Back to Journals » Journal of Pain Research » Volume 15
Non-Pharmacological Management for Vaccine-Related Pain in Children in the Healthcare Setting: A Scoping Review
Authors Wu Y , Zhao Y , Wu L, Zhang P, Yu G
Received 4 May 2022
Accepted for publication 5 August 2022
Published 8 September 2022 Volume 2022:15 Pages 2773—2782
DOI https://doi.org/10.2147/JPR.S371797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Giorgio Veneziano
Yujie Wu,1 Yong Zhao,2 Liping Wu,3 Ping Zhang,1 Genzhen Yu4
1Department of Nursing, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 2School of Public Health and Management, Chongqing Medical University, Chongqing, 400014, People’s Republic of China; 3Department of Nursing, Children’s Hospital of Chongqing Medical University, Chongqing, 400014, People’s Republic of China; 4Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China
Correspondence: Genzhen Yu, Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1095 Jiefang Avenue, Wuhan, Hubei, 430030, People’s Republic of China, Tel +86 13667189216, Email [email protected]
Purpose: To examine how research was conducted on non-pharmacological management in children with vaccine-related pain in the healthcare setting, so as to provide reference for the relief of vaccine-related pain in children.
Methods: This study conducted a scoping review guided by the methodological framework of Arksey and O’Malley. MEDLINE, Cochrane Library, EMBASE, CINAHL, PubMed databases were searched in detail, and search strategy included the keyword “vaccine”, the keyword “pain”, and the keyword “children”. Two researchers conducted literature screening and data extraction independently, and any disagreements were resolved through team consultation.
Results: This study retrieved 1017 literatures, of which 22 were finally included, including 18 randomized controlled studies, 3 quasi-experimental studies and 1 cohort study. Non-pharmacological management measures were summarized in the study, mainly involving taste, tactile, olfactory, visual, exercise, and postural interventions and injection technique. All the above non-pharmacological management were effective in mitigating vaccine-related pain in children. The study population in the included literatures was mainly neonates and infants. Regarding the analgesic effects of taste intervention, breastfeeding was better than sweeteners, and sweeteners were better than sterile water or non-nutritive sucking. However, there was a lack of comparative studies on the analgesic effects of other non-pharmacological management.
Conclusion: There are many non-pharmacological management measures with varying analgesic effects. Diversified non-pharmacological management measures can provide more analgesic choices for children. For reducing vaccine-related pain in newborns and infants, breastfeeding is recommended first, then sweeteners, and then non-nutritious sucking. In addition to the taste intervention, the analgesic effects of other non-pharmacological management measures need further comparative studies. Moreover, medical staff can use a combination of non-pharmacological analgesic measures to maximize the analgesic effect, and medical staff should also fully consider the analgesia willingness of children and parents.
Keywords: vaccine-related pain, non-pharmacological management, vaccination, children, scoping review
Introduction
Vaccines reduce the risk of disease by building a natural defense with the body, which prevent 2 to 3 million deaths from disease each year.1 The Centers for Disease Control and Prevention (CDC) recommends that children aged 0–18 should complete routine vaccinations according to the immunization schedule.2 Moreover, children 5 years and older need to be additionally vaccinated Covid-19 vaccine.3 That means more than a dozen vaccines should be vaccinated in childhood. However, the current prevalence of vaccination is not optimal in children. The National Health Commission of China proposed in the “Healthy Children Action Improvement Plan (2021–2025)” that routine vaccination coverage of children should be kept above 90%.4 In fact, World Health Organization (WHO) data shows that as of October 4, 2021, the global vaccination rates range from 42% to 87%.5 In other words, there is still a long way to go to increase vaccination rates among children.
Children develop at different levels, both physically and psychologically, and are susceptible to pain. The adverse effects of pain on children can be divided into short-term effects and long-term effects, short-term effects such as vaccine hesitancy and needle fear,6 and long-term effects such as pain sensitivity, excessive anxiety, social disorders and avoidance behavior.7 Vaccine-related pain is an important cause of low vaccination rates.8 Most parents believe in the safety of vaccines, vaccine-related pain and the number of vaccinations were key reasons for vaccine hesitancy.9 Therefore, alleviating vaccine-related pain is an integral part in preventing vaccine hesitancy. Vaccine-related pain management is advocated by children, parents, and clinicians,10 and access to pain management is a fundamental human right,11 so there is an urgent need for pain relief in children.
