Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
No increased risk of psoriasis in patients receiving androgen deprivation therapy for prostate cancer: a 17-year population-based study
Authors Liu JM , Lin CY , Chuang HC, Hsu RJ
Received 24 May 2018
Accepted for publication 17 August 2018
Published 28 September 2018 Volume 2018:14 Pages 1831—1837
DOI https://doi.org/10.2147/TCRM.S175244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Jui-Ming Liu,1–3,* Chien-Yu Lin,4,* Heng-Chang Chuang,1 Ren-Jun Hsu3,5,6
1Division of Urology, Department of Surgery, Taoyuan General Hospital, Ministry of Health and Welfare, Taoyuan, Taiwan; 2Department of Medicine, National Yang-Ming University, Taipei, Taiwan; 3Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan; 4Department of Pediatrics, Hsinchu MacKay Memorial Hospital, Hsinchu, Taiwan; 5Biobank Management Center of the Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; 6Department of Pathology and Graduate Institute of Pathology and Parasitology, the Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
*These authors contributed equally to this work
Objective: Androgen deprivation therapy (ADT) use in prostate cancer (PCa) patients has been reported to exacerbate the course of psoriasis. We aimed to assess the impact of ADT on the subsequent risk of psoriasis.
Methods: We utilized data from the National Health Insurance Research Database of Taiwan between 1996 and 2013. In total, 17,168 patients with PCa were identified; 5,141 ADT patients comprised the study group with 5,141 matched non-ADT controls. We used 1:1 propensity score-matched analysis. The demographic characteristics and comorbidities of the patients were analyzed; Cox proportional hazards regression was used to calculate the HRs for the risk of psoriasis.
Results: Eighty-nine (0.87%) patients with newly diagnosed psoriasis were identified. Compared with non-ADT patients, ADT patients had similar risk of subsequent psoriasis with an HR of 0.95 (95% CI 0.63–1.45; P=0.816). However, a higher risk of psoriasis was observed in angiotensin-converting enzyme inhibitors patients (adjusted HR 2.14, 95% CI 1.09–4.20; P<0.05).
Conclusion: ADT use did not increase risk of psoriasis in patients with PCa. Further studies are warranted to assess the clinical significance.
Keywords: prostate cancer, psoriasis, androgen deprivation therapy
Introduction
Prostate cancer (PCa) is one of the most prevalent cancers worldwide and is the cancer with the highest incidence in males in the USA, especially in elderly individuals.1 In 2017, there was estimated to be 161,360 new cases of PCa in the USA, with 26,730 deaths. Androgen deprivation therapy (ADT) is a core component of a combined modality of advanced PCa treatment. Around 500,000 PCa patients have received ADT in the USA in 2000.2 However, long-term deprivation of androgen may cause some systemic adverse effects, such as metabolic complications and cardiovascular disease.3–7 Alterations to the immune system in patients with ADT were also noted.8,9 Increased risk of rheumatoid arthritis in patients with ADT has also been observed.10 The possible role of ADT as a risk factor of systemic diseases remains an important health concern.
Psoriasis is an immune-mediated disorder with a prevalence rate ranging from 0.1% to 2.9% around the world.11,12 Psoriasis is characterized by the hyperproliferation of keratinocytes and immune-mediated inflammation, which are crucial in the pathophysiology.13 The peak incidence of psoriasis is in elderly people; the incidences of psoriasis increased after 30 years of age with a peak in patients aged over 70 years.11 Psoriasis may be triggered by a number of exogenous or endogenous factors, such as stress, infection, or drugs.14,15 The impact of hormone therapy on psoriasis remains largely unclear.
It has previously been reported that a 57-year-old patient developed exacerbation of psoriasis after ADT use in Poland.16 Klil-Drori et al conducted a study on ADT and autoimmune diseases, which showed that ADT was not associated with psoriasis; however, they did not provide further explanation.17 To date, there have been only limited investigations regarding the possible association between ADT and psoriasis. A link between ADT and psoriasis may exist and needs to elucidate. Therefore, the aim of this large-scale, nationwide, population-based study was to investigate the association between ADT and the subsequent risk of psoriasis.
