Back to Journals » International Journal of General Medicine » Volume 15
Nomograms for Death from Pneumocystis jirovecii Pneumonia in HIV-Uninfected and HIV-Infected Patients
Authors Feng Q , Hao J, Li A , Tong Z
Received 17 November 2021
Accepted for publication 1 March 2022
Published 15 March 2022 Volume 2022:15 Pages 3055—3067
DOI https://doi.org/10.2147/IJGM.S349786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Qiuyue Feng,1,2 Jingjing Hao,3 Ang Li,3 Zhaohui Tong1
1Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China; 2Department of Respiratory Medicine, Beijing Huairou Hospital, Beijing, 101400, People’s Republic of China; 3Department of Critical Care Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Correspondence: Zhaohui Tong, Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China, Email [email protected] Ang Li, Department of Critical Care Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China, Email [email protected]
Purpose: Pneumocystis jirovecii pneumonia (PCP) is a major cause of death in immunocompromised patients. Many risk factors for poor prognosis have been reported, but few studies have created predictive models with these variables to calculate the death rate accurately. This study created nomogram models for the precise prediction of mortality risk in human immunodeficiency virus (HIV) uninfected and HIV-infected patients with PCP.
Patients and Methods: A retrospective study was performed over a 10-year period to evaluate the clinical characteristics and outcomes of PCP in HIV-uninfected and HIV-infected adults treated in Beijing, China from 2010 to 2019. Univariate and multivariate logistic regression analyses were used to identify mortality risk factors to create the nomograms. Nomogram models were evaluated by using a bootstrapped concordance index, calibration plots and receiver operating characteristic (ROC) curves.
Results: A total of 167 HIV-uninfected and 193 HIV-infected PCP patients were included in the study. Pneumothorax, duration of fever after admission, CD4+ T cells ≤ 100/μL and trimethoprim-sulfamethoxazole (TMP-SMX) combined with caspofungin (CAS) treatment were independent risk factors for death in HIV-uninfected PCP patients. We derived a well calibrated nomogram for mortality by using these variables. The area under the curve was 0.865 (95% confidence interval 0.799– 0.931). Independent risk factors for death in HIV-infected PCP patients were pneumothorax, platelet (PLT) ≤ 80× 109/L, haemoglobin (HGB) ≤ 90 g/L, albumin (ALB), cytomegalovirus (CMV) coinfection and TMP-SMX combined with CAS treatment. The nomogram showed good discrimination, with a C-index of 0.904 and excellent calibration.
Conclusion: The nomograms which were derived may be useful tools for the precise prediction of mortality in HIV-uninfected and HIV-infected patients, but require validation in clinical practice.
Keywords: Pneumocystis jirovecii pneumonia, nomogram, HIV, HIV-uninfected, mortality
Introduction
Pneumocystis jirovecii pneumonia (PCP), is a fungal infection of the respiratory system caused by Pneumocystis jirovecii. As one of the most common opportunistic infections in patients with the acquired immunodeficiency syndrome (AIDS), it is a major cause of morbidity and mortality.1 In recent years, with the widespread application of glucocorticoids and cytotoxic drugs, advances in tumour chemoradiotherapy, the frequency of connective tissue diseases and organ transplantation, the incidence of PCP in HIV-uninfected immunosuppressed patients has increased significantly.2 PCP typically presents with acute and rapid progressive Respiratory insufficiency. HIV-infected PCP patients follow a more insidious course. In contrast, HIV-uninfected patients with PCP present with abrupt-onset hypoxemic Respiratory failure.3
It has higher mortality in HIV-uninfected patients than in HIV-infected patients (30–60% versus 10–20%).4,5 Many risk factors for poor outcomes in PCP patients have been reported,6 but it is difficult to predict the individual death rate accurately. More efficient prediction tools for estimating the prognosis of PCP patients are needed.
