Back to Journals » Journal of Inflammation Research » Volume 13
NOD1/2 and the C-Type Lectin Receptors Dectin-1 and Mincle Synergistically Enhance Proinflammatory Reactions Both In Vitro and In Vivo
Authors Tukhvatulin AI , Dzharullaeva AS , Erokhova AS , Scheblyakov DV , Naroditsky BS , Gintsburg AL , Logunov DY
Received 11 January 2020
Accepted for publication 20 June 2020
Published 22 July 2020 Volume 2020:13 Pages 357—368
DOI https://doi.org/10.2147/JIR.S245638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Amir I Tukhvatulin, Alina S Dzharullaeva, Alina S Erokhova, Dmitry V Scheblyakov, Boris S Naroditsky, Alexander L Gintsburg, Denis Y Logunov
N. F. Gamaleya National Research Center for Epidemiology and Microbiology, Moscow, Russia
Correspondence: Amir I Tukhvatulin Gamaleya Str.18, Moscow 123098, Russia
Tel/ Fax +74991904373
Email [email protected]
Purpose: Pathogens consist of a wide variety of evolutionarily conserved molecular structures that are recognized by pattern recognition receptors (PRRs) of innate immunity. Reasonably assuming that no single PRR is ever likely to be the sole trigger of the immune response during infection, a great deal remains unknown about collaborative mechanisms and consequential crosstalk effects between multiple PRRs belonging to different families. Here, we aimed to investigate inflammatory response to combined stimulation of cytosolic nucleotide-binding oligomerization domain (NOD) receptors: NOD1, NOD2 and membrane-bound C-type lectin receptors (CLRs): Mincle and Dectin-1 in comparison to individual stimulation both in vitro and in vivo.
Materials and Methods: For in vitro studies, we used human monocytic THP-1 cells endogenously expressing NOD1,2, as well as Mincle and Dectin-1 receptors. Using reporter gene and immunoassay approaches, we measured activity of key proinflammatory transcription factors (NF-κB and AP-1) and cytokine production after addition of specific PRR agonists or their pairwise combinations. In vivo NF-κB activity (bioluminescent detection in NF-κB-Luc transgenic mice), as well as cytokine levels in mouse blood serum, was measured 3 hours after intramuscular injection of PRR agonists.
Results: We detected that combined stimulation of NOD1/2 and C-type lectin receptors (Dectin-1, Mincle) strongly potentiates NF-κB and AP-1 transcription factor activity in human monocytic THP-1 cells, as well as resulting in enhanced levels of IL-8 cytokine production. We demonstrated that RIP2- and Syk-dependent signaling pathways downstream of NOD1/2 and Dectin-1/Mincle, respectively, are essential for the potentiated proinflammatory cell response. Lastly, we confirmed that synergy between NOD and C-type lectin receptors resulting in potentiated levels of NF-κB activation and cytokine (IL-6, KC) production also occurs in vivo.
Conclusion: These findings originally indicate cooperation between NODs and CLRs, leading to potentiated levels of proinflammatory immune response both in vitro and in vivo.
Keywords: pattern recognition receptors, innate immunity, synergy, collaboration
Introduction
The innate immune system is the first line of host defense aimed at early detection and subsequent induction of the protective response to pathogen invasion. The innate immune reactions are triggered by a limited number of germ line-encoded pattern recognition receptors (PRRs) upon binding of evolutionarily conserved structures on pathogens, termed pathogen-associated molecular patterns (PAMPs). Various PRRs have been identified in mammals, including transmembrane Toll-like receptors (TLRs), C-type lectin receptors (CLRs), cytosolic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and retinoic acid-inducible gene (RIG)-I-like receptors (RLRs).1 There has been enormous progress over the past two decades in the identification of PRR members, their agonists, and receptor-specific signaling pathways leading to the formation of inflammatory responses.
