Back to Journals » Journal of Pain Research » Volume 12
Noradrenaline modulates mechanically evoked responses in the rat spinal dorsal horn: an in vivo patch-clamp study
Authors Sonohata M , Doi A , Yasaka T , Uta D, Mawatari M, Yoshimura M
Received 23 July 2018
Accepted for publication 14 February 2019
Published 17 April 2019 Volume 2019:12 Pages 1269—1278
DOI https://doi.org/10.2147/JPR.S181210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael A Ueberall
Motoki Sonohata,1 Atsushi Doi,2 Toshiharu Yasaka,3 Daisuke Uta,4 Masaaki Mawatari,1 Megumu Yoshimura5,6
1Department of Orthopaedic Surgery, Faculty of Medicine, Saga University, Saga, Japan; 2Department of Physical Therapy, Kumamoto Health Science University, Kumamoto, Japan; 3Department of Immunology, Graduate School of Medical and Dental SciencesKagoshima University, Kagoshima, Japan; 4Department of Applied Pharmacology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Toyama, Japan; 5Department of Integrative Physiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; 6Nakamura Hospital, Nogata, Fukuoka, Japan
Purpose: We investigated the effects of noradrenaline (NA) on physiologically evoked synaptic responses of substantia gelatinosa (SG) neurons using anesthetized animals.
Methods: Male Sprague–Dawley rats (6–8 weeks, 200–300 g, n=21) were anesthetized. The lumbar spinal cord was exposed from L3 to L5; subsequently, the rats were fixed to a stereotaxic apparatus. The electrode was advanced at an angle of 30–45 degrees into the SG using a micromanipulator. We recorded excitatory post-synaptic currents (EPSC). Under these conditions, innocuous or noxious mechanical stimuli were applied to the receptive field of the ipsilateral hindlimb with or without NA, respectively.
Results: NA (50 μM) pre-application induced three types of responses for pinch-evoked EPSCs. The number of neurons showing inhibition, facilitation, and no-effect was 15 (71.4%), 2 (9.5%), and 4 (19%), respectively (n=21). Pre-treatment with NA also induced three different types of responses for puff-evoked EPSC (n=21). The number of neurons showing inhibition, facilitation, and no-effect was 9 (42.9%), 9 (42.9%), and 3 (14.2%), respectively. Further, there was a significant difference in the rate distribution (inhibition, facilitation, and no change) between puff- and pinch-evoked responses.
Conclusion: Our present data indicate that NA acts on noxious and innocuous mechanical transmission in the SG. Considering the distinct sensory inputs to the SG, the different actions of NA on the transmission of sensory information imply that NA exerts its analgesic effects in a manner more complicated than previously believed.
Keywords: noradrenaline, in vivo patch-clamp technique, touch, pain, spinal dorsal horn
Introduction
Noradrenaline (NA) is released endogenously not only to the peripheral organs but also to the spinal cord1 and various regions of the central nervous system.2 Such endogenously released NA constitutes an important mechanism as one of the pain inhibitory systems.3–8 As an example, emergency painless injury is believed to be performed predominantly by an activation of noradrenergic or serotonergic and also opioidergic systems.3–8 These systems originate at the brain stem, extend their axons to the spinal cord, and interact with noxious inputs from the periphery.9,10 In the spinal dorsal horn, innoxious (touch) sensations are conveyed preferentially by Aβ fibers and processed at the deep laminae.11 On the other hand, noxious (temperature and pain) sensations conveyed by Aδ and/or C fibers synapse to neurons in the superficial dorsal horn, especially the substantia gelatinosa (SG).12 This sensory information is well known to be modulated by the descending monoaminergic systems, in particular, endogenous NA from the locus coeruleus.13
Our previous in vivo experiments showed that dorsal root stimuli elicit both mono- and poly-synaptic excitatory post-synaptic currents (EPSC) and polysynaptic inhibitory post-synaptic currents (IPSC) in SG neurons.14,15 Exogenous NA application to the spinal cord in in vitro slice preparations has been shown to inhibit monosynaptic EPSC and enhance IPSC.16,17 Furthermore, we already showed that exogenous NA-induced outward currents of the postsynaptic membrane are inhibited by pre-treatment with yohimbine (an α2 antagonist), but not prazosin (an α1 antagonist) or propranolol (a β antagonist).18 However, there is currently no evidence suggesting what type of sensory information (eg, touch or pain sensation) is modulated by NA at the synaptic level.
