Back to Journals » Journal of Pain Research » Volume 14
Non-Particulate Steroids (Betamethasone Sodium Phosphate, Dexamethasone Sodium Phosphate, and Dexamethasone Palmitate) Combined with Local Anesthetics (Ropivacaine, Levobupivacaine, Bupivacaine, and Lidocaine): A Potentially Unsafe Mixture
Authors Choi EJ, Kim DH , Han WK, Lee HJ , Kang I, Nahm FS , Lee PB
Received 17 March 2021
Accepted for publication 3 May 2021
Published 27 May 2021 Volume 2021:14 Pages 1495—1504
DOI https://doi.org/10.2147/JPR.S311573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Robert B. Raffa
Eun Joo Choi,1 Dong-Hyun Kim,1 Woong Ki Han,1 Ho-Jin Lee,2 Imhong Kang,3 Francis Sahngun Nahm,1,4 Pyung-Bok Lee1,4
1Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Korea; 2Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea; 3Department of Anesthesiology and Pain Medicine, Bundang Chuk Hospital, Seongnam, Korea; 4Department of Anesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea
Correspondence: Pyung-Bok Lee
Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, 82, Gumi-Ro 173 Beon-Gil, Bundang-Gu, Seongnam, 13620, Korea
Tel +82-31- 787-7499
Fax +82-31- 787-4063
Email [email protected]
Purpose: Particulate steroids used in epidural steroid injections have been suspected as a cause of post-procedural embolic events. Some particulate steroids have been suspended only when the transforaminal approach is used for an epidural block of the spine. In contrast, non-particulate steroids are generally accepted for safety during epidural steroid injections. However, the safety of using a mixture of non-particulate steroids and local anesthetics is unknown. This study analyzed whether mixtures of commonly used non-particulate steroids and local anesthetics form crystals in solution.
Methods: We mixed non-particulate steroids (betamethasone sodium phosphate, dexamethasone sodium phosphate, and dexamethasone palmitate) and local anesthetics (ropivacaine, levobupivacaine, bupivacaine, and lidocaine) at different ratios. We used fluorescence microscopy to observe whether crystals formed in mixed solutions; we also measured the pH of each steroid, local anesthetic, and the mixtures.
Results: Ropivacaine or levobupivacaine and betamethasone sodium phosphate produced large crystals (> 50 μm). Ropivacaine or levobupivacaine and dexamethasone sodium phosphate produced small crystals (< 10 μm). Lidocaine and all non-particulate steroids produced no identifiable crystals; dexamethasone palmitate and all local anesthetics did not form significant particulates. Betamethasone sodium phosphate and dexamethasone sodium phosphate demonstrated basic pH, while all local anesthetics demonstrated acidic pH. Mixtures showed a wide pH range.
Conclusion: Non-particulate steroids can form crystals upon combination with local anesthetics. Crystal formation may be caused by alkalinization of steroids. The mixing of ropivacaine or levobupivacaine and betamethasone sodium phosphate may require caution during an epidural steroid injection. Lidocaine or bupivacaine is recommended as a local anesthetic. Dexamethasone palmitate is a candidate for a mixture, but additional studies on its safety and effectiveness are needed.
Keywords: epidural space, injections, lidocaine, local anesthetics, particle size, steroids
Introduction
Epidural steroid injection (ESI) is used for the treatment of spinal pain, and the number of treated cases has increased sharply.1 However, questions remain regarding the effects and safety of different ESI approaches (eg, an interlaminar or transforaminal approach) or the use of specific drugs (eg, non-particulate steroids or particulate steroids).2–6 In particular, the cause of catastrophic complications after ESI, such as cerebral or spinal cord infarct, as well as transient ischemic attack,7–9 may be related to the injection of particulate steroids. Particulate steroids may work as an embolus if injected into spinal branches that have anastomosed with the vertebral artery; when cervical transforaminal ESI (TFESI) was performed, these embolic events were reported frequently.7,9–11
These events attracted the attention of the US Food and Drug Administration (FDA); in April 2014, the FDA issued a warning of serious neurologic complications after ESI and implemented label changes.12,13 The Anesthetic and Analgesic Advisory Committee was formed in November of the same year. This committee recommended a contraindication for transforaminal cervical injections with particulate steroids because of the concern of catastrophic complications. In addition, a multidisciplinary working group, which held discussions to establish the safe use of ESI, together with thirteen specialty stakeholder societies, issued 17 suggestions for the safe use of ESI.14 Among these 17 suggestions, five involve the use of particulate steroids (eg, methylprednisolone, triamcinolone acetate, and betamethasone acetate) in ESI. These suggestions recommended the use of non-particulate steroids because TFESI with particulate steroids, regardless of spine level, could cause embolic events. Ultimately, the key aspect of this warning was that the use of material that can cause embolic events during TFESI must be reconsidered.