However, it has now become apparent that no single PRR is the sole trigger of the immune response during infection. Indeed, it has been shown that microbes containing a wide variety of molecules may be recognized by different PRRs simultaneously.2 Scientific findings have revealed the challenges of understanding immune system functions in complex biological processes (infections, sepsis, allergy, etc.) through investigation of molecular mechanisms and immune effects after multiple PRR stimulation. A number of experiments using more physiological approaches have shown synergism between selected pairs of PRRs, resulting in qualitatively distinct immune reactions upon combined stimulation compared to those induced by stimulation of an individual PRR. For instance, synergistic activity among TLRs and NOD receptors was shown to lead to potentiated (greater than additive) levels of transcription factor activation (NF-κB, AP-1, IRFs), production of cytokines (IL-1ɑ, IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-21, IL-22, TNF1, etc.), and antimicrobial peptides (β-defensin-3), providing enhanced antimicrobial protection compared to individually stimulated PRRs.3–6 Interestingly, TLR–NLR cooperation extends to modulation of adaptive immune responses, resulting in enhanced levels of maturation and activation of dendritic cells, elevated adaptive (humoral as well as cellular) immune responses, and synergizes Th1- and Th17-based immune responses.7,8 In contrast, TLR and RLR co-activation in human dendritic cells causes decreased Th1/Th17 responses upon bacterial infection.9 Finally, well-established synergy has also been reported between TLRs and Syk-coupled lectin receptors. In vitro studies revealed that combined stimulation of Dectin-1 and Toll-like receptors (TLR2, 4, 5, 7, 9) significantly enhances activation of NF-κB and increases production of a number of cytokines and chemokines, including TNFɑ, IL-12, MIP-1a, and MIP-2.10–12 Furthermore, human dendritic cells stimulated with a combination of TLR7/8 and Mincle agonists may additionally enhance Th1-based reactions in adaptive immunity.13
Despite synergism in action between members of TLRs and NLRs, as well as TLRs and CLRs being well documented, collaboration between NLRs and CLRs remains unstudied. Accordingly, we herein examined the effects of combined stimulation of NLR (NOD1/2) and Syk-coupled lectin receptors (Dectin-1/Mincle) in comparison to individual PRR stimulation in vitro as well as in vivo.
This is the first report showing that combined stimulation of NOD1/2 and C-type lectin receptors potentiates the activity of key proinflammatory transcription factors (NF-κB, AP-1), as well as IL-8 cytokine production compared to individual PRR agonist activation in human monocytic THP-1 cells. We show a critical role for RIP2 and Syk kinase pathways downstream intracellular signaling of NOD1/2 and C-type lectin receptors, respectively, resulting in potentiated NF-κB/AP-1-dependent SEAP expression as well as IL-8 secretion in response to combined PRR stimulation. Finally, using NF-κB-Luc transgenic mice we demonstrated presence of collaboration between NOD1/2 and C-type lectin receptors in vivo.
In conclusion, our results indicate that positive crosstalk identified between PRRs is not restricted to Toll- and NOD-like receptors or to only Toll-like and C-type lectin receptors pairs but also occurs between NOD1/2 and C-type lectin receptors.
Materials and Methods
Pattern Recognition Receptor Ligands
Stock solutions (1 mg/mL) of specific NOD1 (C12-iE-DAP), NOD2 (L18-MDP) and Mincle (Trehalose-6,6-dibehenate, TDB) and Dectin-1 (Curdlan) agonists were prepared according to the manufacturer’s instructions (Invitrogen, USA). Further dilutions of lipophilic molecules: C12-iE-DAP, L18-MDP and TDB were made using phosphate-buffered saline (PBS) containing 10%v/v DMSO. For in vitro experiments PRR agonists were added directly to the THP-1 cells. The applicability of this dilution and addition method was tested using TDB taken as example and showed comparable activity with the precoating method (Figure S1). Working concentrations of Curdlan were prepared using PBS.
Western Blot Analysis
Receptor expression levels were detected in total protein extracts from THP-1 cells and control HEK-Blue-null1 and HEK-Blue-null2 cells by anti-NOD1 (Thermo Scientific, USA), anti-NOD2 (Santa Cruz Biotechnology, USA), anti-Dectin-1, and anti-Mincle (InvivoGen, USA) primary antibodies. Anti-GAPDH antibodies (Santa Cruz Biotechnology, USA) were used for sample normalization.
For verification of NF-κB activation after PRR stimulation, THP-1 cells were treated for 30 min with TDB (10 µg/mL) and L18-MDP (100 ng/mL) alone or in combination. Nuclear and cytoplasmic cellular extracts were prepared using an NE-PER kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions and normalized using anti-lamin and anti-β-actin antibodies, respectively. The p65 subunit of NF-κB was detected using anti-p65 antibodies (all, Santa Cruz Biotechnology, USA). HRP-conjugated secondary anti-rabbit and anti-mouse antibodies were obtained from GE Healthcare (USA).
Cultured Cells
THP-1 cells, THP1-XBlue-CD14 cells as well as control cells HEK-Blue-null1 and HEK-Blue-null2 with NF-κB/AP-1-inducible secreted embryonic alkaline phosphatase (SEAP) reporter (all InvivoGen, USA) were cultured in RPMI and DMEM, respectively (PAA, USA). Media were supplemented with 10% fetal calf serum (Thermo Scientific, USA), 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM glutamine, 0.1 M NaHCO3 (all PanEco, Russia) at 37°C with 5% CO2.
SEAP Reporter Assay
THP1-XBlue-CD14 cells were seeded in 96-well plates at 105 cells per well in complete RPMI medium. The next day, cells were treated with individual PRR agonists or in combination in indicated doses. SEAP activity was assayed after 18 h of incubation by mixing 50 μL clarified culture supernatants with 150 μL of 60 µM p-nitrophenylphosphate (Sigma-Aldrich, USA) dissolved in SEAP assay buffer. Absorbance was measured at 405 nm in a Wallac 1420 plate reader (PerkinElmer, USA).