Methods
Data source and collection
We conducted a large cohort study using data from National Health Insurance Research Database (NHIRD) of Taiwan. The NHIRD is a database that includes data from the National Health Insurance (NHI) program. The NHI program is the unique medical insurance system of Taiwan, which covers 99.5% of Taiwan’s 23 million residents.18 For this study, we used the Registry for Catastrophic Illness Patient Database (RCIPD), a subdatabase of the NHIRD. In Taiwan, patients diagnosed with PCa, or other major diseases, are entitled to a waiver for medical payment after receiving a catastrophic illness certification. All data in the NHIRD and RCIPD are anonymous and encrypted for research purposes.
Pathological information or imaging findings of PCa are needed for registration into the RCIPD.19 We used the ICD, 9th revision, Clinical Modification (ICD-9-CM) for diagnoses in NHIRD and RCIPD. This study was approved by the Institutional Review Board of the Tri-Service General Hospital (approval number: TSGHIRB NO B-104–21).
Study population
We selected patients with PCa using the RCIPD data between January 1996 and December 2013. The diagnoses of PCa were confirmed by both ICD-9-CM codes (ICD-9-CM: 185)20 and inclusion in the RCIPD. In addition, all patients with PCa who were followed-up for at least 180 days after the initial diagnoses of PCa were then enrolled in this study (Figure 1). The exclusion criteria of this study included PCa diagnosis before 1 January 1997 (n=684); patients who were younger than 40 years at the time of diagnosis (n=144); patients who received a bilateral orchiectomy (n=1,065); history of psoriasis (n=286); and less than 180 days follow-up after the initial diagnoses of PCa (n=1,306). The selected patients in the study population were then divided into two groups: ADT patients and non-ADT patients.
  | Figure 1 Study flowchart of cohort selection. |
Study outcomes and covariates
The use of ADT includes the use of GnRH agonists (leuprolide, goserelin, triptorelin, and buserelin), oral antiandrogens (cyproterone acetate, bicalutamide, and flutamide), and estrogens (diethylstilbestrol). Patients newly diagnosed with psoriasis (ICD-9-CM: 696, 696.1, 696.8) by dermatologists or rheumatologists were identified in the NHIRD. The outcome was the incidence of newly diagnosed psoriasis in both ADT and non-ADT patients. The exact incidence of psoriasis among ADT patients was determined by only including those who received a psoriasis diagnosis after the initiation of ADT and at least 180 days after the PCa diagnosis. Meanwhile, the incidence of psoriasis among non-ADT patients was identified by only including those who received a psoriasis diagnosis at least 180 days after the PCa diagnosis and after the median time to ADT use in this study. Censoring was defined as death on the dates of diagnosis of psoriasis or until the end of the follow-up period on the 31 December 2013, whichever came first.
Covariates including age at diagnosis, alcohol abuse (ICD-9-CM: 303, 305), tobacco-use disorder (ICD-9-CM: 305.1, 649.01, V15.82), obesity (ICD-9-CM: 278), comorbidities, and related medication were according to ICD-9-CM codes and analyzed for both groups. The patients with PCa were classified into the following five age groups: <50 years, 50–60 years, 60–70 years, 70–80 years, and >80 years. Comorbidities that have been reported to be related to psoriasis in the previous literature, including streptococcal infection (ICD-9-CM: 034.0, 038.0, 041.0, 320.2, 482.3, V02.51, V02.52) and HIV disease (ICD-9-CM: 042) were recorded. Related medications including lithium, antihypertensive medications (beta-blockers), antimalarial medications (plaquenil, quinacrine, chloroquine), NSAIDs, and angiotensin-converting enzyme inhibitors (ACEI) were recorded.
Statistical analyses
The baseline characteristics of the patients were first analyzed using descriptive statistics. The two groups (ADT and non-ADT patients) were compared using the chi-squared test for categorical variables. The Kaplan–Meier (KM) curve was used to estimate the cumulative incidences of psoriasis for the two groups and the difference between the ADT group and non-ADT group was calculated with a log-rank test. HRs were calculated using propensity score-matched and multivariable-adjusted Cox proportional hazards models to test the association between ADT and psoriasis.