Mixtures of local anesthetics and non-particulate steroids have been used in common practice. However, the ESI regimen is highly varied, and there are no guidelines. However, the safety of this mixture (ie, whether it makes particulates or not) has not been considered carefully. The first aim of this study was to analyze mixtures of commonly used non-particulate steroids (betamethasone sodium phosphate, dexamethasone sodium phosphate, and dexamethasone palmitate) and local anesthetics to determine the safest combination of steroid and local anesthetic for use. The second aim was to suggest a safe regimen for TFESI (eg, through the use of dexamethasone palmitate).
Materials and Methods
We mixed three non-particulate steroids and four local anesthetics in different ratios. We examined whether these mixtures produced crystals in each mixed solution. In addition, the pH of each mixture was measured.
Non-Particulate Steroids and Local Anesthetics
Three non-particulate steroids were used in this study: (1) betamethasone sodium phosphate 5.2 mg/mL (BSP, Betamethasone Sodium Phosphate Injection®, Daewon Pharm, Seoul, Korea); (2) dexamethasone sodium phosphate 5 mg/mL (DSP, Dexamethasone sodium Phosphate Injection®, Yuhan, Seoul, Korea) as a water-soluble steroid; and (3) dexamethasone palmitate 4 mg/mL (DPA, Limethason Injection®, Mitsubishi Tanabe Pharma Korea, Seoul, Korea) as a fat-soluble steroid. Three local anesthetics were utilized: (1) ropivacaine HCl 0.75% (Ropivacaine, Ropiva®, Hanlim Pharm, Seoul, Korea); (2) levobupivacaine HCl 0.75% (levobupivacaine, Chirocaine Injection®, Abbott Korea, Seoul, Korea); (3) hyperbaric bupivacaine HCl 0.5% (Bupivacaine, Marcaine heavy injection®, AstraZeneca AB, Seoul, Korea), and (4) lidocaine HCl 1% (lidocaine, LidocaineHCl Injection®, Daihan Pharm, Seoul, Korea). Additionally, we mixed each non-particulate steroid with normal saline for comparison with the mixtures of non-particulate steroids and local anesthetics.
Microscopic Analysis of Mixtures
Each non-particulate steroid (0.5 mL) was mixed with normal saline and each local anesthetic (0.5, 1.0, and 1.5 mL) at 1:1, 1:2, and 1:3 ratios on a prepared glass slide. After placement under a cover slip, each slide was examined using a fluorescence microscope (Axioskop 2®; Carl Zeiss Incorporated, Jena, Germany) in four areas (two areas with the most crystals and two areas with the least crystals) at two magnifications (×100 and ×200) within 1 min; a total of eight photos were taken for each slide. Images were analyzed using the Axiovision Rel. 4.8® (Carl Zeiss Inc., Jena, Germany). If crystals were observed in the mixed solution, we grouped the crystals into the following size groups: 0–10, 11–20, 21–50, 51–100, >100, and >500 µm. The number of crystals per size was determined by calculating the average of four areas (two high-density and two low-density) at ×100 magnification. Additionally, if the mixed solutions contained crystals, they were heated at 36–37 °C for 10 min. Then, we reanalyzed the solutions by fluorescence microscopy to determine whether the crystals could melt.
Measurement of pH
First, the pH of each steroid and local anesthetic was measured. Second, each non-particulate steroid (0.5 mL) was mixed with normal saline and each local anesthetic (0.5, 1.0, and 1.5 mL) at 1:1, 1:2, and 1:3 ratios. The pH of each mixture was measured using a glass electrode pH meter (Inolab level 1, WTW Weilheim, Germany) with a biotrode electrode (Hamilton, Bonaduz, Switzerland) at room temperature.