In the present study, therefore, we aimed to investigate the effects of NA on physiologically evoked synaptic responses of SG neurons using the patch-clamp technique in anesthetized animals.
Methods
Ethics statement
All experimental procedures involving the use of animals were approved by the Ethics Committee on Animal Experiments, Kyushu University, and were in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines. The animal experiments were also conducted in accordance with the National Institutes of Health (NIH) guide for the care and use of laboratory animals (NIH Publications No. 80-23, revised 1996).
Preparation
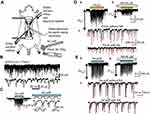
The methods used for the current experiment were modifications of those used in our previous study.18 Briefly, male Sprague–Dawley rats (6–8 weeks of age, 200–300 g, n=21) were anesthetized with urethane (1.2–1.5 g kg−1, intraperitoneally) (Figure 1A). If a withdrawal reflex appeared, a supplemental dose of urethane was administered during surgery and the data collection period. The rectal temperature was maintained at 37–38°C by a heating pad placed beneath the animal. The lumbar spinal cord was exposed from L3 to L5, and then, the rat was fixed to a stereotaxic apparatus (Model ST-7; Narishige, Tokyo, Japan) (Figure 1A). After opening the dura, the dorsal root that entered the spinal cord above the level of the recording sites was slightly shifted using a fine glass retractor to uncover the Lissauer tract, so that a recording electrode could be advanced into the SG from the surface of the spinal cord. The pia-arachnoid membrane was cut to create a window large enough to allow the patch electrode to enter the spinal cord. The surface of the spinal cord was irrigated with 95% O2/5% CO2-equilibrated Krebs solution (mM: NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, glucose 11, and NaHCO3 25) through a glass pipette at 37.5±0.5°C (Figure 1A). At the end of the experiments, the rats were administered an overdose of urethane and were then sacrificed by exsanguination.
In vivo patch-clamp recordings
The patch electrodes were pulled from thin-walled borosilicate glass capillaries (outer diameter 1.5 mm) using a puller (p-97; Sutter Instrument, Novato, CA, USA), and were filled with a patch-pipette solution composed of the following (mM: potassium gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, ATP-Mg 5, Hepes-KOH 5; pH 7.2) (Figure 1A). The electrode with a resistance of 5–12 MΩ was advanced at an angle of 30–45 degrees into the SG using a micromanipulator (Model WR-88; Narishige). We recorded cells which were located at a regular depth of 30–150 μm from the point of contact with the cell to the dorsal surface of the spinal cord.15 After creating a GΩ seal (resistance of at least 10 GΩ), the membrane patch was ruptured by a brief period of further negative pressure, resulting in the whole cell configuration. Under these conditions, we recorded EPSC at the holding potential of −70 mV (Figure 1B).15 According to our previous experiments, all these EPSC were completely eliminated by a non-NMDA receptor antagonist, CNQX (20 μM) (6-cyano-7-nitroquinoxaline-2, 3-dione), indicating that they were mediated by a release of glutamate interacting with non-NMDA receptors.15 Further, at this holding potential, no remaining responses were found, since −70 mV was a reversal potential of IPSC.15 Recordings were obtained using a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Union City, CA, USA), and the data were digitized with an A/D converter (Digidata 1200; Axon Instruments) and stored on a personal computer using a data acquisition program (pCLAMP 7; Axon Instruments). The membrane potentials were not corrected for the liquid junction potential between the Krebs and patch-pipette solutions.