The proportional hazard assumption was tested using Schoenfeld residuals and a log-minus-log graph. The SPSS, version 22.0 for Windows (IBM, Armonk, NY, USA), and the SAS, version 9.2 (SAS Institute, Cary, NC, USA), were used to perform all statistical analyses. The STATA, version 11.2 (StataCorp, College Station, TX, USA), was used to produce KM curve plots with number at risk. Comparison results with a P<0.05 were considered statistically significant.
Results
In total, 17,168 patients with PCa were identified in this study. Of these, 13,683 PCa patients met all inclusion and exclusion criteria. There were 5,588 (32.5%) ADT patients and 8,095 non-ADT patients (Figure 1). The median time from PCa diagnosis to ADT use was 14.6 days. After 1:1 propensity score matching, 5,141 patients were selected in the ADT group and another 5,141 patients were identified as the non-ADT group. The demographic characteristics of the full cohort and the 1:1 propensity score-matched cohort are demonstrated in Table 1. ADT patients were significantly older (74.15±8.34 vs 68.51±10.45 years), had more tobacco-use disorder, and more NSAID medication use. There were no differences in age, comorbidities, and medication use in the propensity score-matched cohort.
  | Table 1 Demographic characteristics of prostate cancer patients according to use of ADT |
Overall, 89 (0.87%) patients were newly diagnosed with psoriasis during a median follow-up of 3.29 years (IQR: 2.42–5.68 years): 39 (0.76%) in the ADT group and 50 (0.97%) in the non-ADT group (Figure 1). Cox proportional hazard regression showed that the crude HR was 0.95 (95% CI 0.66–1.45, P=0.825) for ADT use in patients with PCa compared with non-ADT patients (Table 2). After adjusting for age, comorbidities, and medication use, the adjusted HR of psoriasis was 0.95 (95% CI 0.63–1.45; P=0.816) in ADT patients. Tobacco-use disorder, NSAID, and ACEI use significantly increased the crude HR of psoriasis, but after adjustment, only ACEI use was found to be a significant risk factor for psoriasis (adjusted HR 2.14, 95% CI 1.09–4.20; P<0.05).
The KM curve showed a similar risk of psoriasis in both the ADT and non-ADT patients (Figure 2). We did further analysis on age as a factor and no statistically significant difference was found between the two groups (Table 3). Furthermore, the duration of ADT use was also analyzed, and we found no obvious differences with statistical significance.
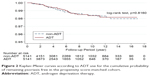  | Figure 2 Kaplan–Meier curves according to ADT use for the cumulative probability of remaining psoriasis free in the propensity score-matched cohort. |
  | Table 3 The risk of psoriasis and ADT by age |
Discussion
We performed a large-scale cohort study to fully investigate the association between ADT and the risk of psoriasis. We enrolled 10,282 patients newly diagnosed with PCa in a propensity score-matched analysis with multivariable regression models adjusted for age, comorbidities, and medication use. Patients with ADT use had no increased risk of psoriasis compared with non-ADT patients. Long-term deprivation of androgen may have impact on systemic diseases; our study revealed that ADT use did not increase risk of psoriasis in patients with PCa.
Several diseases are associated with psoriasis, including cardiovascular disease, dyslipidemia, impaired glucose tolerance, depressive disorder, and inflammatory bowel disorders.21 An increased risk of cardiovascular disease in patients with ADT has been previously reported.3,4 Reduced testosterone levels following ADT has been proved to decrease insulin sensitivity and increase body fat.4,22 In addition, ADT use is indicated to carry a higher risk of anemia and depressive disorders.23,24 Increased risk of rheumatoid arthritis has been observed in patients with ADT.10 Thus, ADT may play a role in the pathogenesis of autoimmune diseases. We hypothesized that ADT may change the risk of psoriasis. However, no statistically significant differences of subsequent psoriasis were found in current study.