Statistical Analysis
Statistical analyses were performed using the SPSS Statistics software (version 21.0; IBM Corp, Armonk, NY, USA). Chi-square analyses were used to compare the proportion of particles 0–10, 11–20–21–50,–, 51–100, and greater than 100, 500 among mixtures according to each ratio in local anesthetics and non-particulated steroids. In all analyses, a two-sided P-value < 0.05, was considered statistically significant.
Results
Microscopic Analysis of Mixtures
All steroids, including BSP, DSP, and DPA, were pure liquids with no identifiable particles (Figure 1). Table 1 shows the number of crystals per size (0–10, 11–20, 21–50, 51–100, >100, and >500 µm) for each mixture of normal saline, local anesthetics, and non-particulate steroids. Each figure shows a microscopic analysis of the mixtures.
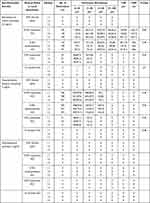 |
Table 1 Particulate Size Distribution in Mixed Solutions (Normal Saline or Local Anesthetics and Non-Particulate Steroids) |
Betamethasone Sodium Phosphate (BSP)
No crystals were observed in the mixture of BSP and normal saline. BSP and ropivacaine produced long rod-like and lucent crystals (Figure 2A). The crystals formed high-density aggregates; the common size of the crystals was 20 µm to >50 µm (Figure 2B, Table 1). BSP and ropivacaine produced crystals >50 µm in size more frequently than BSP and other local anesthetics (Table 1). Only the mixture of BSP and ropivacaine produced crystals > 500-µm in size. There were no significant differences in the number of crystals when the dilution ratios (1:1, 1:2, and 1:3) of ropivacaine with BSP (P = 0.84) were increased (Table 1). This result was observed not only for BSP, but also for other steroids. BSP and levobupivacaine produced not only >50-µm crystals, but also >100-µm crystals (Figure 2C and D), similar in shape to those in the mixture of BSP and ropivacaine. The distribution of the crystal sizes was similar. However, this mixture did not produce crystals >500-µm in size. In addition, there was no significant difference in the dilution ratios of levobupivacaine (P = 0.99). BSP and bupivacaine typically formed dot-shaped crystals <10 µm in size (Figure 2E). These crystals formed lower-density aggregates than ropivacaine or levobupivacaine with BSP. Additionally, there were no differences in the number of crystals based on changes in the ratios. BSP and lidocaine did not produce identifiable crystals, except in one or two cases (< 20 µm) (Figure 2F).
Dexamethasone Sodium Phosphate (DSP)
There were no crystals in the mixture of normal saline and DSP. DSP and ropivacaine formed short rod-shaped crystals (≤10 µm) (Figure 3A and B). Mixtures of DSP and ropivacaine formed small aggregates but exhibited the largest number of crystals among all mixtures. Moreover, DSP and levobupivacaine produced dot-shaped or short rod-like particulates (≤10 µm) (Figure 3C and D). These crystals formed aggregates with a slightly lower density than ropivacaine with DSP. The mixture of DSP and bupivacaine generated dot-shaped crystals <10 µm in number (Figure 3E). Furthermore, DSP and lidocaine did not produce any identifiable crystals (Figure 3F). Overall, mixtures of DSP and local anesthetics produced smaller, more numerous crystals than mixtures of BSP and local anesthetics. There were no differences in the number of crystals based on the changes in the dilution ratios in all mixtures.
Dexamethasone Palmitate (DPA)
No crystals were observed in the mixtures of DPA and normal saline. DPA and all local anesthetics did not form significant crystals; only lipid microspheres were observed (Figure 4).
 |
Figure 4 Microscopic findings of dexamethasone palmitate (DPA) mixed with 0.75% ropivacaine HCl (ropivacaine) (× 100). |
Heating of Particulates
When ropivacaine and levobupivacaine were mixed with BSP or DSP, crystals of various shapes and sizes were produced. After we attempted to melt the produced crystals at 36–37 °C, we analyzed the remaining crystals using fluorescence microscopy. However, all crystals were insoluble at 36–37 °C (Figure 5).
