Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
Non-melanoma skin cancer in renal transplant recipients: a study in a Brazilian reference center
Authors Gonçalves C, Trope B, Ramos-e-Silva M
Received 1 December 2014
Accepted for publication 18 February 2015
Published 7 July 2015 Volume 2015:8 Pages 339—344
DOI https://doi.org/10.2147/CCID.S78456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Carolina Pereira Gonçalves, Beatriz Moritz Trope, Marcia Ramos-e-Silva
Sector of Dermatology, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Background: Non-melanoma skin cancer (NMSC) after kidney transplantation is common and can result in significant morbidity and mortality. Their incidence and risk factors in renal transplant recipients (RTRs) vary depending on geographic location and there is a scarcity of literature describing the features of NMSC in Brazil.
Methods: NMSC data were retrospectively reviewed in charts of RTRs at the Clementino Fraga Filho University Hospital from January 2004 to December 2005, with the objectives of: 1) evaluating the occurrence of NMSC in RTRs transplanted between 2004 and 2005 at a reference center in Brazil; 2) verifying the frequency of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) in these patients according to sex, race, age, and tumor site; and 3) determining the time between transplantation and neoplasia.
Results: We found 202 RTRs, with 165 suitable for the study. There were 19 NMSC in eleven patients (6.67%), at a mean time of 37.7 months after transplantation. The mean follow-up time was 72.7 months. The ratio of SCC:BCC was 1.1:1. White race and age ≥40 years were associated with a higher incidence of NMSC and they appeared predominantly in sun-exposed sites.
Conclusion: Regular dermatological follow-up of RTRs can help to make earlier diagnoses, resulting in better quality of life and lower morbidity and mortality.
Keywords: squamous cell carcinoma, basal cell carcinoma, Brazil
Introduction
Cancer after solid organ transplantation is a rapidly growing public health concern.1 Its overall incidence in renal transplant recipients (RTRs) has been reported to be two to ten fold, and in some cases even 100-fold, when compared to the general population.2,3 Non-melanoma skin cancers (NMSCs) are the most frequent malignancy after kidney transplantation,4–8 especially squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), which account for 95% of skin cancers in organ transplant recipients.9
When compared to the general population, patients undergoing solid organ transplantation are at increased risk for NMSC.10–15 In addition, they develop an excess of NMSC at a relatively young age.5,7,14 Some of them develop multiple lesions14,16 and these tumors have increased metastatic potential.14 This aggressive behavior causes considerable morbidity and potential mortality.7
The majority of studies on this subject describe an inverse ratio of SCC to BCC in comparison to the general population.6,7,11,15,17–19 While BCC is the most frequent NMSC in the general population, SCC is most common in organ transplant recipients.6,9
Immunosuppressors after renal transplantation are responsible for lowering the defense response of skin immunologic system, leading to an increased incidence of skin cancer.20
Risk factors for NMSC in organ transplant recipients include fair Fitzpatrick skin phototype,4,11,18,21 older age at time of transplantation,8,17,18,22–25 male sex,8,15,17–19,21,24,26 cumulative sun exposure,27 duration,8,9,17,23,24,26,28 and level of immunosuppression.9,29
Defining the extent of the problem of NMSC in the general population has been limited by incomplete registration of cases and the scarcity of reliable national incidence data.1 Among kidney transplant recipients, the true rate of NMSC is also greatly underestimated because clinicians tend to stop recording subsequent skin cancers as the patients develop multiple lesions.15 In our country, there is similarly a great paucity of medical literature describing the features of NMSC among RTRs.
The aims of this study are:
- to evaluate the occurrence of NMSC in RTRs at the Clementino Fraga Filho University Hospital (HUCFF) of the Federal University of Rio de Janeiro (UFRJ), who were admitted for transplantation between 2004 and 2005;
- to verify the frequency of BCC and SCC in these patients, according to the variables: sex, race, age, and tumor site; and
- to determine the time between transplantation and the development of the neoplasticism.
Patients and methods
The patient data were gathered from the database of the Renal Transplant Coordination (Sector of Nephrology) and from the Ambulatory of Dermatology and Immunosuppression (Sector of Dermatology). The medical charts of RTRs admitted for renal transplantation between January 2004 and December 2005 were retrospectively reviewed by extracting information on patient characteristics, immunosuppressive medications, and NMSC histopathological confirmation. Approval for this study was obtained from the Ethics Committee of the HUCFF and data compilation took place between April and July 2011.
In order to obtain a sample of patients with at least 1 year of follow-up, patients who died, suffered rejection with graft failure or lost to follow-up less than 1 year after transplantation were excluded from the study.
The bibliographic research was performed using the PubMed database.
The descriptive analysis was presented in tables and data were described as frequency (n) and percentage (%) for categorical data, and mean, standard deviation (SD), median, and minimum and maximum values for numerical data.