Nomograms are graphical models that enable users to calculate the overall probability of a specific clinical outcome for an individual patient.7,8 There are many nomograms used as prediction tools in various diseases, including cancer.9 The nomogram facilitates the clinical implementation and probability calculation of risk factors or other predictor variables. The objective of this study was to combine clinical manifestations, treatment variables, and laboratory variables that were associated with death into two prediction nomograms: we developed and calibrated nomograms that predicted death risk in HIV-uninfected and HIV-infected patients.
Patients and Methods
Study Patients
We conducted a retrospective study to collect clinical data from Beijing Chaoyang Hospital and Beijing Ditan Hospital, Capital Medical University. Approvals to conduct the study were obtained by the hospital research ethics committees. Because of the retrospective nature of the observational study, which lacked interventional aspects, the ethics committee agreed to waive the requirement for informed written consent.
We retrospectively collected the data of patients with confirmed PCP that were Hospitalized for between 1 January 2010 and 31 December 2019 at either of the two hospitals of Capital Medical University, Beijing Chaoyang Hospital and Beijing Ditan Hospital, both tertiary Care University hospitals in Beijing, China. Beijing Ditan Hospital is the largest center for admissions of AIDS patients in Beijing and surroundings, HIV-infected PCP patients are treated and cared there, the treating facilities had no restrictions on access to ICU and ECMO. By screening the eligible adult patients (age ≥18 years) from a computerized Medical chart search system by ICD-10 (International Classification of Diseases, 10th revision) code, medical records were reviewed, and data were extracted and registered in the research forms. Data were entered into Excel spreadsheets for preservation. We diagnosed PCP based on the presence of the following criteria: (1) clinical symptoms, such as fever, dry cough (occasionally expectorant) and progressive dyspnoea; (2) abnormal imaging findings: CT showed a broad range of features, from ground-glass opacity to nodules, cysts, patchy shadows and diffuse interstitial infiltrates; (3) a positive result for P.jirovecii microorganisms or Pneumocystis DNA based on PCR results in an induced sputum, low-tracheal-aspiration or bronchoalveolar lavage fluid (BALF) specimen. Cysts can be stained with Gomori-Grocott, or toluidine blue O. Immunofluorescent monoclonal antibodies have ability to stain both trophic forms and cysts. Confirmed PCP was defined as either the presence of Pneumocystis microorganisms in the respiratory samples upon microscopic examination, or positive results for Pneumocystis DNA based on PCR results and serum tested positive for G test.
Confirmed PCP cases with a first episode were included. Exclusion criteria included current pregnancy, allergy to trimethoprim-sulfamethoxazole (TMP-SMX), and less than one week of hospitalization. The diagnosis of HIV-infection was based on Western blot conducted by the CDC to detect HIV-1 antibody positivity, and clinical stage was assigned according to the Centers for Disease Control and Prevention (CDC) classification.11 All HIV-uninfected PCP patients were tested for HIV to confirm HIV negative status.
Data Collection
The electronic medical charts for each enrolled patient were reviewed to obtain the following information: demographical data, underlying diseases, use of immunosuppressive drugs, clinical manifestations, radiology characteristics, laboratory tests, treatment and in-hospital mortality. Laboratory data used for analysis were those recorded within 48 hours, or the worst values after admission. Febrile days were the unbroken duration of a daily temperature >37.5°C after admission until discharge or death. Microbiological findings included CMV, Epstein–Barr virus (EBV) and bacterial co-infection.
Statistical Analysis
SPSS 20.0 (SPSS Institute, Chicago IL, USA) statistical software was used to perform statistical analysis. Nomogram models were created with R software (version 4.0.3; http://www.Rproject.org) using the “rms” package. Measurement data with a normal distribution are expressed as mean ± SD, while measurement data with a non normal distribution are expressed as median (interquartile range). Count variables are expressed as a percentage (%). The independent sample t-test was used for normally distributed continuous numerical variables. Continuous variables that did not follow a normal distribution or graded variables were tested using the Mann–Whitney U-test, and categorical variables were compared by using the Pearson chi-square test. P-values of < 0.05 were considered as being statistically significant. Variables with statistical significance were selected for the logistic regression analysis of prognosis, and variables with P < 0.05 in univariate regression analyses were incorporated into a multivariate logistic regression model. To identify predictive factors independently associated with in-hospital mortality, nomograms for hospital mortality risk were created based on multivariate logistic regression model outputs. According to nomograms, the score of each patient including in this study was calculated and ROC curve was created. The performance of the nomogram was evaluated through the concordance index and calibration plots with bootstrap samples.