Phosphoprotein Profile in THP-1 Cells
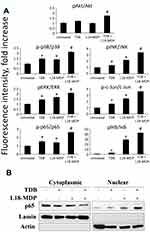
Human Single Bio-Plex Pro™ Cell Signaling Bead Kits and Bio-Plex Pro™ Magnetic Cell Signaling Buffer Assay Kit (all Bio-Rad, USA) were used to detect quantitative changes in total protein concentration, as well as phosphorylated levels of ERK/MAP kinase 1/2 (Thr185/Tyr187), Akt (Ser473), JNK (Thr183/Tyr185), p65 subunit of NF-κB (Ser536), p38 (Thr180/Tyr182), IKKα/β (Ser177/Ser181), and IκB (Ser32) in THP-1 cell lysates according to the manufacturer’s instructions.
Before PRRs stimulation THP-1 cells were serum-starved (2% FCS in RPMI medium) for 24 hours. The next day, cells were seeded in 48-well plates at 4x105 cells per well. Twenty-four hours later, cells were treated with TDB (10 μg/mL) and L18-MDP (100 ng/mL) individually or in combination. Intact (untreated) THP-1 cells were used as a control. Concentrations of ERK/MAP kinase 1/2, Akt, and their phosphorylation levels were measured 15 minutes after PRR agonist addition, whereas similar analysis of p65 subunit NF-κB, IκB, IKKα/β, p38, and JNK were made 40 minutes after PRR stimulation. In indicated time points cells were harvested, washed with PBS, and lysed in MILLIPLEX MAP lysis buffer in the presence of Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, USA). Particulate matter was removed after centrifugation of obtained lysates at 10,000×g for 5 min. Total protein concentration of each clarified lysate was normalized by dilution in Assay Buffer to 25 μL (10 μg total protein/well). A Bio-Plex MAGPIX multiplex reader (Bio-Rad, USA) was used to measure mean fluorescence intensity (MFI) of each sample. For data normalization at the first step, target protein levels were normalized to β-actin concentrations in analyzed samples using the corresponding bead kit (Bio-Rad, USA). Next, MFI of phosphorylated fractions of each protein were normalized to MFI of the total concentration of the corresponding protein.
Cytokine Analysis
THP-1 cells were seeded at 105 cells per well in 96-well plates. Twenty-four hours later, cells were treated with individual PRR agonists or in combinations. Twenty-four hours after stimulation, levels of IL-8 were measured in clarified (1000 rpm for 10 min) supernatants using a Single Human Bio-Plex Pro kit (BioRad, USA) according to the manufacturer’s instructions.
For ex-vivo cytokine assay, BALB/c mice were i.m. injected with PBS or PRR agonists: C12-iE-DAP (1 μg/mouse), L18-MDP (1 μg/mouse), Curdlan (10 μg/mouse), or TDB (10 μg/mouse) individually or in combination: C12-iE-DAP-Curdlan, C12-iE-DAP-TDB, L18-MDP-Curdlan, or L18-MDP-TDB. Three hours later, samples of peripheral blood from the tail vein were collected. Serum was isolated after 20 min of incubation at 37°C and subsequent centrifugation at 1000 rpm for 10 min. Cytokine concentration were measured using 23-plex bead-based Bio-Plex Pro kit (BioRad, USA) according to the manufacturer’s instructions.
Experimental Animals
BALB/c female SPF mice (5–6 weeks old) were purchased from the animal breeding facility BIBCH RAS (Pushchino, Russia). Transgenic female BALB/c-Tg(Rela-luc)31Xen (5–6 weeks old) mice were purchased from Taconic Biosciences (USA). Mice were housed in ventilated polysulfone cages in ventilated Modular Animal Caging System (Alternative Design, USA), and fed regular chow diet with free access to autoclaved tap water. All of the experimental procedures were made in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication #85–23, revised 1996) and approved by the animal ethics committee of N.F.Gamaleya National Research Center for Epidemiology and Microbiology (protocol #20, 2020).
In-Vivo and ExVivo NF-κB Luminescence Analysis
Analysis was performed as previously described.5 Briefly, BALB/c-Tg(Rela-luc)31Xen reporter mice (3 mice per group) were injected intramuscularly (i.m.) with TBD (10 µg/mouse), L18-MDP (1 µg/mouse) individually or in combination. Three hours later mice were anesthetized with 2.5% isoflurane (Abbot, USA) followed by intraperitoneal injection of 3 mg/mouse D-luciferin in 1xPBS (Promega, USA). Luminescence images were collected for 10 s using IVIS Lumina II (Perkin Elmer, USA).
