subsequently, RNA was reverse transcribed into cDNA using reversible transcription kit (Promega). SYBR green quantitative real-time PCR was performed using a real-time PCR system (ABI 7300, Advanced Biosystems). The relative expression of target genes was determined with the comparative Ct method and was normalized to the endogenous level of GAPDH. The primer sequences used for qRT-PCR were as follows: DLL1, forward 5′CGTCATAGCAACTGAGGTGTAA- −3′ and reverse 5′GTTGGGGCATATATCCTTGGAA - −3′; Jagged, forward 5′AAAGTGTGCCTCAAGGAGTATC - −3′ and reverse 5′AATACTGTCAGGTTGAACGGTG - −3′; Notch-1, forward 5′TCATCTCCGACTTCATCTAC - −3′ and reverse 5′GATCAGGATCTGGAAGACAC - −3′; CBF-1, forward 5′GCTGGATCTGGGAATCTCTAGG- −3′ and reverse 5′CAAATTTCCCAGGCGATGGAG - −3′; Hes-1, forward 5′TGAAGAAAGATAGCTCGCGG- −3′ and reverse 5′GTCATGGCATTGATCTGGGT; CK-20 forward 5′CACACGGTGAACTATGGGAG - −3′ and reverse 5′TCCTTAATCTGACTTCGCAGC - −3′. CK-7 forward 5′AGCCGTGAATATCTCTGTGATG - −3′ and reverse 5′CTGACATCACTTTCCAGACTGT - −3′. CDX2 forward 5′TCTCTGAGAGGCAGGTTAAA - −3′ and reverse 5′TGGGACACTTCTCAGAGGAC - −3′; Ber-EP4. forward 5′GGACATAGCTGATGTGGCTTAT - −3′ and reverse 5′CCCATTTACTGTCAGGTCCATT - −3′
Statistical analysis
All data were expressed as the means ± SEM. Graphs were analyzed with GraphPad Prism 5 software. Differences between groups were performed using Student’s t-test or ANOVA statistical analysis. The level of statistical significance was set at 0.05.
Results
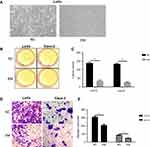
Embryonic microenvironment suppressed colorectal cancer cell survival
We initiated our analysis by confirming that embryonic microenvironment (EM) can affect the growth pattern of colorectal cancer cells (LoVo cell). In the microenvironment without embryonic stem cells (ESC) pre-incubation, colorectal cancer cells displayed multiple layers and clustered morphology. Whereas in the embryonic microenvironment with ESC pre-incubation, colorectal cancer cells grew in single layers, similar to normal colon mucosa cells (Figure 1A). To further confirm this observation, we interrogated the effect of embryonic microenvironment on cell proliferation and migration. The colony formation assay, which was widely used to determine cell proliferation ability, revealed that both LoVo and Caco-2 cells had less colony number in EM condition than control group (without ESC pre-incubation) (Figure 1B and C). Transwell migration assay confirmed that, under EM condition, both LoVo and Caco-2 cells had less migration ability than cells in normal medium (Figure 1D and E). These results suggested that EM condition could inhibit the proliferation and migration of colorectal cancer cells.
Involvement of Notch pathway in embryonic microenvironment-induced tumor inhibition
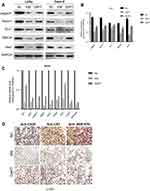
Notch signaling pathway plays an important role in human embryonic development. Activation of Notch signaling pathway is necessary to maintain the undifferentiated state of embryonic cells.20 First, we interrogated the effect of EM on Notch pathway in colorectal cancer cells. We found that protein levels of Notch signal mediators (Jagged1, Jagged2, DLL1, RBPJK and Hes1) were markedly suppressed in colorectal cancer cells (LoVo and Caco-2) when cultured in EM medium (Figure 2A and B), indicating that Notch signaling pathway in colorectal cancer cells was inhibited in such condition. Whereas when DAPT, a Notch inhibitor, was added into medium during ESC pre-incubation, colorectal cancer cells cultured in such EM medium were detected with higher protein level of Notch signal mediators than that in EM without DAPT treatment. Meanwhile, we found the mRNA level of Notch pathway involved modulators shared similar regulation pattern with protein level under EM only or DAPT pre-treated EM treatment (Figure 2C). This intriguing observation indicated that several factors in EM medium were regulated under Notch inhibitor treatment and further regulated Notch pathway of tumor cells.