Results
A total of 622 adult patients with a first episode of PCP were identified in the database from 2010 to 2019. Among these, 202 patients had probable PCP without microbiological results and were excluded from the analysis. Sixty patients were excluded: 26 with TMP-SMX drug allergy and 34 who were hospitalized for less than 1 week. Therefore, 360 patients were included for analysis in the study, 167 (46.3%) of whom were HIV-uninfected, and 193 (53.6%) of whom were HIV-infected.
Patient Characteristics
We compared demographics, clinical characteristics, and auxiliary examinations of the two groups (Table 1) and recorded underlying diseases in the HIV-uninfected group (Table 2). HIV-infected PCP patients were predominantly men (97.4% vs 61.7%; P<0.001), were less often smokers (22.80% vs 35.3%; P=0.009), and were younger (38.12±10.53 vs 53.69±16.32 years; P<0.001) than HIV-uninfected PCP patients. Underlying diseases/conditions in the HIV-uninfected PCP group included chronic diseases (n =89), chronic lung diseases (n=53), solid organ transplantation (n=49: kidney 43, liver 5, cornea 1), connective tissue diseases (n=46), nephrotic syndrome (n=14), solid tumours (n=14), and haematological malignancies (n=8). In the HIV-infected PCP group, past diseases included cardiovascular disease (n=10), diabetes mellitus (n=2), asthma (n=2), chronic hepatitis B (n=1) and schizophrenia (n=1).
 |
Table 1 Demographic Characteristics of HIV-Uninfected and HIV-Infected PCP Patients |
 |
Table 2 Underlying Diseases at Diagnosis of HIV-Uninfected PCP |
The most common clinical finding HIV-uninfected and HIV-infected PCP patients was fever (89.8% vs 86.0%, P=0.271), the triad fever, cough, and dyspnoea (59.3% vs 51.3%, P=0.129), and chest pain (3.60% vs 3.10%, P=0.799). However, duration of fever before admission was significantly shorter in HIV-uninfected patients [7 (3–10) vs 10 (4–15) days, P=0.004], and these patients also had higher temperature [39.0 (38.3–39.5) vs 38.5 (37.6–39.0) °C, P<0.001], a higher prevalence of lung rales (54.5% vs 15.0%, P<0.001), and a lower prevalence of weight loss (18.6% vs 69.4%, P<0.001) compared to HIV-infected patients.
A total of 154 (92.2%) of the HIV-uninfected PCP patients were receiving immunosuppressant therapy for their underlying diseases. Glucocorticoids alone were administered in 147 (88.0%) HIV-uninfected patients, chemotherapeutic agents alone were administered in 19 (11.4%), and glucocorticoids combined with immunosuppressive or chemotherapeutic agents were administered in 115 (68.9%). The median time from initiation of immunosuppressive medication to the PCP diagnosis was 186 days (range: 99–372 days).
Laboratory data were available from 360 patients. The platelet (PLT) count, haemoglobin (HGB) level, CD8+ cell count, C-reactive protein (CRP) level, albumin (ALB) level and oxygenation index were significantly lower in the HIV-uninfected PCP group compared to the HIV-infected PCP group. In contrast, CD4+ T cell counts, the CD4/CD8 ratio, procalcitonin (PCT) and lactic dehydrogenase (LDH) were significantly higher in the HIV-uninfected PCP group. A multivariate logistic regression model identified number of febrile days after admission, CD4+ T cells ≤100/µL, pneumothorax, and TMP-SMX combined with CAS treatment as significantly associated with mortality in the HIV-uninfected PCP group. In the HIV-infected PCP group, PLT≤80x109/L, HGB≤90 g/L, ALB, CMV coinfection, pneumothorax and TMP-SMX combined with CAS treatment were independently associated with in-hospital mortality.