A variety of reasons, such as clinicians’ neglect of pain management in children, family members’ concerns about pharmacological addiction and side effects, the increased economic cost of pharmacological analgesia and the long response time of oral medication, all lead to the limited use of pharmacological management.12 In contrast, non-pharmacological analgesia can reduce the dose of analgesics,13 improve medical compliance,14 and have low cost of use,15 showing great advantages in children’s analgesia. Therefore, the use of non-pharmacological interventions to relieve vaccine-related pain in children may be a reliable approach.
In a patient-focused review,16 analgesic measures were divided into site-specific interventions and patient-led interventions. The review16 found the dose of pharmacological management and the degree of participation in non-pharmacological management affected analgesic efficacy. Children’s participation in non-pharmacological management is determined by their own interests. There are many non-pharmacological interventions with different effects, so it is necessary to list as many non-pharmacological interventions as possible in order to meet the analgesic needs and interests of different children. The purpose of the scoping review was to examine how research was conducted on non-pharmacological management in children with vaccine-related pain in the healthcare setting, so as to provide reference for the relief of vaccine-related pain in children.
Methods
Based on the methodological framework proposed by Arksey and O’Malley,17 this scoping review was performed. In summary, this methodological framework involves 6 corn stages: (a) identifying the research question; (b) identifying relevant studies; (c) study selection; (d) charting the data; (e) collating, summarizing and reporting the results; (f) consultations with consumers, stakeholders and policymakers to retrieve relevant references and insights beyond the literature. Before the literature search, we identified research questions: “What types of non-pharmacological interventions have been reported in vaccine-related pain in children in the healthcare setting, and are there differences between the non-pharmacological management measures?”
Literature Search
Literature search was conducted in January 2022 in the following databases: MEDLINE, Cochrane Library, EMBASE, CINAHL, and PubMed. Search strategy included the keyword “vaccine”, the keyword “pain”, and the keyword “children” . The above keywords use the Boolean operator “AND” in combination. The Inclusion and exclusion criteria were formulated by the PCC mnemonic, which was proposed by Joanna Briggs Institute.18 The PCC mnemonic consists of 3 parts: P for participants, C for concept, and C for context. Regarding participants, this scoping review included all children who experienced vaccine-related pain. Children with oral vaccine formulations were excluded because oral vaccine formulations do not cause any pain. The concept in the study refers to non-pharmacological management. Pain relief measures include pharmacological and non-pharmacological management, the study clarifies as many non-pharmacological management measures as possible for vaccine-related pain. As for context, any healthcare setting is included. There was no restriction on study design or publication date, but the language was limited to English only.
Study Selection
All retrieved literatures were imported into NoteExpress, through which duplicate literatures were deleted. Then, manual screening was performed based on inclusion and exclusion criteria, which mainly consisted of two steps. First, two authors read the title and abstract to preliminarily exclude literatures, and then read the full text to eliminate the literatures that do not meet the requirements. If there was any disagreement regarding literature screening, the research team discussed and determined it. In order to improve the accuracy of literature selection, all members of the research team had studied evidence-based nursing courses and were familiar with the literature screening process.
Data Extraction
Data were extracted by two independent researchers using data extraction tools developed by the research team. Extracted data included general information (year, country, methodology, aim, population, and sample size) and effects of non-pharmacological interventions (control group, experimental group, duration of intervention, pain outcomes and findings). The data were extracted by one researcher and checked by another researcher, and if necessary, the discrepancy was resolved through negotiation with the third member of the research team.
Results
A total of 1017 literatures were retrieved from the preliminary search. After step-by-step screening, 22 literatures met the inclusion criteria. The literature screening process is shown in Figure 1.