Mechanical stimulation and drug application
Both noxious and innocuous mechanical stimuli were applied to the receptive field of the ipsilateral hindlimb with an air puff and toothed forceps (pinch), respectively (Figure 1A). The noxious pinch stimulus was fixed on a rod by placing 30–100 g weights on the forceps (Figure 1A). The duration and strength of the innocuous air-puff stimuli were finely controlled by combining a stimulator (SEN-8203; Nihon Kohden, Tokyo, Japan) and pico injector (PLC-100; PLC Medical System Corporation, Tokyo, Japan) (Figure 1C). NA (50 μM, WAKO, Osaka, Japan) dissolved in Krebs solution was applied to the surface of the spinal cord by exchanging solutions via three-way stopcocks without any change in either the perfusion rate or the temperature. The time necessary for the solution to flow from the stopcock to the surface of the spinal cord was approximately 5 s (Figure 1A).
Statistical analysis
We analyzed both pinch- and air-puff-evoked EPSC enhancements. Further, the rate between these mechanical-stimulus evoked EPSC enhancements without NA (XP or XA-p) and with NA (YP or YA-p) was calculated (Figure 1Da, Db, Ea, and Eb). For detecting the events and the analysis of the Y/X (%) rate, software packages (AxoGraph 4.6; Axon Instruments, Mini Analysis: Synaptosoft, Inc., Decatur, GA, USA, Microsoft Excel 2010, Microsoft, Redmond, WA, USA) were used (Figure 1Dc and Ec). Regarding the classification of “facilitation,” “inhibition,” and “no change,” when the Y/X (%) was larger than 110%, we defined it as “facilitation.” Conversely, if the Y/X was smaller than 90%, it was defined as “inhibition.” In cases where the Y/X was between 90% and 110%, we defined it as “no change.” Statistical significance was determined as p<0.05 using the Wilcoxon-signed rank test for the comparison of each stimulation between the with and without NA conditions, and by the chi-square test for the comparison between pinch and air-puff stimulation, which are indicated by asterisks in the figures. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).19 More precisely, EZR is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.19
Results
Recordings of EPSCs, repetitive pinch, and air-puff responses
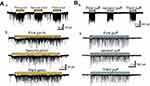
At first, we applied several stimuli to a receptive field with air puffs (Figure 2Aa) or forceps (Figure 2Ba). Three consequent air-puffs or three pinch stimuli elicited EPSC with both their amplitude and frequency being uniform (Figure 2Ab and Bb) and no significant run down was observed. It was obvious that puff-evoked responses were relatively similar and constant in event interval (Figure 2Bb). In contrast, the pinch-evoked EPSC were randomly elicited in amplitude and inter-event interval as shown in Figure 2Ab. As mentioned earlier, this may be due to rapid accommodation of non-nociceptors.
Effects of NA on pinch-evoked EPSCs
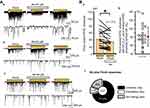
Pre-treatment with NA (50 μM) induced three types of responses for pinch-evoked EPSC (Figure 3A). The first type of response was inhibition of the EPSC in amplitude (Figure 3Aa), the second was facilitation (Figure 3Ab), and the third was a no-significant effect (Figure 3Ac). All pinch-evoked responses between the with and without NA conditions are shown in Figure 3Ba, and the averaged pinch-evoked EPSC amplitudes in both without (XP) and with NA (YP) conditions were 41.5±24.0 pA and 33.6±21.7 pA (average±SD), respectively (Figure 3Ba). The YP/XP rate ranged from 43.3% to 136.3%, and the averaged YP/XP rate was 80.4±22.8% (average ± SD) (Figure 3Bb). The number of neurons showing inhibition, facilitation, and no-effect was 15 (71.4%), 2 (9.5%), and 4 (19%), respectively (n=21; Figure 3Bc).