Samples of lung, liver, kidney, small intestine (referred to as duodenum), colon (referred to as the ascending part), spleen, as well as regional inguinal lymph nodes were isolated from the same mice. Tissue homogenates were normalized to total protein using Pierce BCA Protein Assay Kit (Thermo Scientific, USA). Luminescent signal was measured with Synergy H4 hybrid reader (Bio-Tek, Germany) using Bright-Glo Luciferase Kit (Promega, USA).
Statistical Analysis
Data were analyzed by Mann–Whitney U-test. All variables were expressed as mean ± standard deviation (SD), and values of p< 0.05 were considered statistically significant. All analyses were performed using Excel 2010, Origin2018 and GraphPad Prism version 6.
Results
Combined Stimulation of NOD (NOD1/NOD2) and C-Type Lectin (Dectin-1 or Mincle) Receptors Has a Synergistic Effect on Transcriptional Response in THP-1 Cells
It has been previously shown that stimulation of NOD (NOD1/NOD2) and C-type lectin (Dectin-1 or Mincle) receptors leads to activation of common proinflammatory transcription factors, such as AP-1, NF-κB.14,15 These transcription factors play major roles in the expression of immune effector molecules during innate, as well as adaptive, immune responses. Based on these data, we first examined the effects of combined NOD and CLR stimulation on activity of AP-1 and NF-κB.
For this purpose, we used THP1-XBlue-CD14 cells that express an NF-κB/AP-1-inducible SEAP reporter as well as endogenous NOD1, NOD2, Dectin1, and Mincle receptors (Figure 1A). Cells were treated with individual NOD1/2, Mincle, or Dectin-1 agonists or their pairwise combinations.
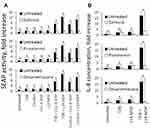
Addition of individual NOD (C12-iE-DAP, L18-MDP) and CLR (Curdlan, TDB) agonists in nontoxic doses from 10 ng/mL up to 10 µg/mL resulted in dose-dependent induction of SEAP reporter gene expression. Levels of NF-κB/AP-1-dependent SEAP expression detected after combined stimulation of receptors from different families (NOD1-Dectin-1, NOD1-Mincle, NOD2-Dectin-1, NOD2-Mincle) were greater than the sum of the levels comparing to individual PRR stimulation (Figure 1B) (marked by asterisks). The most prominent enhancement of SEAP activity levels was seen after combined addition of TDB and L18-MDP (20.5-fold increase over intact cells) at 10 µg/mL doses compared to individual TDB (3.2-fold) or L18-MDP (2.3-fold). It is also worth noting that in many cases only 100-fold increased doses of isolated agonists could result in comparable levels of NF-κB/AP-1-dependent SEAP activity seen after combined stimulation of NLR and CLR. These results indicate that costimulation of NOD1/2 and C-type lectin receptors has a synergistic rather than additive effect on the activity of examined transcription factors. Meanwhile, combined stimulation of PRRs belonging to one family (NOD1/NOD2, Dectin-1/Mincle) resulted in summation but not potentiation of SEAP expression levels compared to individual PRR stimulation (Figure S2).
Additionally, in the context of combined Mincle and NOD2 stimulation, we confirmed potentiation of the NF-κB/AP-1 signaling pathway by conducting protein phosphorylation profiling of these transcription factors and several signaling kinases in THP-1 cells using Bio-pex assay kits. In this experiment, individual addition of TDB (10 µg/mL) or L18-MDP (100 ng/mL) resulted in enhanced phosphorylation levels of p38 (1.5-fold increase over untreated cells with TDB versus 1.4-fold with L18-MDP), ERK/MAP kinase 1/2 (1.4-fold versus 1.7-fold), JNK (1.3-fold versus 2.2-fold), c-Jun subunit of AP-1 transcription factor (1.6-fold versus 2.5-fold), IκB (1.8-fold versus 29.3-fold), and p65 subunit of NF-κB transcription factor (1.5-fold versus 1.4-fold) (Figure 2A). Phosphorylation of Akt was observed only after addition of TDB (1.2-fold over untreated cells), whereas treatment with L18-MDP did not result in substantial response compared to intact cells. Combined stimulation of Mincle and NOD2 exerted significantly stronger effects on intracellular signaling, resulting in enhanced phosphorylation of p38 (2.2-fold increase over untreated cells), ERK/MAP kinase 1/2 (2.1-fold), JNK (3.4-fold), c-Jun subunit of AP-1 transcription factor (3.6-fold), IκB (42.8-fold), p65 subunit of NF-κB transcription factor (2.6-fold), and Akt (1.7-fold). Thus, all proteins presenting enhanced phosphorylation from individual stimulation of either Mincle or NOD2 were even more elevated by combined stimulation of these PRRs.
In order to verify significant increase in activation of NF-κB transcription factor after combined Mincle and NOD2 stimulation we determined translocation of p65 into the nucleus by Western blot analysis (Figure 2B). Treatment of THP-1 cells using TDB (10 µg/mL) or L18-MDP (100 ng/mL) alone in these suboptimal doses had minimal effect on nuclear translocation of p65, whereas combination of PRR agonists resulted marked accumulation of p65 in nucleus.