 |
Figure 5 Insoluble particulates after heating (36–37 °C) (× 400). These particulates were produced from betamethasone sodium phosphate (BSP) mixed with 0.75% ropivacaine HCl (ropivacaine). |
Measurement of pH in Mixtures
Table 2 shows the pH values of each non-particulate steroid and local anesthetic. All non-particulate steroids were basic, BSP was the most basic (pH 8.5), and local anesthetics were acidic; levobupivacaine was the most acidic (pH 5.8). Other local anesthetics showed a pH of < 7.0.
 |
Table 2 The pH of Non-Particulate Steroids, Normal Saline, and Local Anesthetics |
The mixture of BSP and normal saline showed a pH of 7.83 (Table 3). BSP and ropivacaine had pH values between 6.46 (1:3 ratio) and 7.01 (1:1 ratio). BSP and levobupivacaine had the most acidic mixture (pH 6.13–6.92). BSP and bupivacaine or lidocaine were close to neutral pH. The mixture of BSP with local anesthetics was more acidic when higher ratios of local anesthetics were used in the mixture.
 |
Table 3 Measurement of pH in Mixed Solutions (Normal Saline or Local Anesthetics and Non-Particulate Steroids) |
All mixtures of DSP and normal saline or local anesthetics tended to be more acidic than similar mixtures with BSP (rather than DSP). DSP and normal saline had pH values between 6.94 (1:3 ratio) and 7.12 (1:1 ratio). DSP and ropivacaine, levobupivacaine, bupivacaine, and lidocaine had a pH of 6 to < 7. Additionally, the mixture of DSP with local anesthetics was more acidic when higher ratios of local anesthetics were used in the mixture. DPA and local anesthetics produced the most acidic mixtures. DPA and normal saline had pH values between 6.14 (1:3 ratio) and 6.42 (1:1 ratio). The most acidic mixtures, DPA and levobupivacaine, had a pH < 5.
Discussion
In our study, non-particulate steroids could form crystals depending on which local anesthetic they were mixed with. Only lidocaine and all non-particulate steroids did not produce any identifiable crystals. Furthermore, DPA and all local anesthetics did not form substantial crystals. However, when ropivacaine and levobupivacaine were mixed with BSP or DSP, crystals of various shapes and sizes were formed. Among the non-particulate steroids, ropivacaine or levobupivacaine, and BSP formed the largest crystals. Additionally, these crystals were insoluble upon heating at 36–37 °C.
There has been a discussion on safety regarding the use of particulate steroids for TFESI. Rare catastrophic complications after TFESI have been attributed to particulate steroids, including suggestions that the particulate steroid itself could serve as emboli.7–9 The vessel dimensions of the microvascular system vary in size: capillary, 5–8 µm; arteriole, 10–15 µm; metarteriole, 20–50 µm; artery, >50 µm.11 The grouping of particulate sizes in this study was set as a reference for the vessel dimensions of the microvascular system. If particulate steroids, or mixtures of steroids and local anesthetics, were injected into the microvascular system, crystals of >50 µm may play the role of artery emboli.
Benzon et al15 reported the size of crystals formed upon mixing particulate steroids with local anesthetics (1% lidocaine) or normal saline, using the following groups: 0–20, 21–50, 51–1000, and >1000 µm. Particulate sizes of particulate steroids were concentrated in the range of 0–1000 µm. Particulate sizes >1000 µm were reported, although there were very few of these. In our study, when ropivacaine or levobupivacaine and BSP were mixed, the size of the resulting crystals was smaller (0–500 µm or greater) than that during mixing of particulate steroids in a previous report (0–1000 µm). However, small-sized crystals may be potential emboli of vessels. Thus, mixtures of ropivacaine or levobupivacaine and BSP may exhibit a risk similar to that of particulate steroids. Crystals of sizes –51–100 µm or >100 µm were observed only in mixtures of BSP and ropivacaine or levobupivacaine. Crystals > 500 µm in size were observed only in the mixture of ropivacaine and BSP, but there were no crystals >1000 µm in size (Figure 2B and D).