Coinfections in the respiratory tract were detected in HIV-uninfected and HIV-infected PCP patients, although significantly more prevalent in HIV-infected patients [87 (52.0%) vs 136 (70.4%), p<0.001].Fifty-four patients had evidence of infection with 2 or more pathogens simultaneously. A positive serum assay for CMV was identified in 241 patients with similar prevalence in HIV-uninfected and HIV-infected patients [112 (67.1%) vs 129 (66.5%)], EBV in 101 patients [97 (58.1%) vs 4 (2.1%)], and H1N1 virus in 3 patients [2 (1.2%) vs 1 (0.5%)]. Other pathogens found in respiratory samples were Mycobacterium tuberculosis [n=5; 1(0.5%) vs 4(2.1%)], Pseudomonas aeruginosa [n=19; 13 (7.8%) vs 6(3.1%)], Klebsiella pneumoniae [n=4; 4(2.4%) vs 0 (0.0%)], Escherichia coli [n =9; 5(3.0%) vs 4(2.1%)], Aspergillus [n=32,24 (14.3%) vs 8 (4.1%)], Candida [n=48,36 (21.5%) vs.12 (6.2%)], Acinetobacter baumannii [n=7; 5(3.0%) vs 2(1.0%)], and atypical pathogens [n=4; 2(2.1%) vs 2(1.0%)]. Coinfections detected in the blood included CMV [n = 6, 0 (0.0%) vs 6 (3.1%)], Gram-positive coccus septicemia [n = 14, 9 (5.4%) vs 5 (2.6%)], Gram-negative bacillus septicemia [n = 9, 7 (4.2%) vs 2 (1.0%)].
Treatment and Outcome
A total of 354 patients received TMP-SMX, and 125 of whom received TMP-SMX combined with caspofungin (CAS). A total of 41 (83.6%) solid organ transplantation patients did not adhere to TMP-SMX for PCP prophylaxis. Adverse effects of TMP-SMX included liver dysfunction (n=10), gastrointestinal reaction (n=3), minor myelosuppression (n=10), rash (n=22), and minor renal dysfunction (n=11). Adverse effects of TMP-SMX were more common in HIV-uninfected PCP patients [22 (13.2%) vs 21 (10.8%), p=0.504]. TMP-SMX was discontinued in 15 patients due to drug intolerance. A total of 351 (97.5%) patients received suitable antibiotic treatment based on antimicrobial susceptibility tests of respiratory samples or empiric antibiotic therapy. A total of 275 (76.4%) patients received systemic corticosteroids as adjunctive therapy.
PCP in HIV-uninfected patients caused more severe oxygenation impairment (oxygenation index, 287.57±119.28 vs 310.78±100.68 mmHg, p=0.046), and more patients presenting with acute respiratory failure required noninvasive mechanical ventilation [39 (23.4%) vs 2(1.0%), p <0.001] and high-flow nasal cannula [7 (4.2%) vs 4(2.1%), p =0.244], more transfers to the ICU [93 (55.7%) vs 53 (27.5%), p<0.001], and a higher requirement for extracorporeal membrane oxygenation therapy [12 (7.2%) vs 2 (1.0%), p = 0.040]. Furthermore, the mortality rate was higher in the HIV-uninfected PCP group [49 (29.3%) vs 35 (18.1%), p=0.012].