Overall, these data demonstrate synergy between NOD1/2 and C-type lectin receptors in stimulating intracellular signaling pathways, leading to potentiation of NF-κB and AP-1 transcription factor activity.
Synergism Between NOD (NOD1/NOD2) and C-Type Lectin (Dectin-1/Mincle) Receptors Potentiates Levels of IL-8 Cytokine Production in THP-1 Cells
Based on observed differences in activity levels of NF-κB and AP-1 transcription factors, we hypothesized that combined stimulation of NOD1/2 and C-type lectin receptors may also affect downstream immune reactions.
It was previously demonstrated that combined stimulation of TLR2, TLR4, or TLR9 combined with NOD1 or NOD2 significantly enhances production of IL-8 in cultured THP-1 cells.16 Herein, we evaluated whether production of IL-8 in THP-1 cells would be potentiated after combined NOD1/2 and CLR stimulation compared to stimulation of individual PRRs. For individual NOD1/2 and CLR stimulation, we used agonists at working concentrations ranging from 1 to 100 µg/mL. For combined stimulation, we used suboptimal concentrations of PRR agonists ranging from 1 to 10 µg/mL, previously reported to provide maximal potentiation of the NF-κB/AP-1 transcriptional response. As seen in NF-κB/AP-1-dependent reporter expression assay, combined stimulation of NOD1/2 and C-type lectin receptors potentiated expression levels of IL-8 (Figure 3). Addition of TDB (10 µg/mL) in combination with L18-MDP (10 µg/mL) resulted in maximal induction of IL-8 production (29.8-fold increase over intact cells) compared to individual Mincle (2.4-fold) and NOD2 (6.2-fold) agonists. Consistent to SEAP reporter assay, for some doses of PRR agonists, production levels of IL-8 after combined NOD1/2 and CLR stimulation were even higher than for those using 10-fold higher doses for individual PRR stimulation.
These data demonstrate that Mincle and Dectin-1 receptors exhibit remarkable synergism with NOD1/2 receptors to induce cytokine production in human monocytic cells.
Inhibition of RIP2 and Syk-Dependent Signaling Pathways Abrogate Potentiated NF-κB/AP-1 Reporter Gene Expression and IL-8 Production After Combined NOD1/2 and CLR Stimulation in THP-1 Cells
Having demonstrated that costimulation of NOD1/2 and C-type lectin receptors leads to enhanced levels of both NF-κB/AP-1 activity and production of IL-8, we investigated intracellular signaling cascades involved in CLR–NOD potentiation. RIP2 kinase (also known as RIPK, RIPK2) functions downstream of NOD1 and NOD2, whereas Dectin-1 and Mincle receptors utilize Syk-dependent signaling cascades.17,18 To determine whether RIP2 and Syk are important in the enhanced transcriptional response, we employed inhibitors specific for these kinases: gefitinib (RIP2 inhibitor) and piceatannol (Syk inhibitor). We also used synthetic glucocorticoid dexamethasone (DEX) to inhibit transcriptional activity of both NF-κB and AP-1 and evaluate the role of these transcription factors in elevated cytokine production.19 Gefitinib (5 μM), piceatannol (1 μg/mL), and DEX (200 μg/mL) were added to THP1-XBlue-CD14 cells 1 h before PRR agonists addition alone or in combination. Average concentrations of CLR agonists (10 μg/mL) as well as NOD1,2 agonists (1 μg/mL) inducing potentiation effect were used to evaluate NF-κB/AP-1-dependent SEAP activity (Figure 4A). TDB and L18-MDP agonist resulting in maximal potentiation of proinflammatory response were used also to study IL-8 production with or without inhibitors (Figure 4B).
The results of this experiment showed absolute dependency on RIP2 signaling for NF-κB/AP-1 reporter SEAP expression and cytokine secretion after individual NOD1 or NOD2 stimulation. Similar results were achieved using the Syk inhibitor after individual Dectin-1 or Mincle stimulation. During combined stimulation of NOD1/2 and C-type lectin receptor inhibition of RIP2 or Syk, impaired NF-κB/AP-1 response and production of IL-8 similar to those levels induced by individual NOD1/2 or CLR stimulation were observed, respectively. Treatment of cells with DEX completely abrogated the transcriptional response, demonstrating the essential role of enhanced NF-κB/AP-1 activity in potentiation of IL-8 production levels after combined NOD1/2 or CLR stimulation.
Overall, obtained results indicate that RIP2 and Syk kinases play important role in synergistic effects after combined NOD1/2 or CLR stimulation.