In previous studies, when local anesthetics (ropivacaine, bupivacaine, lidocaine) and DSP or BSP were mixed, crystal production was reported.16,17 Alkalization has been suggested as the cause of crystals in mixtures of local anesthetics and non-particulate steroids.17 In our study, all local anesthetics were acidic (pH < 7.0), and non-particulate steroids were basic (DSP, pH 7.6) or strong basic (BSP, pH 8.5). The more basic steroids (BSP, pH 8.5 > DSP pH 7.6 > DPA pH 7.1) tended to produce more crystals. For the same steroid (except DPA), crystal formation was the greatest for ropivacaine (pH 6.1), followed by levobupivacaine (pH 5.8) and bupivacaine (pH 6.4), with non-particulate steroids. Additionally, lidocaine (pH 6.3) did not form crystals with any of the steroids used in this study. A higher fraction of local anesthetic in all mixtures was associated with lower pH, but did not significantly affect the number of crystals. Thus, alkalization of steroids may be more important than the local anesthetic pH in the process of crystal formation.
We used BSP in this study. However, BSP/betamethasone acetate (Celestone Soluspan®; Schering-Plough, Kenilworth, NJ) as a compound drug has been used in the USA Only BSP is available as a cream in the USA or it can be ordered separately from compounding companies. This compound drug has a long-acting duration, although BSP alone (non-particulate, pure liquid) is a short-acting drug. This compound may be preferred for use in clinical settings because of its long-acting duration; however, it has inherent issues, such as potency, accuracy, and sterility. The results of the present study suggest that clinicians should be careful while selecting local anesthetics when they use BSP with local anesthetics; therefore, more attention should be paid to the use of compound drugs with local anesthetics.
DPA, which was used in this study, is not commonly used for an epidural injection, and its safety remains unclear. It is an injectable lipid emulsion, and dexamethasone with lipid microspheres. DPA may not be expected to act as an embolus because the average liposome diameter of DPA is 0.1–0.3 µm, but its safety should be demonstrated.18,19 We measured the pH of DPA, and found that it was pH 7.1. Other non-particulate steroids were basic (DSP, pH 7.6) or strong basic (BSP, pH 8.5), whereas DPA was near neutral. When DPA (pH 7.1) and local anesthetics (pH < 7.0) were mixed, the pH of the resulting mixtures was acidic. Based on our findings, alkalization of steroids may affect crystal formation. It is thought that the almost neutral pH of DPA does not form crystals when mixed with local anesthetics. DPA 4 mg has a potency equivalent to that of DSP (2.5 mg). Thus, DPA exhibits potency that is one-half to one-fifth that of DSP. DPA can be mixed with local anesthetics (ropivacaine, levobupivacaine, bupivacaine, or lidocaine) without the risk of crystal formation. DPA in the clinical setting has been used for intra-articular injection in arthritis patients; it has also been used to treat arthritis in animal models.20,21 Although there have been no reports of epidural steroid injections for spinal pain, their use in lumbar facet joint injections has been reported.22 DPA should be tested for safety to enable its use in the epidural space.
On the basis of the results of this study, the mixture of ropivacaine or levobupivacaine and BSP may be discontinued for TFESI. The use of mixtures of ropivacaine or levobupivacaine and DSP may be possible because these mixtures produced very small crystals. Among the local anesthetics, lidocaine or bupivacaine is recommended when mixed with non-particulate steroids, as neither of them rarely form crystals. Studies have compared the effectiveness of particulate and non-particulate steroids for spinal injection through the transforaminal route.23–26 Physicians believe that particulate steroids have a longer duration than non-particulate steroids. However, these studies showed that the difference in effectiveness was neither clinically nor statistically significant. In addition, many studies have shown that injection of local anesthetics alone is sufficient for pain relief instead of combining corticosteroids with local anesthetics.27,28 Notably, non-particulate steroids are safer substitutes, but caution should be exercised in the selection of local anesthetics based on the results of this study.
We have specific recommendations for the use of non-particulate steroids with local anesthetics in TFESI: ropivacaine or levobupivacaine and BSP may be discontinued in ESI; ropivacaine or levobupivacaine, and DSP may be used because their mixture generated very small particulates; lidocaine or bupivacaine as local anesthetics are recommended; DPA may be optimal, but additional studies regarding its safety and effectiveness are needed. However, the ESI regimen varies, and there are no guidelines that suggest that local anesthetics must always be used. These recommendations are only relevant if mixtures of local anesthetics and non-particulate steroids are used in TFESI.