Since Notch is abnormally activated in many malignant tumors, we further investigated whether the regulation of Notch by EM could affect colorectal cancer cell features. Immunohistochemical staining revealed that several tumor markers of LoVo cells were down-regulated when the cells were cultured in EM medium than that in control group (Figure 2D). The down-regulation of these markers were reversed in the condition of DAPT pre-treated EM. Such observations indicated that the expression level of tumor markers was in line with Notch pathway regulation when cells were cultured in EM or DAPT pre-treated EM.
Inhibition of Notch pathway in embryonic microenvironment ameliorates its suppression on cell growth and migration
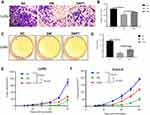
Notch signaling pathway was closely correlated with cell proliferation, apoptosis and migration. Thus, we explored the effect of EM on cell growth and migration. Cell migration assay using Trans-well chambers revealed that EM condition significantly suppressed LoVo cell migration ability, whereas DAPT pre-treated EM condition could increase the migration compared with EM condition only (Figure 3A and B). Colony formation assay was used to determine the proliferation of LoVo cells under EM condition. It showed that EM condition significantly limited colony numbers than control group while DAPT pre-treated EM alleviated the suppression effect by EM (Figure 3C and D).
We further confirmed our observations using in vivo tumor formation assay. The nude mice were injected with colorectal cancer cell lines after embryonic microenvironment treatment. The tumor grew less aggressive slower significantly (p<0.05) when pretreated with EM culture compared with the tumor in no treatment group; however, in DAPT pre-treated EM group, tumor grew faster when compared with EM group (Figure 3E and F). Taken together, both in vitro and in vivo assay suggested that EM condition could inhibit the proliferation and migration of colorectal cancer cells, and manipulation of Notch signal in the EM could regulate the tumorigenicity possibly by changing the Notch signaling pathway in the tumor cells.
Activation of Notch pathway in EM further suppressed its suppression on cell growth and tumor formation
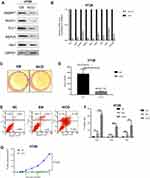
Notch1 intracellular domain (NICD), which translocates to the nucleus and interacts with CSL, could enhance Notch signaling. Next, we explored the effect on cell migration and growth by Notch pathway activation of EM medium both in vitro and in vivo. We found that both protein and mRNA expression of Notch signal mediators (Jagged1, Jagged2, DLL1, RBPJK and Hes1) were greatly suppressed in HT29 cells when cultured in NICD pre-treated EM medium than that in normal EM medium (Figure 4A and B). In addition, in HT29 cells, NICD treatment could suppress the HT29 cell colony formation (Figure 4C and D). Meanwhile, NICD could also stimulate more cells to undergo apoptosis process than EM condition (Figure 4E and F). In vivo tumor formation assay revealed that the tumor size of mice which injected with NICD pre-treated EM cultured cells was smaller significantly (p<0.05) than that of mice injected with normal EM cultured colorectal cells (Figure 4G). These in vitro and in vivo data suggested there might exist a cross-talk and feedback loop between microenvironment and tumor cells.