Inferential analysis was made by the chi square test or Fisher’s exact test to compare categorical data and by the Mann–Whitney test to compare numerical data between the groups with or without NMSC.30
A nonparametric method was used because the variables do not present a Gaussian distribution, as showed by the Kolmogorov–Smirnov test.30 A significance level of 5% was applied and statistical analysis was carried out using SAS® software (v6.11; SAS Institute Inc., Cary, NC, USA).
Results
One hundred and sixty-five of the 202 patients that were admitted for renal transplantation between 2004 and 2005 achieved at least 1 year of follow-up and were included in the study.
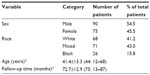
Table 1 provides the frequency (n) and percentage (%) of the epidemiological variables: sex, race, and age, and the follow-up time of the study. The numerical data are described as mean, SD, median, and minimum and maximum values.
  | Table 1 Distribution of epidemiological data |
Of the 165 patients, 75 were female (45.5%) and 90 (54.5%) were male; there were 68 (41.2%) white patients, 71 (43.0%) mixed, and 26 (15.8%) black subjects. The mean age of the patients was 41.4 years (±13.3, range 12–68 years) and the mean follow-up time was 72.7 months (Table 1).
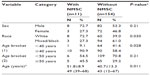
Table 2 provides the frequency (n) and percentage (%) of the epidemiological variables of the patients with and without NMSC and the corresponding P-value of the chi squared test or Fisher’s exact test. The numerical variables were compared using the Mann–Whitney test.
In our study, eleven patients (6.67%) developed 19 NMSC. Among these eleven patients, there were eight men (72.7%) and three women (27.3%). Eight patients were white (72.7%), three were mixed (27.3%), and none of them were black. The mean age of the patients with NMSC was 51.8 years (±8.9, range 39–68 years). The risk factors ‘white race’ (72.7%) and ‘age ≥40 years’ (90.9%) were significantly higher in the group of patients with NMSC than in the group without NMSC (39% and 58.4%, respectively), with P=0.030 and P=0.028, respectively. There was no significant association at the 5% level between the occurrence of NMSC and the epidemiological variables ‘sex’ and ‘age ≥50 years’ (Table 2).
Table 3 shows the distribution of NMSC characteristics found in our study. Among the 19 NMSC that were diagnosed in our patients, ten (53.6%) were SCC and nine (47.4%) were BCC. The ratio of SCC:BCC was 1.1:1. When we compared NMSC found in sun-exposed sites (face, scalp, hands, and arms) with those found in sun-protected sites (trunk, stomach, and legs), the incidence of NMSC in sun-exposed sites (63.2%) was significantly higher than in sun-protected ones (36.8%), with P=0.029. The mean time between transplantation and NMSC diagnosis was 37.7 months (±19.1, range 3–78 months).
Of the nine BCCs, six were in sun-exposed areas and three in sun-protected areas, while for the ten SCCs, six occurred in sun-exposed areas and four in sun-protected areas.
Table 4 presents another characteristic we found in our investigation: the distribution of the immunosuppressive drugs used by patients with and without NMSC. They used combinations of the following drugs: mycophenolate mofetil (MM), sodic mycophenolate (SM), tacrolimus (FK), prednisone (Pred), sirolimus (Sir), and cyclosporine (CsA).
Discussion
Accurate determination of the incidence of post-transplantation malignancies plays a vital role in planning resources to confront this problem and develop prevention strategies.1 It is difficult to compare the incidence of NMSC among RTRs and the general population because of the sub notification of this type of cancer worldwide.15
Usually of good prognosis in the general population, NMSCs in organ transplant recipients are a real concern due to their higher incidence, development of multiple tumors, and aggressive behavior, causing considerable morbidity and potential mortality.7
Our study provides epidemiological aspects related to the occurrence of NMSC in RTRs from a Brazilian renal transplant reference center, with a mean follow-up time of 72.7 months.