Nomogram for Mortality Prediction
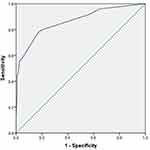
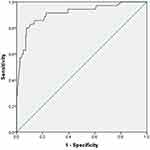
We investigated the association between clinical factors and all-cause mortality by univariate analysis in both groups. Febrile days after admission, PLT≤80(x109/L), HGB≤90 g/L, CD4+ T cells ≤100/µL, LDH≥500 U/L, PCT, ALB, CMV co-infection, EBV co-infection, pneumothorax, TMP-SMX combined with CAS, ICU days, and ECMO were significantly associated with mortality in the HIV-uninfected PCP group (Table 3). In multivariate logistic regression analysis, febrile days after admission, CD4+ cells ≤100/µL, pneumothorax and TMP-SMX combined with CAS therapy were independently associated with in-hospital death, and a combination of these factors most precisely predicted mortality (Table 4). We then created a nomogram for mortality including all of these factors (Figure 1). The area under the curve (AUC) was 0.865 (95% confidence interval 0.799–0.931; Figure 2). The nomogram had a bootstrapped concordance index of 0.865 and was well calibrated (Figure 3). In the same way, we created a nomogram for mortality in the HIV-infected PCP group (Figure 4). The area under the curve (AUC) was 0.910 (95% confidence interval 0.850–0.970; Figure 5). The nomogram had a bootstrapped concordance index of 0.904 and was well calibrated (Figure 6).
 |
Table 3 Prognostic Factors in a Univariate Regression Analysis in Patients with PCP |
 |
Table 4 Multivariate Regression Analysis for Independent Factors Associated with Death After Admission |
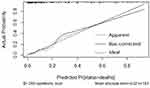 |
Figure 6 Calibration of the nomogram for mortality of the HIV-infected PCP group. |
Discussion
To our knowledge, this is the first study to create predictive nomogram models to calculate mortality risk in PCP patients. The two hospitals are comprehensive teaching hospitals and there is no bias for admission criteria, therapeutic protocols and levels of disease severity. We have affirmed all included laboratory indicators have been tested in the same way, and different reference units have been corrected. This retrospective study describes the clinical characteristics and outcomes of HIV-uninfected and HIV-infected patients with PCP. Baseline characteristics differed substantially between the HIV-uninfected and HIV-infected PCP populations, HIV-uninfected patients being older, as found in a previous report.12 Coinfections, most notably with viruses, especially CMV coinfection, were considerably more prevalent among HIV-uninfected PCP patients, which was consistent with published findings.13 PCP usually presents with atypical symptoms, such as fever, dry cough, and dyspnoea, occurring in up to 86%, 76%, and 81% of cases, respectively.5 In our study, prevalence of fever was similar in the HIV-uninfected and HIV-infected PCP groups. In multivariate regression analysis, febrile days after admission was independently associated with death in HIV-uninfected PCP patients. The results suggested that continuous fever as a predictive factor could enable clinicians to recognize the mortality risk of PCP earlier and avoid further deterioration of the patient’s condition.
The main risk factors for immunosuppression among HIV-uninfected PCP patients in our study were drug-related immunosuppression and transplantation, which are known to impair cell-mediated immunity. Our study shows that 141 (84.4%) HIV-uninfected PCP patients had a low CD4+ T cell counts, and that almost 1/4 (43/167;25.7%) were renal transplant recipients. Solid organ transplant recipients are known to remain at risk for PCP for many years after transplantation,2 but fewer recipients adhered to TMP-SMX for PCP prophylaxis in our study. Glucocorticoid treatment is a well-known risk factor for PCP in HIV-uninfected patients, and accounts for 55–97% of published cases,14,15 including 88.0% in our study. The mechanism for increase susceptibility to PCP in patients treated with glucocorticoids could be due to a decrease in peripheral CD4+ T cells.14 Immunosuppressive agents such as thiopurine could reduce the absolute numbers of lymphocytes by inhibiting cell proliferation. Tacrolimus and cyclosporine can inhibit lymphocyte activation, and cytokines can inhibit lymphocyte function.16
The main radiologic features of PCP identified through CT scanning are extensive ground-glass opacity (GGO) and reticulation.17,18 In our study, the rate of GGOs was higher in the HIV-uninfected PCP group (59.9%), while patients in the HIV-infected PCP group (88.1%) had other radiographic features. Pneumothorax is an unresolved problem in PCP because P. jirovecii infects subpleural spaces to which drug penetration is limited.19 In adults, the incidence of pneumothorax in PCP ranges from 4% to 36%.20 When barotrauma occurs, it usually indicates a poor prognosis and a high mortality rate of 50%-100%.21–23 The pneumothorax rates were 10.2% and 4.7% in HIV-uninfected and HIV-infected patients, respectively; significantly greater in the HIV-uninfected group. Nearly all patients with a poor prognosis developed pneumothorax. In multivariate regression model, pneumothorax was an independent risk factor for mortality in both groups.
