Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Nonsteroidal Anti-Inflammatory Drugs Impact on the Outcomes of Hospitalized Patients with Clostridium difficile Infection
Authors Patel H , Makker J, Vakde T, Shaikh D , Badipatla K, Dunne J, Mantri N , Nayudu SK, Glandt M, Balar B, Chilimuri S
Received 19 July 2019
Accepted for publication 21 November 2019
Published 10 December 2019 Volume 2019:12 Pages 449—456
DOI https://doi.org/10.2147/CEG.S223886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Harish Patel,1,2 Jasbir Makker,1,2 Trupti Vakde,1,3 Danial Shaikh,1,2 Kanthi Badipatla,1,2 James Dunne,4 Nikhitha Mantri,1 Suresh Kumar Nayudu,1,2 Mariela Glandt,1 Bhavna Balar,1,2 Sridhar Chilimuri1,2
1Department of Medicine, Bronx Care Health System, Affiliated with Icahn School of Medicine at Mount Sinai, Bronx, NY, USA; 2Division of Gastroenterology, Bronx Care Health System, Affiliated with Icahn School of Medicine at Mount Sinai, Bronx, NY, USA; 3Division of Pulmonary and Critical Care Medicine, Bronx Care Health System, Affiliated with Icahn School of Medicine at Mount Sinai, Bronx, NY, USA; 4Support Service and Operation, Bronx Care Health System, Affiliated with Icahn School of Medicine at Mount Sinai, Bronx, NY, USA
Correspondence: Jasbir Makker
Bronx Care Health System, Affiliated with Icahn School of Medicine at Mount Sinai, 1650 Selwyn Ave, Bronx, NY, USA
Tel +1 7185181234
Email [email protected]
Purpose: Mouse model experiments have demonstrated an increased Clostridium difficile infection (CDI) severity with Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) use. We aim to evaluate the impact of NSAIDs in humans after a diagnosis of CDI on primary outcomes defined as I) all-cause mortality and II) toxic mega-colon attributable to CDI.
Patients and methods: All hospitalized patients with a diagnosis of CDI were divided into two groups; those with NSAIDs administered up to 10 days after onset of CDI versus no NSAIDs use. The primary outcomes were analyzed between the groups, while controlling for severity of CDI. A logistic regression analysis was performed to identify the predictors of worse outcomes.
Results: NSAIDs were administered in 14% (n=80) of the 568 hospitalized visits for an average of 2.5 days after the CDI diagnosis. All-cause mortality was high in patients who did not receive NSAIDs as compared to those who did receive NSAIDs (16.6% vs 12.5%, p 0.354). Patients who were prescribed NSAIDs were more likely to have toxic mega-colon as compared to those who were not prescribed NSAIDs (2.5% vs 0.6%, p 0.094). Results were not statistically significant, even after controlling for CDI severity. Logistic regression analysis did not identify NSAIDs administration as a significant factor for all-cause mortality in CDI patients.
Conclusion: This retrospective study results, contrary to mouse model, did not show association between NSAID use and CDI related mortality and toxic mega-colon. Shorter duration of NSAIDs use, younger people in study group, and timely CDI treatment may have resulted in contrasting results.