Eleven of the 165 patients developed at least one NMSC during the study period, signifying a frequency of 6.67%. This rate is higher than the 2.5% found in the Brazilian publication by Falsarella et al.31 However, incidence rates vary substantially in the medical literature. We found papers reporting no NMSC in India;32 and incidences of 4.86%3 and 25.3%12 in Spain; 6.4%33 and 10.3%21 in Italy; 15.2%34 in Scotland; 24.4%15 in New Zealand; and even incidences as high as 51.8%, in Australia.7
Among the eleven patients that developed NMSC, 72.7% (n=8) were men and 27.3% (n=3) were women. Although there was no statistical significance at the 5% level in this comparison, the higher number of cases in men is in accordance with the literature.8,15,17–19,21,26
The Fitzpatrick skin phototype scale is used all over the world to estimate skin cancer risk.35 In our study, however, it was not applied because this classification was not used in the patient’s charts. These charts present race classification as ‘white’, ‘mixed’, and ‘black’. The group of patients with NMSC presented the risk factor ‘white race’ (72.7%) significantly higher than the group without NMSC (39%), with P=0.030. This finding is consistent with that in the literature, which demonstrates that fair skin RTRs carry a higher risk of NMSC than darker skin ones.8,9,18,19,23
When we analyze the age of these patients, we observe that the NMSC patients presented the risk factor ‘age ≥40 years’ (90.9%) significantly higher than the group without NMSC (58.4%), with P=0.028. These data are similar to those found in the literature, in which many studies report that NMSC risk grows with age of the patient at the moment of transplantation.8,12,15,17,18,22–24 Some papers show higher risk in older patients in comparison to younger ones, but they set the age of 50 years as the cutoff point.1,19,21 There was no statistical significance in our study when a cutoff point of 50 years was used.
In this study, ten SCC and nine BCC were diagnosed, meaning a SCC:BCC ratio of 1.1:1, which is the same as that found in literature for immunosuppressed patients, while immunocompetent individuals have inverted rates, at 1:4.15,36 Studies in transplant recipients present rates of 1.2:1,15 1.9:1,6 and even higher proportions, including 2:1,7 3.2:1,17 and 7:1.18 The reasons for the variation in ratio were not fully elucidated, but they are probably related to differences in the follow-up time between the studies,8 because in RTRs, the incidence of BCC grows linearly, while the SCC rate grows exponentially.12,34–36 Other possible explanations for the varying SCC:BCC ratios in different studies are regional variations of latitude and sun-exposure habits.9,15
The proportion of NMSC in sun-exposed sites was significantly higher than in sun-protected areas. This is in agreement with the findings of Ramsay et al23 and Ho and Murphy,29 who reported that sun-exposed areas have a higher incidence of NMSC than sun-protected areas.
The mean time between renal transplantation and NMSC diagnosis was 37.7 months or approximately 3 years and 2 months. This is faster than what was found in the majority of studies: Falsarella et al,31 with 3 years and 6 months; Comeau et al,19 with 4 years; Ramsay et al,7 with 4 years and 2 months; Kalinova et al,36 with 5 years; and Mackenzie et al,15 with 5 years and 2 months. Other studies published even higher numbers, including Tessari et al,21 with approximately 7 years; Navarro et al,3 with 7.5 years; Bordea et al,17 with 8 years; and Moloney et al,1 with 8 years and 2 months.
The great variability in incidence rates and in the time between transplantation and NMSC development in the literature are likely due to the same reasons: regional variations of skin phototype,32 geographic characteristics of each country,34 and different lengths of follow-up, since the incidence of NMSC grows with time of immunosuppression.8
Evidence is lacking in human trials in terms of the relationship between skin cancer risk and immunosuppressive agents.37 Two factors impaired the evaluation of the influence of immunosuppressive drugs on NMSC risk in our study: the multiplicity of drug combinations and the lack of information on individual changes of medications post-transplantation.
RTRs have increased risk of NMSC and they usually develop multiple and more aggressive lesions. The factors associated with a higher risk of NMSC in these patients are fair skin phototype, older age at time of transplantation, male sex, cumulative sun exposure, and duration and level of immunosuppression.
Our study was not designed to compare the incidences of NMSC between RTRs and the general population, but to provide an initial analysis of the occurrence of NMSC in a Brazilian renal transplant reference center. Future studies must be performed to further elucidate the factors associated with NMSC in RTRs.
A better understanding of the factors that determine skin cancer risk could be very useful for transplant patients. The possibilities for improvement include prevention and early diagnosis of NMSC, which would help to minimize costs and the inconvenience of multiple surgeries or other modalities of treatments. As can be assumed from all of the above, a close collaboration between dermatologists and transplant teams is recommended for patient education in the prevention and early treatment of evolving skin cancer.