Ziółkowska et al reported on exacerbation of psoriasis after ADT treatment in a 57-year-old patient with PCa in Poland.16 They supposed that alterations in the sex hormones may have triggered psoriasis.16 However, the underlying pathophysiologic mechanisms and the influences of ADT are complicated. In a study from the United Kingdom Clinical Practice Research Datalink, a decreased risk of ulcerative colitis was found in patients with ADT.25 Psoriasis has been reported to be associated with ulcerative colitis.26 Therefore, a possible link between ADT and psoriasis may exist but the complex interactions between ADT and autoimmune diseases are not fully understood. In addition, the typical characteristic of psoriasis is localized hyperproliferation of keratinocytes. T cell-mediated hyperproliferation (Th-1, Th-17, and Th-22 cells) and the overexpression of pro-inflammatory cytokines play important roles in the pathophysiology of psoriasis.13 Inflammatory cytokines are markedly elevated, and cytokine interactions have been reported to activate STAT1, STAT3, and NF-κB in patients with psoriasis.27 In addition, IL-6 and IL-17 have the ability to regulate keratinocyte proliferation in psoriatic lesions via STAT and NF-κB pathway,27 and androgen receptor-dependent, T cell-mediated immunomodulatory activities were observed in patients with ADT.28 ADT use reduced the Th-1 and Th-17 cell levels and also decreased the IL-6 level.29 Therefore, there is evidence to suggest that the use of ADT in PCa patients may reduce the incidence of psoriasis.
Psoriasis is a complex disease, which can be provoked or exacerbated by many endogenous or exogenous factors. Drug exposure is an important triggering factor including lithium, antihypertensive drugs, NSAIDs, antimalarial drugs, and ACEIs.30 This study demonstrated that antihypertensive drugs, NSAIDs, antimalarial drugs, and ACEIs resulted in an increased risk of psoriasis; however, only ACEIs use was identified as a significant independent risk factor for psoriasis (adjusted HR 2.14, 95% CI 1.09–4.20). ACEI-provoked psoriasis has been observed in previous reports.31,32 The peak age of psoriasis was 70 years and over in Taiwan.11,12,33
The strengths of our study lie in the large population-based database, and more than 10,000 PCa patients enrolled in our analysis. In addition, the NHIRD covers ~99% of the 23 million residents of Taiwan, making the analysis broadly representative. However, this study is subject to several limitations. First, details of the laboratory tests are not available in the NHIRD, and so further comparison of laboratory data could not be performed. The levels of sex hormones and inflammatory markers were not investigated. Second, the definite influences of ADT on psoriasis could not be identified directly, even with our cohort design study. Furthermore, the immune system may be altered in PCa patients; therefore, it is valuable to compare the impact of ADT in patients without PCa.34,35 However, it is difficult to compare the effects of androgen deviation in non-PCa patients. Third, although the diagnoses were defined using the ICD-9 coding system, the diagnosis of PCa was validated by RCPID, which was confirmed by specialists after reviewing pathological information or imaging findings. Finally, this is a retrospective study, so further prospective studies are needed to fully evaluate the relationship between ADT use and the risk of psoriasis.
In conclusion, this large-scale nationwide population-based study found that ADT use in patients with PCa does not increase the risk of psoriasis. This finding could provide helpful information for physicians in assessing the risks and benefits of ADT use. Further studies are required to have a better understanding of the relationship between ADT and psoriasis.