Keywords: colitis, NSAIDs, Clostridium difficile infection, nonsteroidal anti-inflammatory drugs, toxic megacolon
Introduction
Clostridium difficile (C. difficile) is a spore-forming gram-positive bacterium. It transmits through fecal-oral route and can be present as an asymptomatic colonization, specifically in individuals on anti-microbial therapy.1,2 In symptomatic patients with diarrhea, abdominal pain and/or fever, Clostridium difficile infection (CDI) is diagnosed by positive stool studies (presence of C. difficile toxins or toxigenic strain of C. difficile in stool), or the presence of typical colonoscopy findings of pseudomembranous colitis.3
CDI is a morbid condition and its prevalence is increasing in hospitalized patients.4 CDI rates have increased across the globe; however, the surge is most predominant in the United States.5 The data from Emerging Infections Program (EIP) have shown that majority of the cases (67%) are health-care-associated CDI, from which 24% are acquired during hospitalization.6
The pathogenesis of CDI entails an amalgam of intestinal microbiota dysbiosis, C. difficile colonization, and an altered immune response.7,8 Risk factors for CDI include prior antibiotic use, acid suppression therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), age above 65 years, inflammatory bowel disease, malignancy with chemotherapy use, recent health-care exposure and non-surgical gastrointestinal procedures.9 In 2005, Dial et al first identified “the current use of NSAIDs” as a risk factor for CDI.10 Subsequently in 2012, the first population-based study on the association of NSAIDs, specifically for Diclofenac, and the risk of the CDI was published.11 This added further interest, and thereafter more reports surfaced including a systemic review and meta-analysis, strengthening the link between NSAIDs and the risk of CDI.12
In recent times, National Health and Nutrition Examination Survey (NHANES) and National Health Interview Survey (NHIS) have reported a nationwide increase in the use of NSAIDs.13,14 With forehand knowledge of a link between NSAIDs and increased CDI susceptibility, and demographic data revealing an increase in NSAIDs use, it is imperative to review the impact of the NSAIDs on the severity and outcomes of the CDI.
NSAIDs, specifically Indomethacin use, can alter intestinal microbiota.15 Immune response dysregulation is a key factor linking the NSAIDs with higher incidence and severity of CDI.16 This phenomenon is primarily through dysregulation of PG gene expression, increased intestinal inflammation and disruption of intestinal epithelial tight junctions by NSAIDS. Muñoz-Miralles et al have demonstrated the negative effect of NSAIDs on antibiotic exposed C. difficile infected mice.17 Antibiotic-exposed mice were inoculated with two different strains of C. difficile spores, and Indomethacin was introduced on a regular basis while monitoring outcomes. The mice exposed to Indomethacin had 100% mortality as compared to the 88% survival in medication naïve C. difficile infected mice. This sets a new horizon for the evaluation of outcomes, severity, and mortality of patients with CDI receiving NSAIDs. We performed a retrospective review to analyze the impact of NSAIDs use on CDI outcomes in hospitalized patients. To our knowledge, there have been no human studies reviewing the impact of NSAIDs on severity outcomes of CDI.
Methods
Our study is retrospective with study period from January 2014 to December 2017. The study protocol was approved by the Institution Review Board (IRB) of Bronx Care Health System. Patient consent was not required by the IRB owing to retrospective nature of the study. The study was performed as per the Declaration of Helsinki.
Patient Selection
All hospitalized patients with positive C. difficile stool test were extracted from the electronic medical record (EMR) system. A positive stool study was defined as i) Positive results of stool C. difficile toxin (Toxin A or Toxin B) and Glutamate Dehydrogenase (GDH) antigen ii) Positive C. difficile nucleic acid amplification test (NAAT) in case of discrepancy of results between C. difficile toxins and GDH. All asymptomatic carriers were excluded.
The Study Group
Medical records were reviewed to identify the date of onset of illness based on presence of symptoms and positive C. difficile stool studies. The information pertaining to indication and duration of NSAIDs was extracted from medication orders in the EMR. We reviewed nursing task completion documentation to confirm administration of the medication. Patients were divided into two groups based on administration of NSAIDs up to 10 days after the onset of CDI. The outpatient prescriptions were also accounted, if stool tests were received after discharge. The primary outcomes of mortality and toxic mega-colon were analyzed between the two groups. Patients who were administered aspirin were also identified, using the same protocol.
Primary Outcomes
EMR of all the patients was reviewed and any of the two primary outcomes – i) all-cause mortality and ii) development of toxic mega-colon that can be attributed to CDI were noted. Colonic dilatation (more than 6 cm) with the worsening sepsis was considered to be diagnostic for the toxic megacolon.
Baseline Characteristics, Laboratory Parameter and Treatment Regimen
Demographic characteristics, comorbid conditions, and first set of the laboratory parameters obtained after diagnosis of CDI were listed. The therapeutic antibiotic regimen used to treat the individual cases of CDI was reviewed.