Discussion
The current view is that tumors arise from stem cells that proliferate indefinitely due to a disorder of self-renewal ability, which may directly contribute to tumorigenesis.21 Tumor cells share many common biological behavior characteristics with normal stem cells.22–24
Embryonic stem cells also have the characteristics of self-renewal and multi-differentiation.25,26 The special matrix microenvironment of embryonic stem cells can provide regulatory molecules for normal growth of stem cells, which can balance proliferation and differentiation when cells are constantly updated. Hendrix et al found that a protein named Nodal, which regulates the development of human embryonic stem cells (hESCs) also inhibits the growth and spread of malignant melanoma.16 Embryonic stem cells are pluripotent, meaning they can grow into more than 200 types of cells in the human body based on signals received from the surrounding microenvironment (surrounding cells, tissues and vessels).27 Malignant cells can also receive signals from the microenvironment to promote their growth and metastasis during the development of cancer.
Colorectal cancer is a world-wide common malignancy with much higher frequency in Asia.28 Currently, the comprehensive treatment program is mainly operated by surgery and supplemented by chemotherapy and radiotherapy, which has achieved certain results, but is still difficult to be satisfied. In this study, we discuss the regulation effects of embryonic microenvironment on inhibition of colorectal cancer cell lines. By treating several different colorectal cancer cells with the microenvironment secreted by hESCs, there showed significant decrease of cell proliferation rate. In concert with this finding, the migration ability assessed by staining is also suppressed by around 30%. These results directly indicated that some key components in embryonic microenvironment are involved in inhibition of tumor cell proliferation.
Following this, we further uncovered the molecular mechanism under this phenotype. Our in vitro study had showed that cells treated with ES cells microenvironment (EM) had much lower transcription and protein level of Notch signaling pathways, indicating that there might exist some extracellular signal which could inhibit the Notch pathway in the tumor cells. Notch signaling pathway is one of the few signal transduction systems that repeatedly regulate cell proliferation and apoptosis. Notch signaling pathway is closely related to cell differentiation, proliferation, apoptosis, adhesion and the transformation of epidermal cells into mesenchymal cells, which is crucial to the normal development of tissue.29 Its classical function is to regulate the differentiation, apoptosis and proliferation of stem and host cells. Abnormal regulation of Notch may cause abnormal tissue development and lead to the occurrence of tumors. It plays an important role in occurrence and development of tumors.30 Notch signaling molecule receptors include notch1-4 and ligands include Jagged1, Jagged2, Delta1, 3, 4. After the binding of receptors and ligands, intracellular protein (NICD) is released, and intracellular protein enters the nucleus to activate the downstream target gene Hes family.31 Interestingly, we found that pre-inhibition of Notch signaling by adding DAPT, a well-known Notch inhibitor, could display a negative feedback regulation of EM-induced Notch signaling suppression. In addition, the functional assay also showed reverse effect after DAPT treatment with the presence of EM culturing in CRC cell lines, including cell proliferation rate, migration ability, colony formation numbers, and also in vivo tumor formation. These results indicated that Notch signal may be the molecular basis of two-way communication between tumor and external environment.
The effects and key molecular mechanism of EM to suppress tumor growth is definitely not due to only one pathway or gene modulation since the composition of EM is very complicated and the molecular pathways have complex networks. Many scientists are also investigating the mechanism in terms of EM modulation on tumor proliferation. For instance, Hendrix et al identified Nodal as a crucial component in EM modulation on cancer cell proliferation underlying the bi-directional communication between human metastatic melanoma cells and zebrafish stem cells.16 Another hypothesis disclosed by Zhou et al was that tumor cells uptook exosomes released by stem cells and re-program their functional profile from normally trophic to pro-tumorigenic.32 Meantime, He et al also claimed that the ES cell microenvironment sufficiently suppressed Stat3 signaling pathway activation in aggressive tumor cells.17 In future studies, it is better to perform omics analysis, for instance metabolomics, proteomics, RNA-sequence for gene signature to further disclose the key genes or functional pathways that affect tumor differentiation or anti-proliferation. And subsequent in vivo functionality validation is also necessary for proof of concept on this hypothesis for future therapeutic approach.
Acknowledgment
We thank all the staff from Southern Medical University, The People’s Hospital of Nanshan District and Harrison International Peace Hospital.