Laboratory indices showed that the lymphocyte count, CD4+ T cells, serum ALB, and oxygenation index were below normal and that (1,3)-β-D-glucan and LDH levels were elevated in PCP patients. WBC count was normal in these patients. These findings are consistent with other studies.24 Previous studies demonstrated that hypoalbuminaemia had a positive correlation with increased lung injury and could be a significant indicator of death in Critically ill patients.25,26 In our study, the mean ALB level was higher in the HIV-infected PCP group, although ALB levels were below normal in both groups. ALB was a significant independent factor of poor prognosis in HIV-infected PCP group only. This finding was similar to that of Kim et al,27 who showed that hypoalbuminaemia was not considered an independent predictor of mortality in HIV-uninfected PCP patients. Overall, these results reflect that treatment strategies for HIV-infected PCP patients should be chosen with the awareness that serum ALB can be a warning of the risk of death.
A decrease in HGB indicates anaemia, and haemoglobin below 90 g/L is moderate to severe anaemia. Anaemia can also be defined as a lowered ability of the blood to carry oxygen. Therefore, we thought that anaemia might cause a worse prognosis in PCP patients. We found that HGB≤90 g/L was an important risk factor for mortality in both groups, and an independent predictor of death in HIV-infected PCP patients. These results were consistent with a previous study,28 which indicated that timely discovery and correction of anaemia were necessary in patients with PJP.
A low oxygenation index is associated with poor outcomes in PCP patients with immunosuppressive disease.29 In our study, the oxygenation index in the HIV-uninfected PCP and HIV-infected PCP groups was 287.57±119.28 vs 310.78±100.68, respectively. A lower oxygenation index, which was also representative of ventilation-perfusion abnormalities, was related to death in both groups.
Despite being associated with intolerance and adverse events, TMP-SMX is still the first-line therapeutic regimen for PCP.10,30,31 In our study, 55.1% of the HIV-uninfected PCP patients and 88.6% of HIV-infected PCP patients were treated with TMP-SMX within 24 hours, but early initiation of therapy targeting P. jirovecii did not improve clinical prognosis.32 Caspofungin is a recognized second-line regimen for the treatment of PCP.33 We found that TMP-SMX combined with CAS treatment was an independent risk factor for death in multivariate analysis in both groups. Other studies have reported failure of echinocandin salvage therapy to improve survival among non-AIDS patients.34,35
The overall mortality rate in HIV-uninfected PCP patients is 31%, but it rises to almost 100% when PCP is not properly and quickly treated.36,37 In several studies, PCP was more often fatal in HIV-uninfected (30–60%) patients than in HIV-infected (7–20%) patients.12,21 We observed in-hospital mortality rates of 29.3% in HIV-uninfected PCP patients, which was higher than the 18.1% mortality rate in HIV-infected patients. To calculate the precise mortality risk of PCP patients, we constructed nomogram models which incorporated clinical parameters and auxiliary examinations.