Definition of Severity of CDI
The severity of the CDI was defined as per the scoring system proposed by Surawicz et al.18
Mild-to-Moderate disease: Diarrhea with no additional features of severe or complicated disease.
Severe Disease: Serum Albumin of less than 3 g/dl plus one of the following
- WBC of above 15,000 cells/mm3
- Abdominal tenderness
Severe and Complicated Disease: Any of the following conditions attributed to CDI
- Intensive care monitoring for CDI
- Hypotension with or without vasopressor use
- Fever of more than 38.5°C
- Ileus or significant abdominal distension
- Mental status change attributed to CDI
- WBC of more than 35,000 cells/mm3 or less than 200 cells/mm3
- Serum Lactate above 2.2 mmol/L
- End organ failure (mechanical ventilation, renal failure)
Statistical Analysis
The patient data collected were stratified across both the groups – CDI with and without NSAID use. Mean and standard deviation were used for continuous variables. Frequency and percentages were reported for categorical variables. Pearson’s Chi-squared test was performed for the categorical variables and ANOVA test was used for continuous variables. A logistic regression analysis was performed to ascertain the predictors of all-cause mortality. A p-value of less than 0.05 was considered to be statistically significant. SPSS version 19 was used to perform the analysis.
Results
There were 568 hospitalized patients diagnosed with CDI during the study period. The diagnosis was predominantly made by a positive stool toxin assay. The mean age of the patients was 56.9 (±16.4) years. Study group comprised predominantly females, 53% (n=303), while males contributed to 47% (n=265) of the cases. The ethnicity of the majority of patients was Hispanic (53%), followed by African American (31%). During hospitalization, the majority of the patients had a mild-to-moderate presentation of CDI (67.4%, n=383), with severe presentation being the next most common (18.3%, n=104). Remaining 14.3% (n=81) were complicated cases of CDI. The anti-microbial management was as per the “in-effect” recommendations. In keeping with mild presentation of CDI being the most common, 61.3% (n=348) of patients were managed with Metronidazole alone and 6.7% (n=38) of patients were treated with oral Vancomycin. The rest were treated with a combination therapy of either Metronidazole along with oral Vancomycin (28.8%, n=164) or Metronidazole with dual-route (oral and rectal) Vancomycin (3.2%, n= 18).
All patients with CDI were divided into two groups, based on the administration of NSAIDs. There were 80 (14%) patients who received NSAIDs after a diagnosis of CDI during the hospitalization. Injectable Ketorolac was the most frequently administered NSAID (56.25%, n=45), and 69% (n=31) received it for analgesia. NSAIDs were prescribed on an average of 2.5 (±2.2) days (Table 1). The baseline characteristics of both the groups were comparable (Table 2), except for the age, presence of hypertension and the renal function tests. The patients in the NSAIDs group were younger (52 years vs 58 years, p 0.003), the majority were hypertensive (81% vs 70%, p 0.043) and had a preserved serum creatinine (1.6 mg/dL vs 2.9 mg/dL, p <0.001) as compared to those who were not given NSAIDs. Most of the NSAIDs (71%, n=57) were prescribed in patients with a mild to moderate presentation of CDI. The distribution of CDI severity in both the groups was similar and statistically not significant (p 0.661) (Table 3).
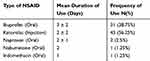 |
Table 1 Type of NSAID Used, Its Duration and Frequency of Use |
 |
Table 2 Baseline Clinical Characteristics Outlayed Between Patients Who Received NSAIDs versus Those Who Did Not Received NSAIDs |
 |
Table 3 Comparison of Severity of CDI for Patients Who Received NSAIDs versus Those Who Did Not Receive NSAIDs |
Overall, there were 5 patients (1%) that had toxic mega-colon and 91 patients (16%) had mortality during hospitalization including all causes (Table 4). The mean length of the stay (LOS) was 5.8 (±13.8) days. Paradoxically, mortality was higher in patients who did not receive NSAIDs (16.6%, n=81) as compared to those who did (12.5%, n=10); however, it was not statistically significant with a p-value of 0.354. The patients who were prescribed NSAIDs were more likely to develop toxic mega-colon as compared to those who did not receive NSAIDs (2.5% vs 0.6%, p 0.094). There was a 20% (1 out of 5) mortality in patients who developed toxic mega-colon. There was no evidence of opioid administration in these patients prior to CDI.