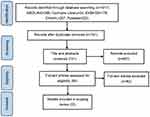 |
Figure 1 Flow chart of literature screening. |
Characteristics of the Included Studies
Of the 22 literatures, more than four-fifths (18/22) were published in the last 10 years. Most studies were conducted in Turkey (n=8) and Iran (n=4), followed by China (n=2). Regarding study types, there were 18 randomized controlled studies, 3 quasi-experimental studies, and 1 cohort study. The sample size for this study ranged from 60 to 537, and the primary study population was infants. The details of the general information are presented in Table 1, and the effects of non-pharmacological interventions are presented in Table 2.
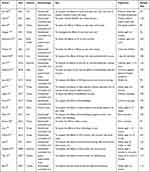 |
Table 1 Details of the General Information |
 |
Table 2 The Effects of Non-Pharmacological Interventions |
Taste Intervention
The most frequent non-pharmacological management for vaccine-related pain was taste intervention (n=9). Most of the studies performed the intervention 2 minutes before the vaccination, and some continued intervention during the vaccination until the end. Combined with the findings of the taste intervention, breastfeeding was superior to sweeteners, and sweeteners were superior to sterile water or non-nutritive sucking in terms of analgesic effects for newborns and infants.19–25 When it comes to the method of feeding, further research showed that breastfed was better than bottle-fed and powdered formula for analgesia.26 Furthermore, the concentration of glucose affects the analgesic effects, 75% glucose group had lower pain scores and less time crying compared to the 25% glucose group.20 As for the comparison between taste intervention and tactile intervention, one study27 implemented the intervention 10 minutes before and 1 minute after vaccination, the results showed that taste intervention (breastfeeding) was more effective in reducing pain than tactile intervention (kangaroo care, swaddling).
Tactile Intervention
BUZZY® is a battery powered plastic vibrating tool, measuring about 8 cm x 5 cm x 2.5 cm, shaped like a bee, the disposable or reusable ice pack is the bee’s wings, which can achieve cooling and vibration function.28 The analgesic effects of BUZZY had been confirmed in infants, preschoolers, and school-aged children,29–31 but they were all compared to standard care, and the application effects in different age groups could not be known. In addition, a study32 showed that topical cold application alone can also reduce pain in infants. Another tool suitable for tactile intervention is Shotblocker, a horseshoe-shaped tool with a thickness of about 2 mm, the lower surface of the tool is a short, blunt skin contact point, and the center of the tool is a hole that exposes the injection site.33 A study34 found that ShotBlocker can reduce NIPS scores and heart rates in term neonates, compared to standard care. The other two tactile interventions without any tools are flick and foot reflexology. Flicking once before vaccination is an extremely simple action, but it can reduce NIPS scores and crying time.35 Compared with flick, foot reflexology takes longer time. Foot reflexology involves applying appropriate pressure to specific areas of the feet. A study36 showed that implementing foot reflexology on 1-year-old infants means less pain, lower heart rates, higher oxygen saturation and shorter crying time.
Olfactory and Visual Intervention
Breast milk is a natural pain reliever containing beta endorphins, especially colostrum, which contains twice the concentration of beta endorphins as plasma.37 In a randomized controlled study,38 breast milk, another mother’s breast milk, and distilled water were placed 3 cm in front of the nose of premature infants. Compared with the other two groups, there were statistically significant differences in heart rates and pain scores in breast milk group. Virtual reality (VR) is a computer technology that synthesizes a simulated environment, which mobilizes multiple senses through immersive experience, enabling users to gain immersion in a simulated three-dimensional space.39 According to a study,40 children wear VR glasses to watch a 3D animated adventure story, and nurses administered the vaccines synchronously according to the actions of the characters in the story. The findings showed that children in the VR group had lower self-reported pain scores than the control group. However, grouping was based on parents’ preferences and children’s acceptance in the study, which may be biased.