Effects of NA on the response of puff-evoked EPSCs
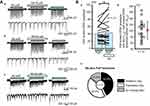
Pre-treatment with NA also induced three different types of responses for puff-evoked EPSCs (Figure 4A). As for pinch-evoked EPSCs, the first type of response was inhibition in amplitude (Figure 4Aa), the second was facilitation (Figure 4Ab), and the third was a no-effect (Figure 4Ac). All air-puff evoked responses between with and without NA conditions are shown in Figure 4Ba, and the averaged puff-evoked EPSC amplitudes in both without (XA-p) and with NA (YA-p) conditions were 40.8±21.7 pA and 43.8±31.5 pA (average±SD), respectively (Figure 4Ba). The YA-p/XA-p rate ranged from 55.7% to 165.6%, and the averaged YA-p/XA-p rate was 100.9±29.4% (average±SD) (Figure 4Bb). The number of neurons showing inhibition, facilitation, and no-effect was 9 (42.9%), 9 (42.9%), and 3 (14.2%), respectively (n=21; Figure 4Bc).
Next, we compared the rates of inhibition and facilitation between puff- and pinch-evoked EPSC by NA (50 μM) with the chi-square test. There was a significant difference in the rate distribution between the puff- and pinch-evoked responses (p<0.05, Figure 5).
Discussion
In the present study, we investigated the effects of NA on SG neurons in the spinal cord using in vivo anesthetized rats. The actions of NA in in vivo preparations were essentially the same as those observed in in vitro examinations as we reported previously.18 NA applied directly to the surface of the spinal cord produced outward currents in the majority of SG neurons and inward currents in a small number of neurons, depression of EPSC in amplitude, and augmentation of IPSC in amplitude. However, in the present in vivo examination, NA enhanced the amplitude of EPSC in a small number of SG neurons, which has never been observed in an in vitro study. Furthermore, we expected that only noxious responses would be depressed by NA. However, as shown in the present study, the puff-evoked responses were also depressed. These unexpected findings might be due to the presence of interneurons and NA applied directly to the spinal cord. At present, no evidence exists on how NA released from descending pain inhibitory system controls sensory transmission to the spinal cord. This issue will be clarified by selective in vivo stimulation of the locus coeruleus.20–23
Difference in the rate of depression by NA on EPSC in amplitude evoked by the pinch and puff stimuli
We found that the NA-induced inhibition rate on the amplitude of the pinch-evoked EPSC (71.4%) was statistically higher than that of the puff-evoked EPSC (42.9%), suggesting that these sensations are carried through distinct synaptic pathways, ie, involving polysynaptic and/or inhibitory interneurons. So far, it has not been elucidated whether these differences in NA action are physiologically significant.
Touch stimuli and SG neurons
Both air-puff and pinch stimuli elicited barrages of EPSC in SG neurons (Figure 2A and B). These results suggest that the central terminal of low-threshold mechanoreceptors (LTMRs) make direct synaptic and/or poly-synaptic contact with not only neurons in the deep laminae but also in the superficial laminae. Morphological and our electrophysiological studies showed that only a few Aβ fibers terminate at the superficial laminae.24,25 In addition, Aδ fibers innervating hair follicles and nociceptors in rats terminate at the SG.26 Therefore, innocuous sensation might be carried by both Aβ and Aδ afferents to the superficial dorsal horn.27,28 SG neurons are known to consist of heterogeneous populations,24,29,30 and some of them are known to receive direct inputs from LTMRs.25,31–33 In addition, there is evidence that certain SG neurons receive inputs from excitatory interneurons in lamina III.34 In this study, recorded SG neurons responded to touch stimuli, suggesting that a small number of SG neurons receive mono- or poly-synaptic inputs from LTMRs. Unfortunately, we could not distinguish between these two types of synaptic inputs using the present conditions.25,31–33
Pain and inhibitory action of NA
NA suppressed the pinch-evoked EPSC in 71.4% (15/21) of the SG neurons (Figure 3Aa and Bc). This inhibitory NA action on the noxious responses could be explained by at least three mechanisms: (1) the presynaptic inhibition of glutamate release from primary afferents, (2) the hyperpolarizing effect on excitatory interneurons, and (3) activation of a subset of inhibitory interneurons. NA is known to pre-synaptically inhibit monosynaptic inputs from Aδ and C fibers to SG neurons,16 and this effect is observed in limited populations of SG neurons such as transient central cells.35 Therefore, in the case that recordings are made from the transient central cells, the main mechanism of inhibitory NA action could be presynaptic inhibition of central terminals of nociceptors. Both in vivo and in vitro studies have shown that NA application hyperpolarizes membrane potentials or induces outward currents in 73–87% of SG neurons.18,24,35–37 Further, most excitatory interneurons in the SG including vertical and radial neurons show outward currents (hyperpolarization) in response to NA.24,38 This evidence suggests that inhibitory NA action may result from inhibition of polysynaptic pathways to SG neurons.