 |
Table 4 Comparison of Primary Outcomes of CDI for Patients Who Received NSAIDs versus Those Who Did Not Receive NSAIDs |
Majority (45%, n=41) of the patients with CDI who died, had severe complicated disease (Table 5). The severity of CDI was a significant predictor of mortality. We did analyze the mortality in both groups after controlling for severity of CDI and found no significant difference in mortality. The administration of NSAIDs did not influence mortality outcomes in patients with mild to moderate severity of CDI.
 |
Table 5 Mortality Outcomes When Controlled for the Severity of CDI Amongst Patients Who Received NSAIDs versus Those Who Did Not Receive NSAIDs |
We also reviewed the need for colectomy for fulminant CDI. Two patients underwent surgery after the diagnosis of CDI. One underwent emergent surgery for bowel obstruction secondary to a colon mass and the second required emergent surgery for bowel perforation after attempted pericardiocentesis. In both cases, surgery could not be attributed to CDI. None of these patients received NSAIDs and did not account towards mortality.
Comparison of CDI severity and outcomes among patients based on exposure to Aspirin after the CDI diagnosis as shown in Table 6 did not reveal any difference between the two groups. Logistic regression analysis did not identify NSAIDs administration as a significant factor for all-cause mortality in CDI patients (Table 7).
 |
Table 6 Comparison of CDI Severity and Outcomes Among Patients Who Did and Did Not Receive Aspirin After the CDI Diagnosis |
 |
Table 7 Binomial Logistic Regression for Predictors of Mortality |
Discussion
NSAIDs are frequently utilized for their anti-inflammatory, analgesic or anti-pyretic properties. Acetaminophen is the most frequently used anti-pyretic and its mechanism of action is different from NSAIDs17; however, NSAIDs are still utilized in refractory cases. Opioids are associated with an increased risk of acquiring CDI19 and worsening the disease outcome,20 hence providers might prefer NSAIDs for analgesia in patients with CDI. NSAIDs have an added anti-inflammatory action as well.
NSAIDs, via mechanism of intestinal microbiota dysbiosis and inflammatory dysregulation, have an increased risk of CDI. The pre-infection dysbiosis increases susceptibility to CDI, whereas the inflammatory dysregulation exacerbates severity of CDI. As supported by a population-based study, NSAIDs exposure has shown to increase the susceptibility of CDI.11 Our study depicted a difference in mortality between the groups who received NSAIDs versus those who did not. Those receiving NSAIDs accounted for a lower mortality, though not statistically significant. These results are contradictory to the laboratory mouse model studies, which revealed a statistically significant increase in mortality. We agree with the underlying pathophysiology of the mouse model study, demonstrating an increase in mortality in antibiotic exposed, treatment deprived murine with persistent use of NSAIDs. However, the strict “causal-effect” demonstrated by the mouse model may differ considerably in humans. There are several possible theories explained below that can explain why our study results were not consistent with the results of the mouse model investigation.
NSAIDs are commonly used medications and account for 12% of all ambulatory prescriptions. Over a period of 5 years (2005 to 2010), a 40% increase in NSAIDs use was reported.14 Four percent to 14% of hospitalized patients across various clinical settings and geographical locations are prescribed NSAIDs.21,22 NSAIDs contribute to 11% of adverse drug events during hospitalization.23 Though not reported, we expect a difference in prescribing patterns of NSAIDs, in terms of dosage, duration and indication in the in-hospital versus ambulatory setting. The minimum duration of NSAID exposure required to cause inflammatory dysregulation is not clear. As reported in our study, NSAIDs were prescribed in 14% of the patients and the duration was limited to an average of 2.5 days.
NSAIDs are generally prescribed more commonly in the elderly. Age above 70 years is considered to be one of the risk factors for severe CDI.24 On the contrary in our study, patients who received NSAIDs were younger than those who did not (52 years versus 58 years). The age difference could be one of the reasons for higher mortality in the group that did not receive NSAIDs.