Other Intervention
After 15 minutes of elastic resistance band exercise, the self-reported pain of female adolescents was significantly lower than that of males, suggesting that exercise analgesia can be encouraged in female adolescents before vaccination.41 WHO recommended that children should be held by a caregiver during vaccination.42 Further study found that the supine position was more effective in reducing acute pain than the upright position when vaccinating 2-month-old infants.43 Pre-injection aspiration was originally intended to reduce the risk of intravascular vaccine injection, but there are no large blood vessels at the injection site, so guidelines recommend rapid injection without aspiration before vaccine injection.44 For infants 4–6 months, rapid injection without aspiration was less painful than slow injection, and the combination of manual compression provided better analgesia.45,46
Discussion
The management measures for vaccine-related pain in children mainly involve taste, tactile, olfactory, visual, exercise, and postural interventions and injection technique. The pain outcomes of different studies were generally similar, mainly including a series of physiological parameters such as heart rates, blood oxygen saturation, blood pressure, as well as pain scales, crying time and behavioral variations. Non-pharmacological interventions are known to be safe and effective, but their application in vaccine-related pain needs further promotion.
Mother’s unique odor stimulates the release of cholecystokinin to support pain relief,47–49 which may explain why both breastfeeding and breast milk odor can relieve pain in children. Breastfeeding is thought to promote mother-infant bonding and provide psychological comfort,50 which is especially suitable for neonates and infants. The analgesic mechanism of sweeteners is controversial, but its analgesic effects have been confirmed in many studies. It is important to note that the long-term effects of sweeteners on development and neurological function are not yet known,51 the dose, concentration and duration of taste intervention require further study to clarify.
When referring to analgesic mechanisms of BUZZY, on the one hand, cold can directly affect peripheral nerves and slow down the spread of pain, on the other hand, cold can indirectly reduce pain by relieving edema, swelling and muscle spasm.52 The analgesic mechanism of Shotblocker, flick, and foot reflexology is similar. In the Gate Control Theory,53 the pressure exerted by massage, friction and pressure on the skin stimulates smaller diameter, faster-transmitting nerve cells, temporarily blocking the pain signal by closing the door to the central nervous system, thereby reducing the pain experienced.35 However, the tactile intervention studies included in this scoping review were all compared to standard care, and could not compare the advantages and disadvantages of different tactile interventions.
Exercise is considered an analgesic, and its analgesic mechanisms include activation of the endogenous opioid system,54 increase of serotonin, influence of descending inhibitory pathways, and decrease of sensory neuron activation.55 When experiencing vaccine-related pain, 2-month-old infants do not have fully developed neck muscles for effective head control, and do not like being held in upright position. Older children, whose head muscles are fully developed, may prefer upright position.43 As for the pain-relieving mechanism of rapid injection without aspiration, it can be explained by the shorter residence time of the needle in the skin tissue and less needle shaking.56
Limitations
The language of the included literature was restricted to English only, which may lead to bias. Moreover, some of the included studies were compared to standard care, so that the effects of different non-pharmacological interventions could not be compared.
Recommendations for Clinical Practice and Future Research
From existing studies comparing different tactile interventions, we can easily draw the following conclusion: for newborns and infants requiring vaccination, breastfeeding is recommended first, followed by sweeteners and non-nutritive sucking. Although there are few comparative studies of other non-pharmacological interventions, their analgesic effects have been confirmed. Diversified analgesic measures can provide more options for children’s analgesia. In addition, analgesic measures should be humanized, and it is suggested that the will and preferences of the children and their parents should be fully considered when choosing an analgesic measure. Moreover, considering that different non-pharmacological interventions may have coordinated analgesic effects, a combination of non-pharmacological interventions is recommended to maximize the analgesic effects.
The study population of the current study focused on neonates and infants, non-pharmacological interventions in older children is lacking. In the future, a multi-center, large-sample, randomized controlled study should be carried out for older children. To enhance the targeting of non-pharmacological interventions, it is recommended that future studies divide children into age groups, and then compare the analgesic effects of different interventions, so as to obtain the best management measures of each age group.
Conclusions
The scoping review clarified the analgesic efficacy of different non-pharmacological interventions, which can provide reference for the relief of vaccine-related pain in children in the healthcare setting. When choosing a non-pharmacological analgesia measure, it is recommended to comprehensively consider the interests of children, the attitude of children’s parents, and the effects of non-pharmacological analgesia measures, in order to adopt the best mode of analgesia and effectively reduce vaccine-related pain.