Combined Stimulation of NOD2 and Mincle Receptors Potentiates NF-κB Activation In Vivo
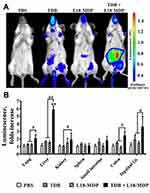
To determine whether elevated activity of proinflammatory transcription factors after combined stimulation of NOD1/2 and C-type lectin receptors observed in vitro also occurs in vivo, we used BALB/c-Tg(Rela-luc)31Xen mice carrying an NF-κB-dependent luciferase reporter gene. For evaluation of NF-κB activity in vivo we used Mincle and NOD2 agonists which demonstrated the most significant synergistic effects in vitro upon combined application. Based on preliminary experiments (data not shown) we chose suboptimal doses of TDB (10 μg/mouse) and L18-MDP (1 μg/mouse) in order to retain the capacity of bioluminescence assay to respond at higher rates. Mice were i.m. injected with TDB and L18-MDP, alone or in combination. Control mice received PBS. Immunostimulatory activity of PRR agonists was measured 3 hours after administration referring to the maximum of the bioluminescence kinetic curve.20 The obtained data show that individual Mincle or NOD2 agonists result in elevation of NF-κB-dependent luminescence at abdominal region comparing to PBS injection (Figure 5A). However, the maximal luminesce intensity was registered after administration of both PRR agonists. Having detected differences in whole-body bioluminescence imaging between individual and combined Mincle and NOD2 stimulation we quantified luciferase activity in a number of organs: liver, lungs, spleen, kidneys, small intestine, colon, and inguinal lymphatic nodes (Figure 5B). Interestingly, individual injection of TDB and L18-MDP induced NF-κB-dependent luciferase expression in different organs. Injection of TDB led to significant NF-κB-dependent signal in inguinal lymphatic nodes (1.9-fold increase over PBS-treated mice), whereas L18-MDP resulted in significant reporter expression in kidneys (1.5-fold increase).
Combined stimulation of PRRs resulted in maximal induction in most studied organs: liver (5.9-fold increase), colon (2.5-fold increase), lungs (2.1-fold increase), kidneys (1.9-fold increase), and inguinal lymphatic nodes (3.6-fold increase).
The obtained results indicate that the registered in-vitro effect of NF-κB potentiation using solubilized Mincle and NOD2 agonists is also present in vivo.
Combined Stimulation of NOD (NOD1/NOD2) and C-Type Lectin (Dectin1 or Mincle) Receptors Potentiates Cytokine Production In Vivo
Lastly, we examined the biological outcome of enhanced NF-κB activity upon combined CLR and NOD stimulation in terms of cytokine production in vivo.
To address this goal, BALB/c mice were i.m. injected with NOD agonists: C12-iE-DAP (1 μg/mouse), L18-MDP (1 μg/mouse) and CLR agonists: Curdlan (10 μg/mouse), TDB (10 μg/mouse), individually or in combination. Control mice were i.m. injected with 100 μL PBS. Three hours later, mice were euthanized, and blood samples were collected for measurement of cytokine levels in serum using bead-based immunoassays.
Data revealed that each combination of NOD1/2 and CLR agonists (C12-iE-DAP-Curdlan, L18-MDP-Curdlan, C12-iE-DAP-TDB, L18-MDP-TDB) potentiated production of two of 23 measured cytokines (IL-6, KC) compared to stimulation of either receptor alone (Figure 6). Importantly, combined stimulation of PRRs belonging to one family (NOD1-NOD2, Dectin-1-Mincle) resulted in summation, but not potentiation, of cytokine production compared to individual PRR stimulation (Figure S3).
In summary, these results clearly indicate that combined stimulation of NOD1/2 and C-type lectin receptors shows synergistic effects on cytokine production, not only in vitro but also in vivo.
Discussion
Microorganisms consist of a wide variety of PAMPs that are detected by different PRRs of the host innate immune system.2 This redundancy in the stimulation of PRRs, along with the multiplicity of signaling cascades, could be one mechanism of the immune system that allows it to shape the intensity of downstream immune reactions, resulting in adequate response to microbial threats.
One promising example of where significant progress has been made regarding crosstalk between different PRRs is the “two-step” mechanism of IL-1β and IL-18 production.21 Stimulation of surface PRRs results in expression of inactive precursors of IL-1β and IL-18. In case of bacterial invasion, several PAMPs (flagellin, LPS, bacterial DNA, MDP) appear in the cytosol, where they are recognized by several inflammasome sensor molecules (NLRP1, NLRP3, NLRC4, AIM, etc.). Further cleavage of pro-IL-1β and pro-IL-18 by activated caspase-1 leads to formation of mature proinflammatory cytokines: IL-1β and IL-18, shown to be crucial for enhanced host defense.
Along with IL-1β and IL-18 production, a number of factors are required for host defense (e.g., other cytokines and chemokines, and antimicrobial peptides) and are found to be synergistically induced upon combined stimulation of PRRs. One of the mechanisms underlying the synergistic effects of PRR cooperation relies upon utilization of common intracellular signaling cascades, culminating in activation of several common proinflammatory transcription factors (NF-κB, AP-1, etc.). In several reports, synergistic collaborations have been reported between members of Toll-like and NOD-like, as well as Toll-like and C-type lectin receptor families.3–5,12,13 However, the collaboration between members of NOD-like and Syk-coupled lectin receptors has so far not been identified.