Acknowledgment
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for funding this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154(3):498–504. | |
Végsô G, Tóth M, Hídvégi M, et al. Malignancies after renal transplant during 33 years at a single center. Pathol Oncol Res. 2007;13(1):63–69. | |
Navarro MD, López-Andréu M, Rodríguez-Benot A, Agüera ML, Del Castillo D, Aljama P. Cancer incidence and survival in kidney transplant patients. Transplant Proc. 2008;40(9):2936–2940. | |
Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol. 2011;165(5):953–965. | |
Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplant. 2009;87(11):1667–1671. | |
Buell JF, Hanaway MJ, Thomas M, Alloway RR, Woodle ES. Skin cancer following transplant: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc. 2005;37(2):962–963. | |
Ramsay HM, Fryer AA, Hawley CM, Smith AG, Harden PN. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 2002;147(5):950–956. | |
Mudigonda T, Levender MM, O’Neill JL, West CE, Pearce DJ, Feldman SR. Incidence, risk factors, and preventative management of skin cancers in organ transplant recipients: a review of single- and multicenter retrospective studies from 2006 to 2010. Dermatol Surg. 2013;39(3 Pt 1):345–364. | |
Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients – where do we stand today? Am J Transplant. 2008;8(11):2192–2198. | |
Bouwes Bavinck JN, Euvrard S, Naldi L, et al; EPI-HPV-UV-CA group. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127(7):1647–1656. | |
Zavos G, Bokos J, Papaconstantinou J, et al. Study of “de novo” malignancies among Greek renal transplant recipients. Transplant Proc. 2003;35(4):1399–1403. | |
Fuente MJ, Sabat M, Roca J, Lauzurica R, Fernández-Figueras MT, Ferrándiz C. A prospective study of the incidence of skin cancer and its risk factors in a Spanish Mediterranean population of kidney transplant recipients. Br J Dermatol. 2003;149(6):1221–1226. | |
Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys. 2011;508(2):159–163. | |
Harden PN, Reece SM, Fryer AA, Smith AG, Ramsay HM. Skin cancer surveillance in renal transplant recipients: questionnaire survey of current UK practice. BMJ. 2001;323(7313):600–601. | |
Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25(1):300–306. | |
Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155(5):916–925. | |
Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplant. 2004;77(4):574–579. | |
Keller B, Braathen LR, Marti HP, Hunger RE. Skin cancers in renal transplant recipients: a description of the renal transplant cohort in Bern. Swiss Med Wkly. 2010;140:w13036. | |
Comeau S, Jensen L, Cockfield SM, Sapijaszko M, Gourishankar S. Non-melanoma skin cancer incidence and risk factors after kidney transplant: a Canadian experience. Transplant. 2008;86(4):535–541. | |
Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56(1):127–136. | |
Tessari G, Naldi L, Boschiero L, et al. Incidence and clinical predictors of a subsequent nonmelanoma skin cancer in solid organ transplant recipients with a first nonmelanoma skin cancer: a multicenter cohort study. Arch Dermatol. 2010;146(3):294–299. | |
Bernat García J, Morales Suárez-Varela M, Vilata JJ, Marquina A, Pallardó L, Crespo J. Risk factors for non-melanoma skin cancer in kidney transplant patients in a Spanish population in the Mediterranean region. Acta Derm Venereol. 2013;93(4):422–427. | |
Ramsay HM, Fryer AA, Hawley CM, Smith AG, Nicol DL, Harden PN. Factors associated with nonmelanoma skin cancer following renal transplant in Queensland, Australia. J Am Acad Dermatol. 2003;49(3):397–406. | |
Rubel JR, Milford EL, Abdi R. Cutaneous neoplasms in renal transplant recipients. Eur J Dermatol. 2002;12(6):532–535. | |
Kessler M, Jay N, Molle R, Guillemin F. Excess risk of cancer in renal transplant patients. Transpl Int. 2006;19(11):908–914. | |
Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplant. 1996;61(5):715–721. | |
Moloney FJ, Almarzouqi E, O’Kelly P, Conlon P, Murphy GM. Sunscreen use before and after transplant and assessment of risk factors associated with skin cancer development in renal transplant recipients. Arch Dermatol. 2005;141(8):978–982. | |
Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1(8630):124–129. | |
Ho WL, Murphy GM. Update on the pathogenesis of post-transplant skin cancer in renal transplant recipients. Br J Dermatol. 2008;158(2):217–224. | |
Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York, NY: John Wiley and Sons; 1973. | |
Falsarella PM, Alves-Filho G, Mazzali M. Skin malignancies in renal transplant recipients: a Brazilian center registry. Transplant Proc. 2008;40(3):767–768. | |
George L, John GT, Jacob CK, Eapen P, Pulimood S, George R. Skin lesions in renal transplant recipients: a single center analysis. Indian J Dermatol Venereol Leprol. 2009;78(3):255–261. | |
Formicone F, Fargnoli MC, Pisani F, Rascente M, Famulari A, Peris K. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37(6):2527–2528. | |
Mackintosh LJ, Geddes CC, Herd RM. Skin tumours in the West of Scotland renal transplant population. Br J Dermatol. 2013;168(5):1047–1053. | |
Ravnbak MH. Objective determination of Fitzpatrick skin type. Dan Med Bull. 2010;57(8):B4153. | |
Kalinova L, Majek O, Stehlik D, Krejci K, Bachleda P. Skin cancer incidence in renal transplant recipients – a single center study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(3):257–260. | |
Francis S, Berg D. Reducing skin malignancy risk in organ transplant recipients. Skin Therapy Lett. 2013;18(1):1–3. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.