Funding
There was no funding for this study.
Disclosure
All authors report no conflicts of interest in this work.
References
1. World Health Organization. Vaccines and immunization (WHO); 2021. Available from: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1.
2. Centers for Disease Control and Prevention (CDC). Child and adolescent immunization schedule; 2020. Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
3. Centers for Disease Control and Prevention (CDC). U.S. COVID-19 vaccine product information; 2020. Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/index.html.
4. National Health Commission of China. Healthy children action improvement plan (2021–2025); 2020. Available from: http://www.gov.cn/zhengce/zhengceku/2021-11/05/content_5649019.htm.
5. World Health Organization. Vaccines and immunization (WHO). Vaccination coverage globally; 2021. Available from: https://immunizationdata.who.int/.
6. MacDougall T, Cunningham S, Whitney L, et al. Improving pediatric experience of pain during vaccinations: a quality improvement project. Int J Health Care Qual Assur. 2019;32(6):1034–1040. doi:10.1108/IJHCQA-07-2018-0185
7. Valeri BO, Ranger M, Chau CMY, et al. Neonatal invasive procedures predict pain intensity at school age in children born very preterm. Clin J Pain. 2016;32(12):1086–1093. doi:10.1097/AJP.0000000000000353
8. Ignis IO, Tomini S. Vaccination coverage: vaccine-related determinants & anthropometric measures in children resident in a rural community in Nigeria. Curr Drug Saf. 2021;17(3):199–210. doi:10.2174/1574886316666211029153212
9. Alawneh I, Saymeh A, Yasin A, et al. Vaccines attitudes, concerns, and information sources reported by parents of young children among north Palestinian parents. Adv Prev Med. 2020;2020:8028172. doi:10.1155/2020/8028172
10. Kuntz JL, Firemark A, Schneider J, et al. Development of an intervention to reduce pain and prevent syncope related to adolescent vaccination. Perm J. 2019;23:17–136. doi:10.7812/TPP/17-136
11. International Pain Summit of the International Association for the Study of Pain. Declaration of Montréal: declaration that access to pain management is a fundamental human right. J Pain Palliat Care Pharmacother. 2011;25(1):29–31. doi:10.3109/15360288.2010.547560
12. Poonai N, Zhu R. Analgesia for children in acute pain in the post-codeine era. Curr Pediatr Rev. 2018;14(1):34–40. doi:10.2174/1573396313666170829115631
13. Pancekauskaitė G, Jankauskaitė L. Paediatric pain medicine: pain differences, recognition and coping acute procedural pain in paediatric emergency room. Medicina. 2018;54(6):94. doi:10.3390/medicina54060094
14. Bergomi P, Scudeller L, Pintaldi S, et al. Efficacy of non-pharmacological methods of pain management in children undergoing venipuncture in a pediatric outpatient clinic: a randomized controlled trial of audiovisual distraction and external cold and vibration. J Pediatr Nurs. 2018;42:e66–e72. doi:10.1016/j.pedn.2018.04.011
15. Wente SJ. Nonpharmacologic pediatric pain management in emergency departments: a systematic review of the literature. J Emerg Nurs. 2013;39(2):140–150. doi:10.1016/j.jen.2012.09.011
16. Lee VY, Caillaud C, Fong J, et al. Improving vaccine-related pain, distress or fear in healthy children and adolescents-a systematic search of patient-focused interventions. Hum Vaccin Immunother. 2018;14(11):2737–2747. doi:10.1080/21645515.2018.1480238
17. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi:10.1080/1364557032000119616
18. Peters MD, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141–146. doi:10.1097/XEB.0000000000000050