Renal insufficiency is an important predictor of the need for the colectomy and mortality outcome for patient with CDI.25 Patient with elevated altered renal function are often not a good candidate for the NSAIDs therapy. In our study patients with high serum creatinine are less likely to have NSAIDs administration and that can bias the endpoint of the mortality outcome. This is a limitation to the study and by but excluding such patients with elevated serum creatinine, the study will lose its comprehensive inclusion of all the patients.
To understand the differences between results in our study and mouse model study by Munoz et al,17 we further reviewed their findings. The clinical outcomes of weight loss and survival were studied among three different groups – a) mice exposed to CDI only, b) mice exposed to Indomethacin only and c) mice exposed to both CDI and Indomethacin. Study was conducted at two different sites with one utilizing low dose and other with higher dose of Indomethacin. The clinical outcome of decreased weight was more pronounced after 4 days of high dose Indomethacin use. In our study, NSAIDs were used for an average of 2.5 days, and there was no significant difference in duration of NSAIDs used between the group. It is possible that owing to short duration of NSAIDs use, immune dysregulation may not have had time to impact the primary outcomes in our study.
The mouse model study by Munoz et al17 did not depict cytotoxicity in colonic mucosa. Colonic cytotoxic titers and spores of C. difficile in the cecum were assessed and were found to be similar in CDI groups with and without Indomethacin. They concluded that there was no effect of Indomethacin on C. difficile colonization or cytotoxicity of colon cells; however, there was exacerbation of severe manifestations in CDI mice receiving Indomethacin. In our study, we also noticed similar results with 2.5% of patients who were administered NSAIDs subsequently developed toxic-megacolon, as opposed to 0.6% in those without NSAIDs exposure. Due to a small sample size of patients affected by toxic mega-colon (five patients), this difference did not achieve statistical significance. There was no prior opioid exposure in these patients. Toxic mega-colon is a clinical marker of severe colonic inflammation that is seen more commonly in infections with a virulent strain or with the concomitant use of NSAIDs. Our data is limited by unavailability of C. difficile strain typing, and in view of the small proportion of patients with toxic mega-colon, it did not affect the overall mortality.
The severity of CDI predicts the mortality.26 In our study as well, 45% of the mortality in the CDI was from complicated clinical manifestations. However, majority of our patients had mild to moderate disease, with majority of NSAIDs administered in the same group accordingly. To overcome the bias of severity of illness, we correlated mortality to NSAIDs administration after controlling for severity of illness (Table 5). NSAIDs use was not a predictor of mortality in patients with mild to moderate, severe or the complicated CDI.
NSAIDs use has been reported to exacerbate the severity of soft tissue infections.27 Clinicians have identified NSAIDs as a risk factor for worse clinical outcomes in pulmonary and tonsillar infections.28,29 The negative effect of the NSAIDs on pneumonia can be from delayed anti-microbial therapy or immune dysregulation.28 It has also been reported that administration of NSAIDs increases septic complications without exacerbating the severity of sepsis.30 Contradictory to these findings, Eisen opined that NSAIDs have a beneficial effect on sepsis due to inhibition of neutrophilic factor (NF)-kB, especially in conjunction with aspirin.31 The antibacterial activity of NSAIDs by unknown mechanism is evident in the literature.32 NSAIDs have also shown a synergistic antimicrobial effect on drug-resistant organisms.33 In patients with mild urinary tract bacterial infections, NSAIDs have proven to decrease the use of antibiotics, though increasing the complications in patients with severe disease.34 Hence, there is no definitive consensus on the effect of NSAIDs on anti-bacterial or anti-inflammatory activity in patients with bacterial sepsis, an argument that supports the contrasting results of our study when compared to the mouse model testing.
Aspirin exposure is not linked to increased rates of C. difficile,10 however, there is some evidence that shows decrease in incidence of CDI.35 In our study, there was no statistically significant difference in the primary outcome of mortality or the development of toxic mega-colon between the two groups based on Aspirin exposure (Table 6). There were only three patients who were on synchronous use of aspirin and NSAIDs. The sample size was too small to learn about its impact on the primary outcome of the CDI.