Generally, NA binds to three types of G-protein-coupled metabotropic receptors: α1, α2, and β.38 Activation of both α1 and β receptors facilitates neuronal excitability, whereas activation of the α2 receptor inhibits neuronal excitability.38 While the inhibitory NA actions described earlier are mediated by the α2 receptor,38 there is also evidence that the activation of the α1 receptor depolarizes inhibitory interneurons in the dorsal horn.35,39–41 Baba et al reported that inhibitory interneurons in deeper laminae, which express α1 receptors, are activated by NA, and these neurons may directly innervate SG neurons.39,40 Another study reported that a subset of inhibitory SG neurons expressing the glutamic acid decarboxylase 67 is depolarized by NA. This excitatory effect is mediated by the activation of α1 receptors.41 Lu and Perl reported that a depolarizing effect of NA mediated by the α1 receptor is observed in islet cells (inhibitory interneurons).35 These results suggest that NA activates a subset of inhibitory interneurons in the spinal dorsal horn. These inhibitory interneurons are known to form axo-axonic synapses onto the central terminals of certain primary afferents including nociceptors to provide presynaptic inhibition.42,43 Inhibitory interneurons are also known to provide postsynaptic inhibition to excitatory interneurons in the dorsal horn.29,35 Therefore, NA application-induced activation of inhibitory interneurons may inhibit primary afferent input to the SG neurons recorded and/or excitatory polysynaptic pathways from primary afferents to the SG neurons recorded. Together with the inhibitory actions of NA, these pre- and/or post-synaptic inhibitions provided by inhibitory cells that are activated by NA may be associated with changes in the amplitude of EPSC.32
Touch, pain, and excitatory action of NA
We observed the excitatory action of NA for both puff- (9/21 [42.9%]) and pinch- (2/21 [9.5%]) evoked EPSC (Figures 3Ab, Bc and 4Ab, Bc). Most inhibitory interneurons including islet cells and central cells are depolarized by NA;24,35 these interneurons possibly provide both presynaptic inhibition at primary afferents and postsynaptic inhibition to the excitatory interneurons that could form polysynaptic pathways. Such excitatory NA action has been rarely reported.26 An explanation as to why we could observe excitatory NA action in this study may be a few anatomical factors that establish the differences between in vitro and in vivo preparations. The first difference may relate to the limitation of neuronal circuits in in vitro preparations, which were 500-μm-thick transverse slices.39,40 For example, islet cells, a major subset of inhibitory interneurons, are spreading their dendrites and axons to distances of more than 400 (occasionally >1,000) μm in rostrocaudal directions.24,29,35,44 The long distances of both dendrites and axons may be physically disrupted in transverse slices.39,40 Therefore, the excitatory action of NA in vivo may be influenced by such inhibitory circuits. The second difference may relate to the condition of preserved poly-synaptic pathways because in our previous in vitro experiments, the effects of NA were tested on identified mono-synaptic Aδ or C afferent fibers, in contrast the noxious and innoxious responses tested in the present in vivo study might consist of both mono- and poly-synaptic components. This evidence complicates the interpretation of the NA effects. This notion should be more rigorously elucidated in vivo in combination with in vitro and immunohistochemical experiments.
Technical considerations
The present study elucidates the differences in descending inhibitory actions of NA on touch and pain sensory inputs to SG neurons in the spinal dorsal horn. While data obtained from in vivo experiments are more physiologically relevant compared to those from in vitro slice experiments, the characteristic features of recorded neurons were not determined in this study because of experimental limitations. Since SG neurons include heterogeneous populations, the characterization of the physiological, morphological, and neurochemical features of each recorded neuron will provide a better understanding of noradrenergic descending neuromodulation in neuronal circuits processing touch and pain in the spinal dorsal horn.