Disclosure
The authors declare that there are no conflicts of interest to report in this work.
References
1. Fitzmaurice C, Allen C;
2. Tougeron D, Fauquembergue E, Latouche JB. Immunotherapy for colorectal cancer. Bull Cancer. 2013;100(9):871–885. doi:10.1684/bdc.2013.1800
3. Xiang B, Snook AE, Magee MS, Waldman SA. Colorectal cancer immunotherapy. Discov Med. 2013;15(84):301–308.
4. Singh PP, Sharma PK, Krishnan G, Lockhart AC. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol Rep (Oxf). 2015;3(4):289–297. doi:10.1093/gastro/gov053
5. Lynch D, Murphy A. The emerging role of immunotherapy in colorectal cancer. Ann Transl Med. 2016;4(16):305. doi:10.21037/atm.2016.04.05
6. Koido S, Ohkusa T, Homma S, et al. Immunotherapy for colorectal cancer. World J Gastroenterol. 2013;19(46):8531–8542. doi:10.3748/wjg.v19.i46.8531
7. Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–1313. doi:10.1158/0008-5472.CAN-08-2741
8. Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1(3):241–242. doi:10.1016/j.stem.2007.08.012
9. Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67(10):4827–4833. doi:10.1158/0008-5472.CAN-06-3557
10. Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406(6795):536–540. doi:10.1038/35020115
11. Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006;103(10):3752–3757. doi:10.1073/pnas.0506977103
12. Costa FF, Seftor EA, Bischof JM, et al. Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics. 2009;1(2):387–398. doi:10.2217/epi.09.25
13. Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25(1):43–51. doi:10.1002/btpr.139
14. Postovit LM, Margaryan NV, Seftor EA, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U S A. 2008;105(11):4329–4334. doi:10.1073/pnas.0800467105
15. Kasemeier-Kulesa JC, Teddy JM, Postovit LM, et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237(10):2657–2666. doi:10.1002/dvdy.21613
16. Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7(4):246–255. doi:10.1038/nrc2108
17. He N, Feng G, Li Y, et al. Embryonic stem cell preconditioned microenvironment suppresses tumorigenic properties in breast cancer. Stem Cell Res Ther. 2016;7(1):95. doi:10.1186/s13287-016-0360-x
18. Jang J, Ku SY, Kim JE, et al. Notch inhibition promotes human embryonic stem cell-derived cardiac mesoderm differentiation. Stem Cells. 2008;26(11):2782–2790. doi:10.1634/stemcells.2007-1053
19. Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. A three-dimensional model to study the epigenetic effects induced by the microenvironment of human embryonic stem cells. Stem Cells. 2006;24(3):501–505. doi:10.1634/stemcells.2005-0459
20. Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Curr Opin Pharmacol. 2007;7(3):303–309. doi:10.1016/j.coph.2007.02.004
21. Li JW, Yang D, Yang D, et al. Tumors arise from the excessive repair of damaged stem cells. Med Hypotheses. 2017;102:112–122. doi:10.1016/j.mehy.2017.03.005
22. Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi:10.1038/35102167
23. Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72.
24. Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66(9):4553–4557. doi:10.1158/0008-5472.CAN-05-3986
25. Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–523. doi:10.1038/nature06968
26. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi:10.1016/j.cell.2008.02.008
27. Delgado-Olguin P, Recillas-Targa F. Chromatin structure of pluripotent stem cells and induced pluripotent stem cells. Brief Funct Genomics. 2011;10(1):37–49. doi:10.1093/bfgp/elq038
28. Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull. 2013;105:29–42. doi:10.1093/bmb/lds040
29. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi:10.1038/nrm2009
30. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776.
31. Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268(5208):225–232.
32. Zhou S, Abdouh M, Arena V, Arena M, Arena GO. Reprogramming malignant cancer cells toward a benign phenotype following exposure to human embryonic stem cell microenvironment. PLoS One. 2017;12(1):e0169899. doi:10.1371/journal.pone.0169899
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.