In this study, we used known, chemically defined agonistic molecules to explore collaborative responses induced by NOD1/2 and C-type lectin receptors (Dectin-1, Mincle).
First, because both NOD1/2 and C-type lectin receptors activate NF-κB and AP-1, we investigated the effects of combined stimulation of NODs and CLRs on activity levels of these transcription factors. Using THP-1 cells expressing endogenous NOD1, NOD2, Dectin-1, and Mincle receptors with an introduced SEAP reporter gene, we demonstrated for the first time that combined stimulation of either NOD1 or NOD2 with either Dectin-1 or Mincle leads to significant greater-than-additive potentiation of NF-κB/AP-1 activation relative to individual stimulation. We confirmed collaborative interconnection between studied receptors by evaluating IL-8 cytokine production. Of note, combined stimulation of PRRs belonging to the same family (NOD1-NOD2 or Dectin-1-Mincle) does not potentiate levels of NF-κB/AP-1-dependent SEAP expression. This finding, in accordance with previously published data, indicates that collaboration should be favored between PRRs localized in different cellular compartments, including cytosolic (NOD receptors) and surface membrane-bound (C-type lectin receptors), which could be useful for discrimination of most dangerous invasive from non-invasive, e.g., commensal bacteria.
Next, we elucidated the role of downstream signaling pathways of NOD and CLR in the observed potentiation phenomenon. We evaluated whether potentiated levels of NF-κB/AP-1 and cytokine production in THP-1 cells is dependent on the activity of RIP2 and Syk kinases, using specific inhibitors Gefitinib and Piceatannol, correspondingly. We showed that in the absence of additional NOD1/2 signaling during CLR stimulation, inhibition of NF-κB/AP-1 transcriptional activity and production of IL-8 by Gefitinib was reduced to levels equal to those induced by individual CLR stimulation. Similar results were obtained using the Piceatannol inhibitor in terms of NOD stimulation. These results reveal critical roles of RIP2- and Syk-dependent signaling in mediating synergy between NOD and C-type lectin receptors. Using dexamethasone (DEX) we showed the critical role of both the NF-κB and mitogen-activated protein kinase (MAPK)–AP-1 pathways for potentiation of IL-8 production.19 Thus, this is the first report showing that the effect of potentiated cytokine production triggered by combined activation of NOD and C-type lectin receptors in vitro is mediated by potentiated NF-κB/AP-1 activation.
Finally, we showed that synergy between NOD1/2 and Syk-coupled lectin receptors, which leads to enhanced activation of NF-κB, AP-1 and production of IL-8 in THP-1 cells in vitro, is also observed in vivo. Using transgenic NF-κB-Luc mice we evaluated NF-κB activity after i.m. injection of individual TDB and L18-MDP molecules or their combination. Interestingly, that the most significant enhancement of NF-κB activation after combined Mincle and NOD2 stimulation was observed in liver. It is known that both Mincle and NOD2 receptors are expressed by hepatocytes as well as innate immune cells presented in liver. Upon recognition of various PAMPs and DAMPs in portal tracts these receptors play indispensable role in pathogenesis of hepatic inflammation and subsequent injury.22,23 In this regard focused potentiation of main proinflammatory transcription factor NF-κB in liver tissue upon combined Mincle and NOD2 stimulation provides the basis to suggest that these receptors could play cooperative role in liver immune pathologies. Synergistic activation of NF-κB was complemented with potentiated levels of cytokine levels: IL-6 and KC in mouse serum after combined NOD and CLR stimulation. According to published data showing that production of both IL-6 and KC is mediated by activity of NF-κB and AP-1, elevated cytokine levels support the idea that combined NOD and CLR stimulation also affects potentiation of NF-κB and AP-1 in vivo.24,25
In summary, this is the first report demonstrating synergistic collaboration between NOD1/2 and C-type lectin receptors both in vitro and in vivo. However, further studies are required to elucidate the beneficial effects of NOD-like and C-type lectin receptor collaboration during infection. These observations could help to improve our knowledge about mechanisms of activation and functions of the innate immune system. This fundamental knowledge could be easily implemented for practical development of vaccine adjuvants and therapeutic drugs for immunopathological conditions.