This study has some limitations. First, we thought that alkalization of steroids may play a more important role in the process of crystal formation. However, further research is needed to determine the exact cause. Second, the characteristics of other formulations can be different, although the local anesthetics and non-particulate steroids used in this study were the same ingredients. We cannot generalize this result for all other marketed formulations. Third, the concentration of the local anesthetic agent used for our experiment was higher than its concentration used for an epidural block. We only changed the volume of the local anesthetic without using normal saline. The crystal formation may be different with varying concentrations of local anesthetic. Fourth, we attempted to melt the crystals from all mixing regimens at 36–37 °C. and all crystals were insoluble at 36–37 °C. This is consistent with previous reports.17,29 However, because an in vivo analysis was not performed, it is unclear whether crystals may dissolve during actual ESI procedures. In addition, we could not prove the safety (eg, neurotoxicity and role of emboli) of these crystals when they reach the arterial supply to the spinal cord or brain during procedures.
Conclusion
In conclusion, non-particulate steroids have been thought to be safer than particulate steroids because of previous catastrophic embolic events in ESI, especially TFESI, which resulted from particulate steroid usage. However, because some non-particulate steroids can form particulates when mixed with local anesthetics, these combinations should be avoided. Mixtures of non-particulate steroids with local anesthetics that do not form crystals or form less of them are recommended.
Acknowledgments
This work was supported by a research grant from Seoul National University Bundang Hospital (02-2015-014) in 2015–2016.
Disclosure
Eun joo Choi, Dong-Hyun Kim, Woong Ki Han, Ho-Jin Lee, Imhong Kang, Francis Sahngun Nahm, Pyung-Bok Lee have no conflict of interest.
References
1. Manchikanti L, Alan DK, Almon S, et al. Comprehensive evidence-based guidelines for facet joint interventions in the management of chronic spinal pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2020;23(5;3S):S1–127. doi:10.36076/ppj.2020/23/S1
2. Manchikanti L, Falco FJ, Diwan S, Hirsch JA, Smith HS. Cervical radicular pain: the role of interlaminar and transforaminal epidural injections. Curr Pain Headache Rep. 2014;18(1):389. doi:10.1007/s11916-013-0389-9
3. Bensler S, Sutter R, Pfirrmann CW, Peterson CK. Is there a difference in treatment outcomes between epidural injections with particulate versus non-particulate steroids? Eur Radiol. 2017;27(4):1505–1511. doi:10.1007/s00330-016-4498-9
4. Makkar JK, Singh PM, Jain D, Goudra B. Particulate vs non-particulate steroids for transforaminal epidural steroid injections: systematic review and meta-analysis of the current literature. Pain Physician. 2016;19(6;7):327–340. doi:10.36076/ppj/2016.19.327
5. McCormick ZL, Cushman D, Marshall B, et al. Pain reduction and repeat injections after transforaminal epidural injection with particulate versus nonparticulate steroid for the treatment of chronic painful lumbosacral radiculopathy. PM R. 2016;8(11):1039–1045. doi:10.1016/j.pmrj.2016.03.011
6. Hong JH, Park EK, Park KB, Park JH, Jung SW. Comparison of clinical efficacy in epidural steroid injections through transforaminal or parasagittal approaches. Korean J Pain. 2017;30(3):220–228. doi:10.3344/kjp.2017.30.3.220
7. Huntoon MA. Anatomy of the cervical intervertebral foramina: vulnerable arteries and ischemic neurologic injuries after transforaminal epidural injections. Pain. 2005;117(1):104–111. doi:10.1016/j.pain.2005.05.030
8. Malhotra G, Abbasi A, Rhee M. Complications of transforaminal cervical epidural steroid injections. Spine. 2009;34(7):731–739. doi:10.1097/BRS.0b013e318194e247
9. Scanlon GC, Moeller-Bertram T, Romanowsky SM, Wallace MS. Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine. 2007;32(11):1249–1256. doi:10.1097/BRS.0b013e318053ec50
10. Shah RV. Paraplegia following thoracic and lumbar transforaminal epidural steroid injections: how relevant is physician negligence? J Neurointerv Surg. 2014;6(3):166–168. doi:10.1136/neurintsurg-2013-010903
11. Tiso RL, Cutler T, Catania JA, Whalen K. Adverse central nervous system sequelae after selective transforaminal block: the role of corticosteroids. Spine J. 2004;4(4):468–474. doi:10.1016/j.spinee.2003.10.007
12. Racoosin JA, Seymour SM, Cascio L, Gill R. Serious neurologic events after epidural glucocorticoid injection–the FDA’s risk assessment. N Engl J Med. 2015;373(24):2299–2301. doi:10.1056/NEJMp1511754
13. FDA Drug Safety Communications. FDA drug safety communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. Available from: https://www.fda.gov/media/88483/download.