Prevention of CDI remains the mainstay intervention to decrease CDI related mortality.36 The suspicion of C. difficile should incite prompt initiation of treatment while awaiting laboratory confirmation.18 The severity of illness guided antibiotic regimen has shown to improve mortality. Oral Vancomycin and Metronidazole are the first-line therapy. Early treatment in the patient with CDI, especially nosocomial infections, has shown mortality benefit.37 Chen et al demonstrated mortality benefit of oral Vancomycin in C. difficile inoculated mice.38 Murine models, as studied by Munoz et al, were not exposed to any CDI focused antimicrobial regimen. The majority of the patients in our retrospective review were treated with CDI targeted antibiotics prior to the diagnosis, based on pre-treatment evaluation. This CDI directed management could impact mortality outcomes in such patients.
In our study, possibly due to shorter duration of NSAIDs use, timely implementation of CDI treatment, poor understanding of precise pathogenesis of dysregulation of inflammation caused by NSAIDs, and small sample size of our study population, we were not able to link NSAIDs use to CDI related mortality in hospitalized patients. Our study proved to be a clinical paradox to outcomes revealed in the mouse model experiments.
Conclusion
This retrospective study results, contrary to mouse model study, did not show any association between NSAID use and CDI outcomes of mortality and toxic mega-colon. It is possible that contrasting results in our study resulted due to shorter duration of NSAIDs use, younger people in study group, and more importantly timely administration of CDI treatment. Nevertheless, further studies are needed to validate our findings.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi:10.1086/651706
2. Marciniak C, Chen D, Stein AC, Semik PE. Prevalence of Clostridium difficile colonization at admission to rehabilitation. Arch Phys Med Rehabil. 2006;87(8):1086–1090. doi:10.1016/j.apmr.2006.03.020
3. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48.
4. Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6):929–931. doi:10.3201/eid1406.071447
5. King A, Mullish BH, Williams HRT, Aylin P. Comparative epidemiology of Clostridium difficile infection: England and the USA. Int J Qual Health Care. 2017;29(6):785–791. doi:10.1093/intqhc/mzx120
6. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–834. doi:10.1056/NEJMoa1408913
7. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol. 2013;6(1):53–68. doi:10.1177/1756283X12454590
8. Peniche AG, Savidge TC, Dann SM. Recent insights into Clostridium difficile pathogenesis. Curr Opin Infect Dis. 2013;26(5):447–453. doi:10.1097/01.qco.0000433318.82618.c6
9. Eze P, Balsells E, Kyaw MH, Nair H. Risk factors for Clostridium difficile infections - an overview of the evidence base and challenges in data synthesis. J Glob Health. 2017;7(1):010417. doi:10.7189/jogh.07.010417
10. Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid–suppressive agents and the risk of community-acquired Clostridium difficile–associated disease. JAMA. 2005;294(23):2989–2995. doi:10.1001/jama.294.23.2989
11. Suissa D, Delaney JA, Dial S, Brassard P. Non-steroidal anti-inflammatory drugs and the risk of Clostridium difficile-associated disease. Br J Clin Pharmacol. 2012;74(2):370–375. doi:10.1111/j.1365-2125.2012.04191.x
12. Permpalung N, Upala S, Sanguankeo A, Sornprom S. Association between NSAIDs and Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Canadian J Gastroenterol Hepatol. 2016;2016:7431838. doi:10.1155/2016/7431838
13. Davis JS, Lee HY, Kim J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi:10.1136/openhrt-2016-000550
14. Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general US population. Pharmacoepidemiol Drug Saf. 2014;23(1):43–50. doi:10.1002/pds.v23.1
15. Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;22(2):