19. Lima AG, Santos VS, Nunes MS, et al. Glucose solution is more effective in relieving pain in neonates than non-nutritive sucking: a randomized clinical trial. Eur J Pain. 2017;21(1):159–165. doi:10.1002/ejp.912
20. Yilmaz G, Caylan N, Oguz M, et al. Oral sucrose administration to reduce pain response during immunization in 16–19-month infants: a randomized, placebo-controlled trial. Eur J Pediatr. 2014;173(11):1527–1532. doi:10.1007/s00431-014-2358-7
21. Liaw JJ, Zeng WP, Yang L, et al. Nonnutritive sucking and oral sucrose relieve neonatal pain during intramuscular injection of hepatitis vaccine. J Pain Symptom Manage. 2011;42(6):918–930. doi:10.1016/j.jpainsymman.2011.02.016
22. Hatfield LA, Gusic ME, Dyer AM, et al. Analgesic properties of oral sucrose during routine immunizations at 2 and 4 months of age. Pediatrics. 2008;121(2):e327–e334. doi:10.1542/peds.2006-3719
23. Thyr M, Sundholm A, Teeland L, et al. Oral glucose as an analgesic to reduce infant distress following immunization at the age of 3, 5 and 12 months. Acta Paediatr. 2007;96(2):233–236. doi:10.1111/j.1651-2227.2007.00021.x
24. Gajbhiye M, Rao SK, Singh HP. Comparative study between analgesic effect of breast feeding and oral sucrose in full term newborns. J Clin Diagn Res. 2018;12(12):SC09–SC12.
25. Erkul M, Efe E. Efficacy of breastfeeding on babies’ pain during vaccinations. Breastfeed Med. 2017;12(2):110–115. doi:10.1089/bfm.2016.0141
26. Hatami BZ, Hemati K, Sayehmiri K, et al. Effects of breast milk on pain severity during muscular injection of hepatitis B vaccine in neonates in a teaching hospital in Iran. Arch Pediatr. 2018;25(6):365–370. doi:10.1016/j.arcped.2018.06.001
27. Fallah R, Naserzadeh N, Ferdosian F, et al. Comparison of effect of kangaroo mother care, breastfeeding and swaddling on Bacillus Calmette-Guerin vaccination pain score in healthy term neonates by a clinical trial. J Matern Fetal Neonatal Med. 2017;30(10):1147–1150. doi:10.1080/14767058.2016.1205030
28. Moadad N, Kozman K, Shahine R, et al. Distraction using the BUZZY for children during an IV Insertion. J Pediatr Nurs. 2016;31(1):64–72. doi:10.1016/j.pedn.2015.07.010
29. Ueki S, Matsunaka E, Takao K, et al. The effectiveness of vibratory stimulation in reducing pain in children receiving vaccine injection: a randomized controlled trial. Vaccine. 2021;39(15):2080–2087. doi:10.1016/j.vaccine.2021.03.013
30. Sapci E, Bilsin KE, Gungormus Z. Effects of applying external cold and vibration to children during vaccination on pain, fear and anxiety. Complement Ther Med. 2021;58:102688. doi:10.1016/j.ctim.2021.102688
31. Khanjari S, Haghani H, Khoshghadm M, et al. The effect of combined external cold and vibration during immunization on pain and anxiety levels in children. J Nurs Midwifery Sci. 2021;8(4):231–237. doi:10.4103/jnms.jnms_128_20
32. Gungor T, Ozturk Sahin O. Analysis of two non-pharmacological pain management methods for vaccine injection pain in infants: a randomized controlled trial. Agri. 2021;33(1):15–22. doi:10.14744/agri.2020.54289
33. Yildirim D, Dinçer B. Shotblocker use in emergency care: a randomized clinical trial. Adv Emerg Nurs J. 2021;43(1):39–47. doi:10.1097/TME.0000000000000330
34. Caglar S, Büyükyılmaz F, Coşansu G, et al. Effectiveness of ShotBlocker for immunization pain in full-term neonates: a randomized controlled trial. J Perinat Neonatal Nurs. 2017;31(2):166–171. doi:10.1097/JPN.0000000000000256
35. Karaca CE, Kardas OF, Aydin D. Effect of flick application on pain level and duration of crying during infant vaccination. Ital J Pediatr. 2016;42(1):8. doi:10.1186/s13052-016-0218-y