Acknowledgment
This study was supported by the Taoyuan General Hospital, Ministry of Health and Welfare (Grants No PYT10702), the Tri-Service General Hospital (Grants No TSGH-C106-148 and ATSGH-C107-213) and the Ministry of Science and Technology Taiwan (Grants No MOST 104–2320-B-016–012-MY3).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;2017(67):7–30. | ||
Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93(6):2042–2049. | ||
Levine GN, D’Amico AV, et al; American Heart Association Council on Clinical Cardiology and Council on Epidemiology and Prevention, the American Cancer Society, and the American Urological Association. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194–201. | ||
Shastri BR, Yaturu S. Metabolic complications and increased cardiovascular risks as a result of androgen deprivation therapy in men with prostate cancer. Prostate Cancer. 2011;2011:391576–391579. | ||
Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115(11):2388–2399. | ||
Owen PJ, Daly RM, Livingston PM, Fraser SF. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis. 2017;20(2):137–145. | ||
Mundell NL, Daly RM, Macpherson H, Fraser SF. Cognitive decline in prostate cancer patients undergoing ADT: a potential role for exercise training. Endocr Relat Cancer. 2017;24(4):R145–R155. | ||
Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. | ||
Kalina JL, Neilson DS, Comber AP, et al. Immune modulation by androgen deprivation and radiation therapy: implications for prostate cancer immunotherapy. Cancers. 2017;9(2):E13. | ||
Yang DD, Krasnova A, Nead KT, et al. Androgen deprivation therapy and risk of rheumatoid arthritis in patients with localized prostate cancer. Ann Oncol. 2018;29(2):386–391. | ||
Chang YT, Chen TJ, Liu PC, et al. Epidemiological study of psoriasis in the National Health Insurance database in Taiwan. Acta Derm Venereol. 2009;89(3):262–266. | ||
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and management of psoriasis and associated comorbidity (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. | ||
Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–255. | ||
Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25(6):606–615. | ||
Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–ii23. | ||
Ziółkowska E, Biedka M, Zyromska A, Makarewicz R. Psoriasis exacerbation after hormonotherapy in prostate cancer patient-Case report. Rep Pract Oncol Radiother. 2010;15(4):103–106. | ||
Klil-Drori AJ, Tascilar K, Yin H, Aprikian AG, Azoulay L. Androgen deprivation therapy and the incidence of autoimmune diseases. J Clin Oncol. 2015;33:e16010. | ||
Bureau of National Health Insurance DoH, Executive Yuan. The National Health Insurance Statistics; 2013. Available from: http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&webdata_id=1942&WD_ID=296. Accessed 18 September, 2014. | ||
Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. | ||
Liu JM, Lin PH, Hsu RJ, et al. Complementary traditional Chinese medicine therapy improves survival in patients with metastatic prostate cancer. Medicine. 2016;95(31):e4475. | ||
Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25(6):58–518. | ||
Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013;189(1 Suppl):S34–S44, discussion S3–S4. | ||
Chung SD, Kao LT, Lin HC, Xirasagar S, Huang CC, Lee HC. Patients receiving androgen deprivation therapy for prostate cancer have an increased risk of depressive disorder. PLoS One. 2017;12(3):e0173266. | ||
Hicks BM, Klil-Drori AJ, Yin H, Campeau L, Azoulay L. Androgen deprivation therapy and the risk of anemia in men with prostate cancer. Epidemiology. 2017;28(5):712–718. | ||
Klil-Drori AJ, Tascilar K, Yin H, Aprikian A, Bitton A, Azoulay L. Androgen deprivation therapy and the incidence of inflammatory bowel disease in patients with prostate cancer. Am J Epidemiol. 2016;184(1):15–22. | ||
Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23(5):561–565. | ||
Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. | ||
Ardiani A, Gameiro SR, Kwilas AR, Donahue RN, Hodge JW. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget. 2014;5(19):9335–9348. | ||
Morse MD, Mcneel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71(5):496–504. | ||
Tsankov N, Angelova I, Kazandjieva J. Drug-induced psoriasis. Recognition and management. Am J Clin Dermatol. 2000;1(3):159–165. | ||
Kim GK, del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated?: Understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3(1):32–38. | ||
Wolf R, Tamir A, Brenner S. Psoriasis related to angiotensin-converting enzyme inhibitors. Dermatologica. 1990;181(1):51–53. | ||
Tsai TF, Wang TS, Hung ST, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–46. | ||
Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control. 2009;20(7):1091–1105. | ||
Weng PH, Huang YL, Page JH, et al. Polymorphisms of an innate immune gene, toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysis. PLoS One. 2014;9(10):e110569. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