14. Rathmell JP, Benzon HT, Dreyfuss P, et al. Safeguards to prevent neurologic complications after epidural steroid injections: consensus opinions from a multidisciplinary working group and national organizations. Anesthesiology. 2015;122(5):974–984. doi:10.1097/ALN.0000000000000614
15. Benzon HT, Chew TL, McCarthy R, Benzon HA, Walega DR. Comparison of the particle sizes of the different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106(2):331–338. doi:10.1097/00000542-200702000-00022
16. Watkins TW, Dupre S, Coucher JR. Ropivacaine and dexamethasone: a potentially dangerous combination for therapeutic pain injections. J Med Imaging Radiat Oncol. 2015;59(5):571–577. doi:10.1111/1754-9485.12333
17. Hwang H, Park J, Lee WK, et al. Crystallization of local anesthetics when mixed with corticosteroid solutions. Ann Rehabil Med. 2016;40(1):21–27. doi:10.5535/arm.2016.40.1.21
18. Phillips NC, Thomas DP, Knight CG, Dingle JT. Liposome-incorporated corticosteroids. II. Therapeutic activity in experimental arthritis. Ann Rheum Dis. 1979;38(6):553–557. doi:10.1136/ard.38.6.553
19. Shaw IH, Knight CG, Thomas DP, Phillips NC, Dingle JT. Liposome-incorporated corticosteroids: I. The interaction of liposomal cortisol palmitate with inflammatory synovial membrane. Br J Exp Pathol. 1979;60(2):142–150.
20. Hoshi K, Mizushima Y, Shiokawa Y, et al. Double-blind study with liposteroid in rheumatoid arthritis. Drugs Exp Clin Res. 1985;11(9):621–626.
21. Bonanomi MH, Velvart M, Weder HG. Fate of different kinds of liposomes containing dexamethasone palmitate after intra-articular injection into rabbit joints. J Microencapsul. 1987;4(3):189–200. doi:10.3109/02652048709021812
22. Hellmich D, Kob A, Deubler R, Schroder C, Rose P, Elsasser R. Acute treatment of facet syndrome by CT-guided injection of dexamethasone-21-palmitate alone and in combination with mepivacaine. Clin Drug Investig. 2004;24(10):559–567. doi:10.2165/00044011-200424100-00001
23. Dreyfuss P, Baker R, Bogduk N. Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med. 2006;7(3):237–242. doi:10.1111/j.1526-4637.2006.00162.x
24. Lee JW, Park KW, Chung SK, et al. Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol. 2009;38(11):1077–1082. doi:10.1007/s00256-009-0735-5
25. Kennedy DJ, Plastaras C, Casey E, et al. Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med. 2014;15(4):548–555. doi:10.1111/pme.12325
26. Nebojsa NK, Alexei L, Kenneth DC. Transforaminal vs interlaminar epidural steroid injections: differences in the surgical rates and safety concerns. Pain Med. 2014;15(11):1975–1976. doi:10.1111/pme.12572
27. Joshua AH, Alan DK, Amit M, et al. Lack of superiority of epidural injections with lidocaine with steroids compared to without steroids in spinal pain: a systematic review and meta-analysis. Pain Physician. 2020;24(6):S239–S270. doi:10.1152/ajplegacy.1975.229.6.1726
28. Manchikanti L, Nebojsa NK, Allan P, Alan DK, Mahendra S, Joshua AH. Does epidural bupivacaine with or without steroids provide long-term relief? A systematic review and meta-analysis. Curr Pain Headache Rep. 2020;24(6):26. doi:10.1007/s11916-020-00859-7
29. Koitabashi T, Sekiguchi H, Miyao H, Kawasaki J, Kawazoe T. Precipitation of pH-adjusted local anesthetics with sodium bicarbonate. Masui. 1995;44(1):15–20.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