36. Koç T, Gözen D. The effect of foot reflexology on acute pain in infants: a randomized controlled trial. Worldviews Evid Based Nurs. 2015;12(5):289–296. doi:10.1111/wvn.12099
37. Ombra MN, Musumeci M, Simpore J, et al. beta-Endorphin concentration in colostrums of Burkinabe and Sicilian women. Nutrition. 2008;24(1):31–36. doi:10.1016/j.nut.2007.09.004
38. Rad ZA, Aziznejadroshan P, Amiri AS, et al. The effect of inhaling mother’s breast milk odor on the behavioral responses to pain caused by hepatitis B vaccine in preterm infants: a randomized clinical trial. BMC Pediatr. 2021;21(1):61. doi:10.1186/s12887-021-02519-0
39. Fowler CA, Ballistrea LM, Mazzone KE, et al. Virtual reality as a therapy adjunct for fear of movement in veterans with chronic pain: single-arm feasibility study. JMIR Form Res. 2019;3(4):e11266. doi:10.2196/11266
40. Althumairi A, Sahwan M, Alsaleh S, et al. Virtual reality: is it helping children cope with fear and pain during vaccination? J Multidiscip Healthc. 2021;14:2625–2632. doi:10.2147/JMDH.S327349
41. Lee VY, Booy R, Skinner R, et al. The effect of exercise on vaccine-related pain, anxiety and fear during HPV vaccinations in adolescents. Vaccine. 2018;36(23):3254–3259. doi:10.1016/j.vaccine.2018.04.069
42. World Health Organization. Reducing pain at the time of vaccination: WHO position paper, September 2015-recommendations. Vaccine. 2016;34(32):3629–3630.
43. Yin H, Cheng S, Yang C, et al. Comparative survey of holding positions for reducing vaccination pain in young infants. Pain Res Manag. 2017;2017:1–7. doi:10.1155/2017/3273171
44. Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood vaccination: an evidence-based clinical practice guideline (summary). CMAJ. 2010;182(18):1989–1995. doi:10.1503/cmaj.092048
45. Göl İ, Altuğ ÖS. Effects of rapid vaccine injection without aspiration and applying manual pressure before vaccination on pain and crying time in Infants. Worldviews Evid Based Nurs. 2017;14(2):154–162. doi:10.1111/wvn.12206
46. Ipp M, Taddio A, Sam J, et al. Vaccine-related pain: randomised controlled trial of two injection techniques. Arch Dis Child. 2007;92(12):1105–1108. doi:10.1136/adc.2007.118695
47. Hebb AL, Poulin JF, Roach SP, et al. Cholecystokinin and endogenous opioid peptides: interactive influence on pain, cognition, and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1225–1238. doi:10.1016/j.pnpbp.2005.08.008
48. Porter RH, Winberg J. Unique salience of maternal breast odors for newborn infants. Neurosci Biobehav Rev. 1999;23(3):439–449. doi:10.1016/S0149-7634(98)00044-X
49. Mangat AK, Oei JL, Chen K, et al. A review of non-pharmacological treatments for pain management in newborn infants. Children. 2018;5(10):130. doi:10.3390/children5100130
50. Shah PS, Herbozo C, Aliwalas LL, et al. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2012;12:D4950. doi:10.1002/14651858.CD004950.pub3
51. Sun L, Lin Y, Chen Z, et al. Research progress on sweetening agents for reducing preterm infants’ pain during medical procedure. Chin J Nurs. 2016;51(4):471–474. doi:10.3761/j.issn.0254-1769.2016.04.018
52. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57–65. doi:10.1080/00325481.2015.992719
53. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi:10.1126/science.150.3699.971
54. Koltyn KF. Analgesia following exercise: a review. Sports Med. 2000;29(2):85–98. doi:10.2165/00007256-200029020-00002
55. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi:10.1097/AIA.0b013e318034194e
56. Taddio A, McMurtry CM, Shah V, et al. Reducing pain during vaccine injections: clinical practice guideline. CMAJ. 2015;187(13):975–982. doi:10.1503/cmaj.150391
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