This study has some limitations. First, it is a retrospective study of two centres with a reasonably small study population, and included only patients with laboratory-proven PJP, precluding analysis of patients with presumptive PCP, who may have had different clinical manifestations or severity of the disease. Retrospective studies may be biased in terms of the data collected, such as physical examination data and the normal ranges in lab tests. A larger, prospective study is necessary particularly in order to validate the nomogram models that were derived in this analysis. Second, the most common underlying diseases/conditions in the HIV-uninfected PCP group was renal transplant (n=43), which may not be fully representative; however, this is balanced by the fact that HIV-uninfected patients with underlying chronic diseases (n=89) constituted the largest group of PCP patients. Third, our analysis only includes PCP patients who were hospitalized for more than 7 days. Some patients were not enrolled in this study because they left within 7 days after admission for any reason.
Conclusion
Our nomogram models may provide a useful, convenient and applicable tool to evaluate the individualized prognosis of mortality in HIV-uninfected and HIV-infected PCP patients, but require validation in prospective studies in our and other settings.
Abbreviations
ALB, albumin; BMI, body mass index; CAS, caspofungine; CMV, cytomegalovirus; CRP, C-reactive protein; EBV, Epstein–Barr virus; ECMO, extracorporeal membrane oxygenation GGO, ground-glass opacity; HFNC, high-flow nasal cannula; HIV, human immunodeficiency virus; HGB, hemoglobin; ICU, intensive Care unit; IMV, invasive mechanical ventilation; LDH, lactate dehydrogenase; NIMV, non-invasive mechanical ventilation; OI, oxygenation index; PCT, procalcitonin; PCP, Pneumocystis jirovecii pneumonia; Rh, Rhesus; TMP-SMX, trimethoprim-sulfamethoxazole; WBC, white blood cells.
Data Sharing Statement
The data used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted according to the Declaration of Helsinki. For transplant patients, all organs were donated voluntarily with written informed consent, and that this was conducted in accordance with the Declaration of Istanbul. The Medical Ethics Committee of Beijing Chaoyang Hospital approved this study and waived the informed written consent given its observational nature.
Acknowledgments
We would like to thank the staff of the Beijing Chaoyang Hospital and Beijing Ditan Hospital medical records department for the help in locating all of the patient’s charts for this study.
Authors’ Information
Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, People’s Republic of China. Department of Critical Care Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, People’s Republic of China. Department of Respiratory Medicine, Beijing Huairou Hospital, Beijing 101400, People’s Republic of China.
Funding
This work was supported by the Research Award Fund for Clinical Medicine of Beijing Hospital Authority, China (Grant No: ZYLX201802), in part by a grant from Capital Development Special Program of Beijing Hospital Authority, China (Grant NO. 2018-1-2171). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Huang L, Cattamanchi A, Davis JL, et al. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8(3):294–300.
2. Guo F, Chen Y, Yang SL, Xia H, Li XW, Tong ZH. Pneumocystis pneumonia in HIV-infected and immunocompromised non-HIV infected patients: a retrospective study of two centers in China. PLoS One. 2014;9(7):e101943.
3. Bateman M, Oladele R, Kolls JK. Diagnosing Pneumocystis jirovecii pneumonia: a review of current methods and novel approaches. Med Mycol. 2020;58(8):1015–1028.
4. Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25(2):297–317.
5. Roux A, Gonzalez F, Roux M, et al. Update on pulmonary Pneumocystis jirovecii infection in non-HIV patients. Med Mal Infect. 2014;44(5):185–198.
6. Rego de Figueiredo I, Vieira Alves R, Drummond Borges D, et al. Pneumocystosis pneumonia: a comparison study between HIV and non-HIV immunocompromised patients. Pulmonology. 2019;25(5):271–274.
7. Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Semin Urol Oncol. 2002;20(2):108–115.
8. Kattan MW, Giri D, Panageas KS, et al. A tool for predicting breast carcinoma mortality in women who do not receive adjuvant therapy. Cancer. 2004;101(11):2509–2515.