Clinical application
NA is considered to relate to a couple of mechanisms and diseases. It serves as an endogenous substance, such as in the descending inhibitory systems;13 on the other hand, it serves as a critical factor in complex regional pain or neuropathic pain syndrome.45–47 In a neuropathic pain animal model, overexpression of α2 receptors on the dorsal root ganglia, which directly extend their axons as presynaptic terminals to dorsal horn SG neurons, render the sympathetic nervous system dominant.45–47 These data are critical to elucidating the mechanisms of both allodynia and hyperalgesia in the spinal dorsal horn.
Conclusion
Our present data indicate that NA acts on noxious and innocuous mechanical transmission in the SG. Considering the distinct sensory inputs to the SG, the different actions of NA on transmission for sensory information imply that NA exerts its analgesic effects in a manner more complicated than previously believed.
Abbreviation list
EPSC, excitatory post-synaptic currents; IPSC, inhibitory post-synaptic currents; NA, noradrenaline; NIH, National Institutes of Health; SG, substantia gelatinosa.
Acknowledgments
This study was performed when MS, AD, TY, and DU were graduate students at Kyushu University under the instruction of co-author MY. This work was supported by Grants-in-Aid for Scientific Research (13780655, 14658268, 15029247, and 15300135) to MY from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–1826. doi:10.1161/CIRCRESAHA.114.302589
2. Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol. 2015;25(21):R1051–R1056. doi:10.1016/j.cub.2015.09.039
3. Dickenson AH, Le Bars D. Supraspinal morphine and descending inhibitions acting on the dorsal horn of the rat. J Physiol. 1987;384:81–107.
4. Wei F, Gu M, Chu YX. New tricks for an old slug: descending serotonergic system in pain. Sheng Li Xue Bao. 2012;64(5):520–530.
5. Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi:10.1016/j.pneurobio.2006.08.001
6. Sircuta C, Lazar A, Azamfirei L, Baranyi M, Vizi ES, Borbely Z. Correlation between the increased release of catecholamines evoked by local anesthetics and their analgesic and adverse effects: role of K(+) channel inhibition. Brain Res Bull. 2016;124:21–26. doi:10.1016/j.brainresbull.2016.03.009
7. Borbely Z, Csomo BK, Kittel A, Gerber G, Varga G, Vizi ES. Effect of rat spinal cord injury (hemisection) on the ex vivo uptake and release of [(3)H]noradrenaline from a slice preparation. Brain Res Bull. 2017;131:150–155. doi:10.1016/j.brainresbull.2017.04.007
8. Vegh D, Somogyi A, Banyai D, et al. Effects of articaine on [(3)H]noradrenaline release from cortical and spinal cord slices prepared from normal and streptozotocin-induced diabetic rats and compared to lidocaine. Brain Res Bull. 2017;135:157–162. doi:10.1016/j.brainresbull.2017.10.011
9. Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somat Mot Res. 1992;9(2):157–173. doi:10.3109/08990229209144768
10. Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: an immunocytochemical-retrograde transport study. Brain Res. 1981;226(1–2):187–199.
11. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi:10.1016/j.cell.2009.09.028
12. Abraira V, Ginty D. The sensory neurons of touch. Neuron. 2013;79(4):618–639. doi:10.1016/j.neuron.2013.07.051
13. Westlund K, Bowker R, Ziegler M, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263(1):15–31.
14. Narikawa K, Furue H, Kumamoto E, Yoshimura M. In vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation. J Neurophysiol. 2000;84(4):2171–2174. doi:10.1152/jn.2000.84.4.2171
15. Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J Physiol (Lond). 1999;521(Pt 2):529–535.
16. Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682–689.