16. Maseda D, Zackular JP, Trindade B, et al. Nonsteroidal anti-inflammatory drugs alter the microbiota and exacerbate Clostridium difficile colitis while dysregulating the inflammatory response. MBio. 2019;10(1):e02282–02218. doi:10.1128/mBio.02282-18
17. Muñoz-Miralles J, Trindade BC, Castro-Córdova P, et al. Indomethacin increases severity of Clostridium difficile infection in mouse model. Future Microbiol. 2018;13(11):1271–1281. doi:10.2217/fmb-2017-0311
18. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi:10.1038/ajg.2013.4
19. Mora AL, Salazar M, Pablo-Caeiro J, et al. Moderate to high use of opioid analgesics are associated with an increased risk of Clostridium difficile infection. Am J Med Sci. 2012;343(4):277–280. doi:10.1097/MAJ.0b013e31822f42eb
20. Chowdhry M, Bhaty M, Huggett A, et al. Sa1843 - opioid use in Clostridium Difficile infection is associated with severe disease and prolonged hospitalization. Gastroenterology. 2018;154(6):S–416. doi:10.1016/S0016-5085(18)31666-4
21. Kitchen J, Kane D. Non-steroidal anti-inflammatory drug prescriptions in hospital inpatients: are we assessing the risks? Ir J Med Sci. 2010;179(3):357–360. doi:10.1007/s11845-010-0496-0
22. Alvarez PA, Nguyen DT, Schutt R, et al. In-hospital use of non-steroidal anti-inflammatory drugs in patients with heart failure in academic centers in the United States. Int J Risk Saf Med. 2016;28(4):181–188. doi:10.3233/JRS-170736
23. Giardina C, Cutroneo PM, Mocciaro E, et al. Adverse drug reactions in hospitalized patients: results of the FORWARD (Facilitation of Reporting in Hospital Ward) study. Front Pharmacol. 2018;9:350. doi:10.3389/fphar.2018.00350
24. Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15(3):415–422. doi:10.3201/eid1503.080312
25. Pepin J, Vo TT, Boutros M, et al. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Dis Colon Rectum. 2009;52(3):400–405. doi:10.1007/DCR.0b013e31819a69aa
26. Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32(3):220–228. doi:10.1086/658336
27. Stevens DL. Could nonsteroidal antiinflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21(4):977–980. doi:10.1093/clinids/21.4.977
28. Basille D. Non-steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia: a cohort study. Lung. 2017;195(2):201–208. doi:10.1007/s00408-016-9973-1.
29. Lepelletier D, Pinaud V, Le Conte P, et al. Is there an association between prior anti-inflammatory drug exposure and occurrence of peritonsillar abscess (PTA)? A national multicenter prospective observational case-control study. Eur J Clin Microbiol Infect Dis. 2017;36(1):57–63. doi:10.1007/s10096-016-2770-1
30. Le Turnier P, Boutoille D, Joyau C, Veyrac G, Asseray N. Bacterial infections and NSAIDs exposure? Seek septic complications. Eur J Intern Med. 2017;41:e33–e34. doi:10.1016/j.ejim.2017.03.004
31. Eisen DP. Manifold beneficial effects of acetyl salicylic acid and nonsteroidal anti-inflammatory drugs on sepsis. Intensive Care Med. 2012;38(8):1249–1257. doi:10.1007/s00134-012-2570-8
32. Yin Z, Wang Y, Whittell Louise R, et al. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem Biol. 2014;21(4):481–487. doi:10.1016/j.chembiol.2014.02.009
33. Chan EWL, Yee ZY, Raja I, Yap JKY. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J Glob Antimicrob Resist. 2017;10:70–74. doi:10.1016/j.jgar.2017.03.012
34. Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015;351:h6544. doi:10.1136/bmj.h6544
35. Garellek J, Tin K, Srikanthan R, et al. Aspirin, statins and stool PH levels in preventing clostridium difficile infection. Gastroenterology. 2017;152(5):S950. doi:10.1016/S0016-5085(17)33232-8
36. Gerding DN, Muto CA, Owens RC
37. Zahar JR, Schwebel C, Adrie C, et al. Outcome of ICU patients with Clostridium difficile infection. Crit Care. 2012;16(6):R215. doi:10.1186/cc11852
38. Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135(6):1984–1992. doi:10.1053/j.gastro.2008.09.002
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