9. Newton AD, Li J, Jeganathan AN, Mahmoud NN, Epstein AJ, Paulson EC. A Nomogram to Predict Lymph Node Positivity Following Neoadjuvant Chemoradiation in Locally Advanced Rectal Cancer. Dis Colon Rectum. 2016;59(8):710–717.
10. Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498.
11. Castro KG, Ward JW, Slutsker L, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Clin Infect Dis. 1993;17(4):802–810.
12. Monnet X, Vidal-Petiot E, Osman D, et al. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care. 2008;12(1):R28.
13. Kim T, Moon SM, Sung H, et al. Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis. 2012;44(9):670–677.
14. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13.
15. Roblot F, Godet C, Le Moal G, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21(7):523–531.
16. Yuping L, Chengshui C, Shaoling Z, et al. Clinical significance of absolute value of lymphocytes and CD4+T cells in patients with pneumocystis carinii pneumonia after renal transplantation [J]. Chin J Tubere Respir Dis. 2007;30(5):383–384.
17. Vogel MN, Vatlach M, Weissgerber P, et al. HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromised patients. Eur J Radiol. 2012;81(6):1315–1320.
18. Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol. 2012;198(6):W555–W561.
19. Azoulay E, Parrot A, Flahault A, et al. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am J Respir Crit Care Med. 1999;160(2):493–499.
20. Ling C, Qian S, Wang Q, et al. Pneumocystis pneumonia in non-HIV children: a 10-year retrospective study. Clin Respir J. 2018;12(1):16–22.
21. Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128(2):573–579.
22. Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50(1):7–15.
23. Wachter RM, Luce JM, Safrin S, Berrios DC, Charlebois E, Scitovsky AA. Cost and outcome of intensive care for patients with AIDS, Pneumocystis carinii pneumonia, and severe respiratory failure. JAMA. 1995;273(3):230–235.
24. Hardak E, Neuberger A, Yigla M, et al. Outcome of Pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology. 2012;17(4):681–686.
25. Dubois MJ, Orellana-Jimenez C, Melot C, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: a prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34(10):2536–2540.
26. Tiwari LK, Singhi S, Jayashree M, Baranwal AK, Bansal A. Hypoalbuminemia in critically sick children. Indian J Crit Care Med. 2014;18(9):565–569.
27. Kim SJ, Lee J, Cho YJ, et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J Infect. 2014;69(1):88–95.
28. Wu L, Zhang Z, Wang Y, et al. A Model to Predict In-Hospital Mortality in HIV/AIDS Patients with Pneumocystis Pneumonia in China: the Clinical Practice in Real World. Biomed Res Int. 2019;2019:6057028.
29. Chen M, Tian X, Qin F, et al. Pneumocystis Pneumonia in Patients with Autoimmune Diseases: a Retrospective Study Focused on Clinical Characteristics and Prognostic Factors Related to Death. PLoS One. 2015;10(9):e0139144.
30. Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii Pneumonia. Infect Dis Clin North Am. 2010;24(1):107–138.
31. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011;32(6):775–782.
32. Ko RE, Na SJ, Huh K, Suh GY, Jeon K. Association of time-to-treatment with outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. Respir Res. 2019;20(1):213.
33. Armstrong-James D, Stebbing J, John L, et al. A trial of caspofungin salvage treatment in PCP pneumonia. Thorax. 2011;66(6):537–538.
34. Kamboj M, Weinstock D, Sepkowitz KA. Progression of Pneumocystis jiroveci pneumonia in patients receiving echinocandin therapy. Clin Infect Dis. 2006;43(9):e92–e94.
35. Kim T, Hong HL, Lee YM, et al. Is caspofungin really an effective treatment for Pneumocystis jirovecii pneumonia in immunocompromised patients without human immunodeficiency virus infection? Experiences at a single center and a literature review. Scand J Infect Dis. 2013;45(6):484–488.
36. Avino LJ, Naylor SM, Roecker AM. Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population. Ann Pharmacother. 2016;50(8):673–679.
37. Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget. 2017;8(35):59729–59739.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.