17. Liu T, Fujita T, Kumamoto E. Acetylcholine and norepinephrine mediate GABAergic but not glycinergic transmission enhancement by melittin in adult rat substantia gelatinosa neurons. J Neurophysiol. 2011;106(1):233–246. doi:10.1152/jn.00838.2010
18. Sonohata M, Furue H, Katafuchi T, et al. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol (Lond). 2004;555(Pt 2):515–526. doi:10.1113/jphysiol.2003.054932
19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi:10.1038/bmt.2012.244
20. Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J Neurophysiol. 2014;111(12):2570–2588. doi:10.1152/jn.00920.2013
21. Park JW, Rv B, Park J. Noradrenergic modulation of dopamine transmission evoked by electrical stimulation of the locus coeruleus in the rat brain. ACS Chem Neurosci. 2017;8(9):1913–1924. doi:10.1021/acschemneuro.7b00078
22. Elisa R-O, Cañadas F, Carvajal F, Cardona D. In vivo stimulation of locus coeruleus: effects on amygdala subnuclei. Acta Neurobiol Exp. 2017;77(3):261–268.
23. Doi A, Ramirez J-M. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30(24):8251–8262. doi:10.1523/JNEUROSCI.5361-09.2010
24. Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina {II} of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151(2):475–488. doi:10.1016/j.pain.2010.08.008
25. Yasaka T, Tiong SY, Polgár E, et al. A putative relay circuit providing low-threshold mechanoreceptive input to lamina I projection neurons via vertical cells in lamina {II} of the rat dorsal horn. Mol Pain. 2014;10(1):3. doi:10.1186/1744-8069-10-3
26. Li L, Rutlin M, Abraira VE, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–1627. doi:10.1016/j.cell.2011.11.027
27. Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. doi:10.1038/nrn2947
28. Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355(6355):75–78. doi:10.1038/355075a0
29. Yasaka T, Kato G, Furue H, et al. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol (Lond). 2007;581(Pt 2):603–618. doi:10.1113/jphysiol.2006.123919
30. Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol (Lond). 2002;540(Pt 1):189–207.
31. Petitjean H, Pawlowski S, Fraine S, et al. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep. 2015;13(6):1246–1257. doi:10.1016/j.celrep.2015.09.080
32. Duan B, Cheng L, Bourane S, et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159(6):1417–1432. doi:10.1016/j.cell.2014.11.003
33. Lu Y, Dong H, Gao Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123(9):4050–4062. doi:10.1172/JCI70026
34. Kato G, Kawasaki Y, Koga K, et al. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29(16):5088–5099. doi:10.1523/JNEUROSCI.6175-08.2009
35. Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol (Lond). 2007;582(Pt 1):127–136. doi:10.1113/jphysiol.2007.131565
36. North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol (Lond). 1984;349:43–55.
37. Yajiri Y, Yoshimura M, Okamoto M, Takahashi H, Higashi H. A novel slow excitatory postsynaptic current in substantia gelatinosa neurons of the rat spinal cord in vitro. Neuroscience. 1997;76(3):673–688.
38. Bylund DB, Eikenberg DC, Hieble JP, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46(2):121–136.
39. Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92(2):473–484.
40. Baba H, Goldstein PA, Okamoto M, et al. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology. 2000;92(2):485–492.
41. Gassner M, Ruscheweyh R, Sandkuhler J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain. 2009;145(1–2):204–210. doi:10.1016/j.pain.2009.06.021
42. Zeilhofer HU, Wildner H, Yevenes G. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92(1):193–235. doi:10.1152/physrev.00043.2010
43. Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience. 2014;283:95–106. doi:10.1016/j.neuroscience.2014.09.032
44. Spike RC, Todd AJ. Ultrastructural and immunocytochemical study of lamina {II} islet cells in rat spinal dorsal horn. J Comp Neurol. 1992;323(3):359–369. doi:10.1002/cne.903230305
45. Kasai M, Mizumura K. Increase in spontaneous action potentials and sensitivity in response to norepinephrine in dorsal root ganglion neurons of adjuvant inflamed rats. Neurosci Res. 2001;39(1):109–113.
46. Perl ER. Causalgia, pathological pain, and adrenergic receptors. Proc Natl Acad Sci USA. 1999;96(14):7664–7667.
47. McLachlan E, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363(6429):543–546. doi:10.1038/363543a0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.