Abbreviations
AP-1, activator protein 1; CLR, C-type lectin receptors; DEX, dexamethasone; ECL, enhanced chemiluminescence; ELISA, enzyme-linked immunosorbent assay; FCS, fetal calf serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HRP, horseradish peroxidase; LPS, lipopolysaccharide; mDAP, meso-diaminopimelic acid; MFI, mean fluorescence intensity; MPLA, monophosphoryl lipid A; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NOD, sensors nucleotide-binding oligomerization domain; PAMPs, pathogen-associated molecular patterns; PRR, pattern recognition receptors; RIG, retinoic acid-inducible gene; RIPA, radioimmunoprecipitation assay; RLR, RIG-like receptors; SD, standard deviation; SEAP, secreted embryonic alkaline phosphatase; Syk, spleen tyrosine kinase; TDB, trehalose-6,6-dibehenate; TDM, trehalose-6,6-dimycolate; TLR, toll-like receptors; RIP2, receptor-interacting serine/threonine-protein kinase 2.
Disclosure
The authors declare that they have no conflicts of interest regarding this manuscript.
References
1. Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi:10.1093/intimm/dxp017
2. Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukocyte Biol. 2006;80(2):267–277. doi:10.1189/jlb.1105626
3. Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28(2):246–257. doi:10.1016/j.immuni.2007.12.012
4. Uehara A, Takada H. Synergism between TLRs and NOD1/2 in oral epithelial cells. J Dent Res. 2008;87(7):682–686. doi:10.1177/154405910808700709
5. Tukhvatulin AI, Gitlin II, Shcheblyakov DV, et al. Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to salmonella enterica serovar typhimurium infection. Infect Immun. 2013;81(10):3855–3864. doi:10.1128/IAI.00525-13
6. van Heel DA, Ghosh S, Butler M, et al. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35(8):2471–2476. doi:10.1002/eji.200526296
7. Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, et al. Powerful complex immunoadjuvant based on synergistic effect of combined TLR4 and NOD2 activation significantly enhances magnitude of humoral and cellular adaptive immune responses. PLoS One. 2016;11(5):e0155650. doi:10.1371/journal.pone.0155650
8. Schwarz H, Posselt G, Wurm P, Ulbing M, Duschl A, Horejs-Hoeck J. TLR8 and NOD signaling synergistically induce the production of IL-1β and IL-23 in monocyte-derived DCs and enhance the expression of the feedback inhibitor SOCS2. Immunobiology. 2013;218(4):533–542. doi:10.1016/j.imbio.2012.06.007
9. Negishi H, Yanai H, Nakajima A, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol. 2012;13(7):
10. Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J Exp Med. 2003;197(9):1107–1117. doi:10.1084/jem.20021787
11. Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38(2):500–506. doi:10.1002/eji.200737741
12. Viriyakosol S, Fierer J, Brown GD, Kirkland TN. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on toll-like receptor 2 and dectin-1. Infect Immun. 2005;73(3):1553–1560. doi:10.1128/IAI.73.3.1553-1560.2005
13. van Haren SD, Dowling DJ, Foppen W, et al. Age-specific adjuvant synergy: dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol. 2016;197(11):4413–4424. doi:10.4049/jimmunol.1600282
14. Ostrop J, Lang LR. Contact, collaboration, and conflict: signal integration of Syk-coupled C-type lectin receptors. J Immunol. 2017;198(4):1403–1414. doi:10.4049/jimmunol.1601665
15. Kim YK, Shin JS, Nahm MH. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J. 2016;57(1):5–14. doi:10.3349/ymj.2016.57.1.5
16. Uehara A, Yang S, Fujimoto Y, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol. 2005;7(1):53–61. doi:10.1111/j.1462-5822.2004.00433.x
17. Hasegawa M, Fujimoto Y, Lucas PC, et al. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 2008;27(2):373–383. doi:10.1038/sj.emboj.7601962
18. Drummond RA, Saijo S, Iwakura Y, Brown GD. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41(2):276–281. doi:10.1002/eji.201041252
19. Bhattacharyya S, Ratajczak CK, Vogt SK, et al. TAK1 targeting by glucocorticoids determines JNK and IκB regulation in Toll-like receptor–stimulated macrophages. Blood. 2010;115(10):1921–1931. doi:10.1182/blood-2009-06-224782
20. Ansaldi D, Hod EA, Stellari F, et al. Imaging pulmonary NF-kappaB activation and therapeutic effects of MLN120B and TDZD-8. PLoS One. 2011;6(9):e25093. doi:10.1371/journal.pone.0025093
21. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7(1):31–40. doi:10.1038/nri1997
22. Body-Malapel M, Dharancy S, Berrebi D, et al. NOD2: a potential target for regulating liver injury. Lab Invest. 2008;88(3):318–327. doi:10.1038/labinvest.3700716
23. Greco SH, Torres-Hernandez A, Kalabin A, et al. Mincle signaling promotes Con A hepatitis. J Immunol. 2016;197(7):2816–2827. doi:10.4049/jimmunol.1600598
24. Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90(21):10193–10197. doi:10.1073/pnas.90.21.10193
25. Thobe BM, Frink M, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Src family kinases regulate p38 MAPK-mediated IL-6 production in Kupffer cells following hypoxia. Am J Physiol Cell Physiol. 2006;291(3):C476–482. doi:10.1152/ajpcell.00076.2006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.